Abstract
In animals and plants, pathogen recognition triggers the local activation of intracellular signaling that is prerequisite for mounting systemic defenses in the whole organism. We identified that Arabidopsis thaliana isoform CPK5 of the plant calcium-dependent protein kinase family becomes rapidly biochemically activated in response to pathogen-associated molecular pattern (PAMP) stimulation. CPK5 signaling resulted in enhanced salicylic acid–mediated resistance to the bacterial pathogen Pst DC3000, differential plant defense gene expression, and synthesis of reactive oxygen species (ROS). Using selected reaction monitoring MS, we identified the plant NADPH oxidase, respiratory burst oxidase homolog D (RBOHD), as an in vivo phosphorylation target of CPK5. Remarkably, CPK5-dependent in vivo phosphorylation of RBOHD occurs on both PAMP- and ROS stimulation. Furthermore, rapid CPK5-dependent biochemical and transcriptional activation of defense reactions at distal sites is compromised in cpk5 and rbohd mutants. Our data not only identify CPK5 as a key regulator of innate immune responses in plants but also support a model of ROS-mediated cell-to-cell communication, where a self-propagating mutual activation circuit consisting of the protein kinase, CPK5, and the NADPH oxidase RBOHD facilitates rapid signal propagation as a prerequisite for defense response activation at distal sites within the plant.
Keywords: disease resistance, plant innate immunity, ROS signaling
Receptor-mediated recognition of pathogen-associated molecular patterns (PAMPs) triggers the activation of inducible defenses against microbial pathogens in both plants and animals. Some of the earliest PAMP-induced intracellular signaling responses are shared in these two kingdoms (1), including changes in ion fluxes, an increase in the intracellular calcium concentration, the activation of protein kinases, or the synthesis of reactive oxygen species (ROS). These rapid responses are a prerequisite for the subsequent transcriptional reprogramming and alterations in hormone status that ultimately lead to resistance (2, 3). In contrast, the role of calcium-regulated protein kinase signaling in local and systemic immune responses is less well characterized. In the animal system, stimulation of Toll-like receptors TLR2 or TLR4 is known to result in the recruitment and activation of distinct calcium-responsive kinases Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC) and subsequent substrate-specific phosphorylation and signal transmission (4, 5). In Arabidopsis, equivalents to the CaMKII and PKC classes of calcium sensor protein kinases have not been identified. Instead, certain members of the plant calcium-dependent protein kinases (CDPKs; abbreviated as CPK for members of the Arabidopsis thaliana gene family) have been shown to mediate calcium-directed phosphorylation during plant defense activation (6, 7). CDPKs, which have a combined calmodulin-like calcium sensor and protein kinase effector domain (8), are attractive candidates for the translation of PAMP-induced intracellular changes in calcium concentrations into distinct local and distal immune responses. Several CDPKs (including NtCDPK2 and 3 in tobacco, StCDPK4 and 5 in potato, and several isoforms in Arabidopsis) are biochemically activated within a few minutes of exposure to pathogen-related stimuli and participate in the induction of early defense responses (9–12). In Arabidopsis, overexpression of AtCPK1 affects disease resistance toward various fungal pathogens (13). Also, the ectopic expression of constitutively active CDPK variants results in increased production of ROS, changes in gene expression, and altered phytohormone synthesis (9–11, 14). Furthermore, in combination with transcriptional profiling, CPK4, 5, 6, and 11 were identified as enzymes whose function was correlated with innate immune signaling mediated by the PAMP receptor FLS2 (14). CDPK silencing experiments in tobacco and potato and the characterization of double, triple, and/or quadruple cpk mutant lines in Arabidopsis has revealed compromised immune responses, including reduced pathogen-induced ROS production. However, these studies have not revealed in vivo mechanisms enabling CDPKs to activate rapid local, as well as sustained systemic defense responses via phosphorylation of an in vivo target. Also, these studies lack in planta experiments, in which the function of both the kinase and its phosphorylation target are mutually assessed in the absence of ectopic overexpression. Such studies do exist for some CDPK phosphorylation targets identified in abiotic stress signaling (15).
Plausible, but as yet in vivo biochemically undemonstrated, defense-related CDPK targets being discussed in the literature are plant NADPH oxidases (11, 12, 14, 16, 17). NADPH oxidases are integral membrane proteins encoded by the respiratory burst oxidase homolog (RBOH) gene family. The plant RBOH proteins differ from their mammalian counterparts by possessing an extended N-terminal domain with two EF-hand calcium-binding motifs and phosphorylation sites (18–22). RBOH enzymes catalyze the synthesis of the superoxide anion (O2−), which subsequently dismutates into hydrogen peroxide. MS analysis has revealed that the RBOHD protein becomes phosphorylated in response to the bacterial PAMP molecule flagellin in vivo (23, 24). Furthermore, rbohd mutants lack pathogen- or PAMP elicitor–induced ROS production, display altered disease susceptibility on infection with bacterial pathogens (18, 23, 25, 26), and are compromised in the systemic signal propagation required for long-distance signaling (27). Enhanced ROS production was observed on the transient ectopic overexpression of constitutively active CDPK variants lacking their regulatory protein domains (10, 11).
In this study, we identify CPK5 as a biochemically activated CDPK with a dual function in PAMP immune signaling and pathogen resistance and provide in vivo evidence for a rapid CPK5/RBOHD-mediated, ROS-based, signal propagation that is required for the constitution of distal immune reactions.
Results
CPK5 Is Rapidly Biochemically Activated on PAMP Stimulation and Mediates Cell Death.
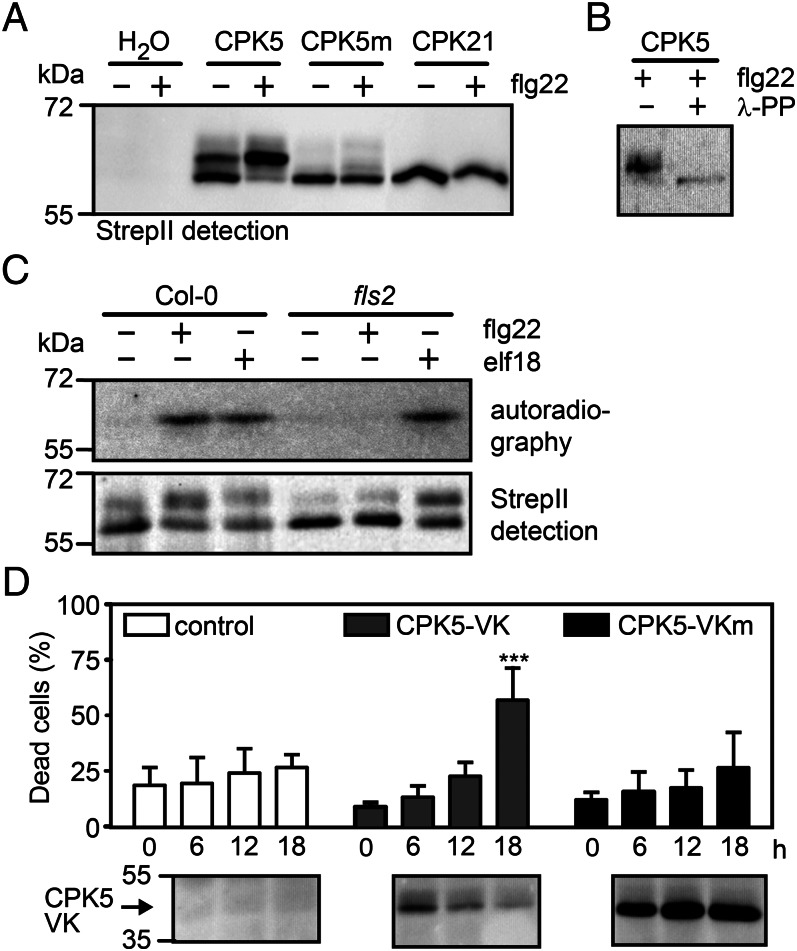
We conducted a combined gain-of-function/biochemical activation screen in Nicotiana benthamiana and Arabidopsis to select these members of the CPK family that become rapidly activated on PAMP stimulation. In a first round of selection, constitutively active, StrepII-tagged CPK variants were transiently expressed in leaf mesophyll cells of N. benthamiana leaves. Clones, representing members of the four CDPK subgroups (6), consisting of the N-terminal variable and protein kinase domains only (VK) and lacking their autoinhibitory region and calcium-binding domain (28), were created. Developing cell death symptoms, detected by Trypan blue staining as previously described for defense-related tobacco NtCDPK2-VK, were assessed (10, 29, 30). Only a few of the CDPKs tested, all belonging to subgroup I of the AtCPK gene family (6), were able to confer cell death symptoms in N. benthamiana leaves. Among these were CPK5-VK and CPK6-VK, but not CPK28-VK (SI Appendix, Fig. S1 A and B). In an in vitro protein kinase assay using affinity-purified enzymes, the activity was found to be lower than that of full-length CPK5 but to lack the calcium dependence seen in the full-length enzyme (SI Appendix, Fig. S2). Subsequently, full-length AtCPK5 was transiently expressed in Arabidopsis mesophyll protoplasts, and posttranslational enzyme modification and in-gel protein kinase activity were assessed after addition of the PAMPs flg22 (a 22 amino acid epitope of bacterial flagellin) or elf18 (18-amino acid peptide from bacterial elongation factor Tu). CPK5 became rapidly phosphorylated and activated. Flg22-induced activation of CPK5 was abolished in the fls2 mutant (lacking the cognate receptor for flg22) or when a kinase-deficient CPK5m variant was expressed in protoplasts (Fig. 1 A–C). A statistically significant increase in cell death development was also observed in Arabidopsis protoplasts upon ectopic expression of the constitutively active CPK5-VK, but not of a kinase-deficient CPK5-VKm (Fig. 1D).
Fig. 1.
CPK5 is rapidly biochemically activated on PAMP elicitation and induces cell death. (A) Flg22 induces biochemical modification of CPK5. On transient expression in Col-0 Arabidopsis mesophyll protoplasts, full-length CPK5 (StrepII tagged) showed biochemical modification, detected 10 min after treatment with buffer (−) or 200 nM flg22 (+) by immunoblot analysis. This effect was not seen with kinase-deficient CPK5m or CPK21. (B) Flg22-induced modification is caused by CDPK phosphorylation. CPK5 prepared as above was affinity purified and incubated for 10 min with λ-phosphatase before immunoblot analysis. (C) PAMP elicitation triggers CPK5 kinase activity. On expression in Col-0 or fls2 protoplasts, CPK5 protein kinase activation was analyzed 10 min after treatment with buffer (−), 200 nM flg22, or elf18 (+) by histone in-gel kinase assay (Upper) and immunoblot analysis (Lower). (D) Constitutively active CPK5-VK induces cell death in Arabidopsis. Transient expression of CPK5-VK, but not kinase-deficient CPK5-VKm, resulted in enhanced cell death development, detected by fluorescence microscopy after staining with fluorescein diacetate and propidium iodide (Upper). Error bars, SD (n = 3); two-way ANOVA, Bonferroni posttest, ***P < 0.001. Protein expression was monitored by immunoblot analysis (Lower).
CPK5 Mediates Plant Defense Responses and Bacterial Resistance.
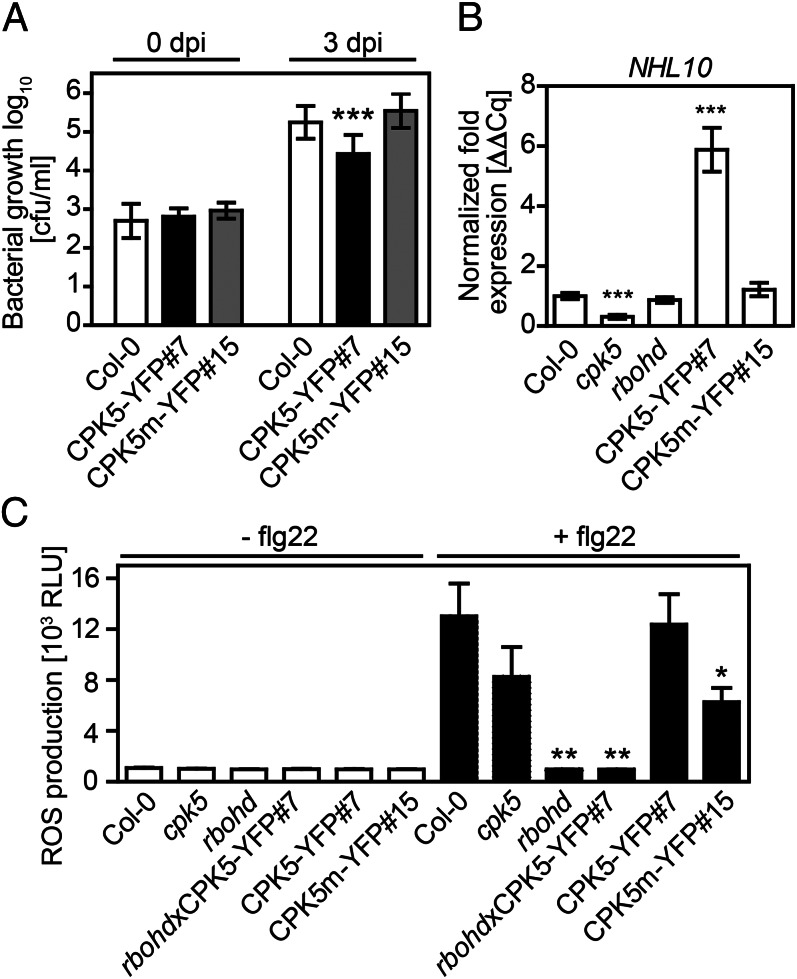
To explore the function of CPK5 in pathogen resistance in planta, independent transgenic Arabidopsis lines were generated in the Col-0 background that express full-length CPK5 or its kinase-deficient variant fused C-terminally to YFP. A high expressing line (35S::CPK5-YFP#2) displayed stunted growth and spontaneous cell death development detectable by Trypan blue staining. These symptoms were not as extreme in line 35S::CPK5-YFP#7 with lower CPK5-YFP expression levels and were absent in line CPK5m-YFP#15 overexpressing the kinase-deficient variant (SI Appendix, Fig. S3A). Interestingly, the enzyme was constitutively activated in both lines overexpressing CPK5-YFP but not in the line overexpressing the kinase-deficient CPK5m-YFP variant (SI Appendix, Fig. S3B). When we evaluated bacterial growth after infiltration of Pseudomonas syringae pv. tomato DC3000, enhanced resistance was observed in line 35S::CPK5-YFP#7 compared with both Col-0 and the kinase-deficient 35S::CPK5m-YFP#15 line (Fig. 2A). These data identify CPK5 as a PAMP-induced and biochemically activated positive regulator of plant resistance to biotrophic pathogens.
Fig. 2.
CPK5 mediates early defense reactions and pathogen resistance. (A) CPK5, but not kinase-deficient CPK5m, expressed in transgenic Col-0 plants, improves resistance to the bacterial pathogen Pst DC3000. Bacterial counts were taken in 6-wk-old Col-0, 35S::CPK5-YFP#7 and 35S::CPK5m-YFP#15 plants at day 0 and day 3 after inoculation of Pst DC3000. c.f.u., colony forming units. Error bars, SD (n = 12); one-way ANOVA, Dunnett posttest, ***P < 0.001. (B) CPK5 controls expression of the early flg22-induced gene NHL10. Basal gene expression was analyzed by qRT-PCR in 6-wk-old plants of Col-0, cpk5, and rbohd mutants and in transgenic CPK5- and CPK5m-expressing lines. Error bars, SEM (n ≥ 12); ***P < 0.0001. (C) CPK5 triggers flg22-induced ROS production. ROS production was determined over 60 min via a luminol-based assay with and without treatment with 200 nM flg22 in plants as in B and the cross resulting from the rbohd mutant and the transgenic CPK5-expressing line #7. RLU, relative light units; error bars, SEM (n = 8); one-way ANOVA, Dunnett posttest, *P < 0.05; **P < 0.01.
We then assessed the function of CPK5 in early PAMP-induced responses by quantitative RT-PCR (qRT-PCR) analysis of defense-related genes. NHL10 (NDR1/HIN1-LIKE10) was previously reported as an early transcribed CDPK- and MAPK-dependent gene (14). These genes showed enhanced expression in line 35S::CPK5-YFP#7, whereas reduced NHL10 transcript levels were observed in cpk5 (Fig. 2B). Furthermore, transcript accumulation in line 35S::CPK5-YFP#7 could be detected for PHI1 [PHOSPHATE-INDUCED 1; reported as a PAMP-induced CDPK-specific gene (14)], FRK1 (FLG22-INDUCED RECEPTOR KINASE 1), ICS1 (ISOCHORISMATE SYNTHASE 1), and PR1 (PATHOGENESIS-RELATED PROTEIN 1; SI Appendix, Fig. S4). Stunted growth, leaf rim chlorosis, and enhanced expression of ICS1 and PR1 genes suggested that CPK5 mediates bacterial resistance via the activation of salicylic acid (SA)-dependent signaling pathways (31). Indeed, quantification of the phytohormone SA in WT and transgenic uninfected plants revealed ∼10- and 3.5-fold higher concentrations of free SA in lines 35S::CPK5-YFP#2 (high overexpressing line with strong growth phenotype) and #7 (moderate overexpression of CPK5), but not in line #15 (carrying the kinase-deficient variant; SI Appendix, Fig. S5). Stunted growth, leaf chlorosis, and CPK5-dependent enhancement of SA levels were restored to the WT phenotype in plants resulting from a cross between 35S::CPK5-YFP#7 × 35S::NahG, expressing the bacterial SA-degrading enzyme salicylate hydroxylase NahG (SI Appendix, Fig. S5).
We also found that CPK5 positively regulated rapid, flg22-induced ROS production in Arabidopsis leaf discs recorded immediately after PAMP stimulation (Fig. 2C). Compared with Col-0, there was reduced ROS accumulation after addition of flg22 in the cpk5 mutant and in discs expressing the kinase-deficient form CPK5m, indicating a potential dominant negative effect of CPK5 on the ROS generation machinery. Furthermore, the high concentration of flg22-induced increase of ROS production observed in both Col-0 and in the CPK5 overexpressing line 35S::CPK5-YFP#7 provides evidence that a preexisting ROS generating machinery is controlled by rapid posttranslational modifications. No decrease in rapid flg22-induced ROS production was observed in leaf discs of the cpk6 mutant line (SI Appendix, Fig. S6A), despite the fact that the CPK6 isoform, when ectopically expressed in Arabidopsis protoplasts, showed flg22-induced biochemical activation (SI Appendix, Fig. S1 B and C) and led to cell death symptoms when expressed in N. benthamina (SI Appendix, Fig. S1B). As already described (23), no flg22-induced ROS production occurred in the rbohd mutant.
NADPH Oxidase RBOHD Is a Direct In Vivo Phosphorylation Target of CPK5.
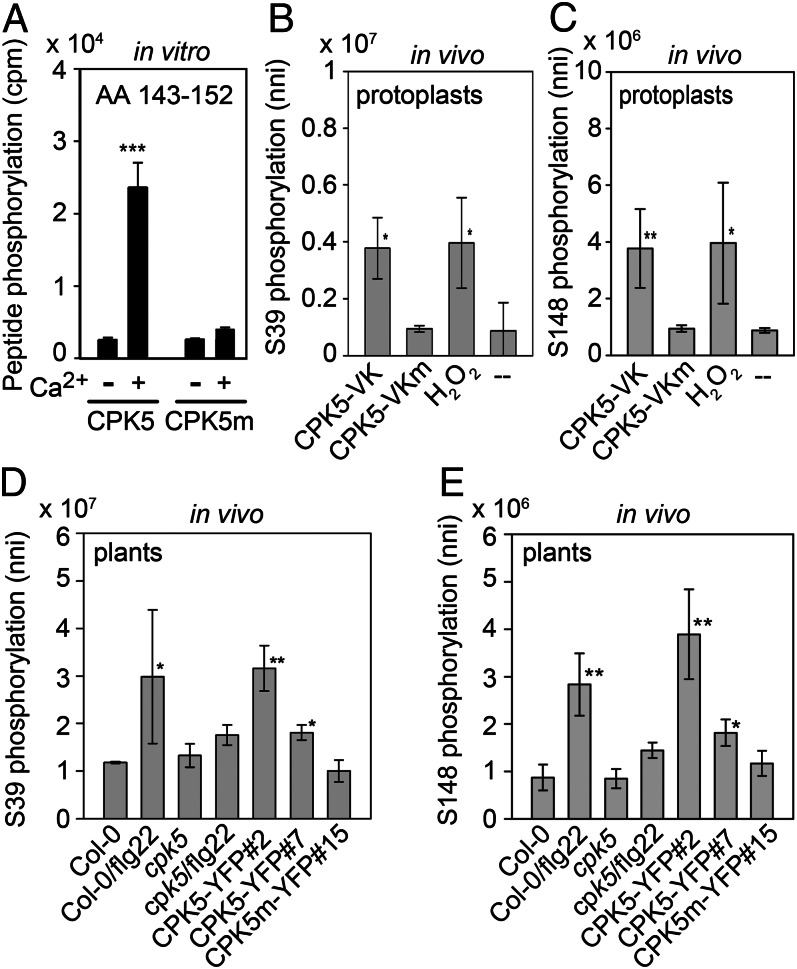
Although quantitative phosphoproteomics has identified flg22-induced, differentially phosphorylated amino acids in the N-terminal region of RBOHD in vivo (23, 24), the nature of the kinase is still unknown. We therefore assessed whether RBOHD is a direct phosphorylation substrate for CPK5. In in vitro protein kinase assays with enzyme purified from N. benthamiana leaves, we could demonstrate calcium-dependent phosphorylation of an 11-amino acid RBOHD (aa 143–152) peptide encompassing Ser148 by purified WT CPK5 but not by the kinase-deficient CPK5m (Fig. 3A). The RBOHD peptide had been selected from a preliminary screen in which synthetic peptides were simultaneously used as substrates for different CDPKs and phosphorylation was analyzed by MS (32). However, CDPKs can be prone to nonspecific substrate acceptance in in vitro protein kinase assays with recombinant enzymes or substrate proteins or by loss of plant cell type–specific expression pattern of the enzyme due to ectopic overexpression. We therefore assessed the in vivo phosphorylation of RBOHD by selected reaction monitoring (SRM) MS analysis of RBOHD peptides with known phosphorylation sites. Leaf material, in the presence or absence of PAMP stimulation and resulting from CPK5 overexpressing and mutant lines, was analyzed for the presence of differentially phosphorylated amino acids in the NADPH oxidase protein. In our in vivo study, four differentially phosphorylated peptides derived from the RBOHD N terminus were identified by comparing CPK5-overexpressing lines 35S::CPK5-YFP#2, 35S::CPK5-YFP#7, cpk5, and Col-0: GAF(pS)GPLGRPK (36-46, peptide I), VF(pS)R (146-149, peptide II), T(pS)(pS)AAIHALK (161-170, peptide III), and IL(pS)QML(pS)QK (341–349, peptide IV; SI Appendix, Fig. S7 A and B). Among these, peptides I, III, and IV had been detected previously in phosphoproteomic analysis of plasma membrane proteins after flg22 treatment (23, 24). Our analysis identified six serine residues, of which phosphorylation was increased at S39, S148, S163, and S347 in the CPK5 overexpressing lines but was unaltered or reduced in cpk5 (SI Appendix, Fig. S7B). In contrast, phosphorylation at S162 and S343 was enhanced in cpk5 and reduced in CPK5 overexpressing lines. These results suggest that other protein kinases besides CPK5 participate in the regulation of RBOHD phosphorylation in vivo, and a hierarchical order of phosphorylation at neighboring amino acids may exist. Interestingly, phosphorylation at Ser148 not only corresponds to the peptide that we have selected as in vitro substrate for CPK5 (Fig. 3A) but also to Ser97 from potato StRBOHB, the closest homolog to AtRBOHD (11). Potato StRBOHB Ser97 was phosphorylated in vitro by recombinant StCDPK4/5, as well as by ectopically expressed, constitutively active variants of these enzymes in N. benthamiana. A second reported StRBOHB phosphorylation site, at S82 (corresponding to AtRBOHD S133), did not overlap with our in vivo analysis (11).
Fig. 3.
CPK5 phosphorylates N-terminal serine residues of RBOHD in vitro and in vivo. (A) Protein kinase assay with full-length CPK5 and kinase-deficient CPK5m (affinity purified from Nicotiana benthamiana) in the absence (−) or presence (+) of 10 µM calcium using an N-terminal RBOHD peptide (aa 143–152) as substrate. Error bars, SEM (n =3); ***P < 0,0001. (B–E) Quantification of CPK5-dependent in vivo phosphorylation of RBOHD at S39 (B and D) and S148 (C and E) via SRM mass spectrometry. (B and C) Peptide phosphorylation after transfection of protoplasts with CPK5-VK, kinase-deficient CPK5-VKm, or 10 min after addition of 200 μM H2O2. (D and E) Peptide phosphorylation [normalized ion intensities (nni)] in rosette leaves of 4-wk-old Col-0 and cpk5 plants before and 1 h after treatment with 200 nM flg22 and of transgenic lines 35S::CPK5-YFP#2, 35S::CPK5-YFP#7, and 35S::CPK5m-YFP#15. Error bars, SD (n = 3); one-way ANOVA, Holm-Sidak posttest, **P < 0.01; *P < 0.5.
The PAMP-induced, CPK5-dependent in vivo phosphorylation of RBOHD was further validated by quantifying ion intensities for peptides I (pS39) and II (pS148) based on the coelution with stable-isotope labeled synthetic standard peptides (Fig. 3 B–E; SI Appendix, Fig. S7C). Increased levels of pS39 and pS148 were observed in CPK5-VK transfected protoplasts in the absence of elicitation in transgenic CPK5-overexpressing lines with and without elicitation, as well as after flg22 treatment of Col-0 plants and protoplasts. In contrast, flg22-induced pS39 and pS148 phosphorylation was compromised in cpk5 plants and no change in pS39 and pS148 levels occurred when kinase-deficient CPK5m and CPK5-VKm were expressed in plants and protoplasts, respectively. These results demonstrate a requirement for CPK5 in full PAMP- induced RBOHD phosphorylation in vivo. Remarkably, exposure of protoplasts to hydrogen peroxide also resulted in increased amounts of S39 and S148 phosphorylation. A coexpression analysis of CPK5-VK and RBOHD in N. benthamiana with mutant RBOHD carrying single amino acid substitutions confirmed that the identified phosphorylation sites are required for full ROS production (SI Appendix, Fig. S8).
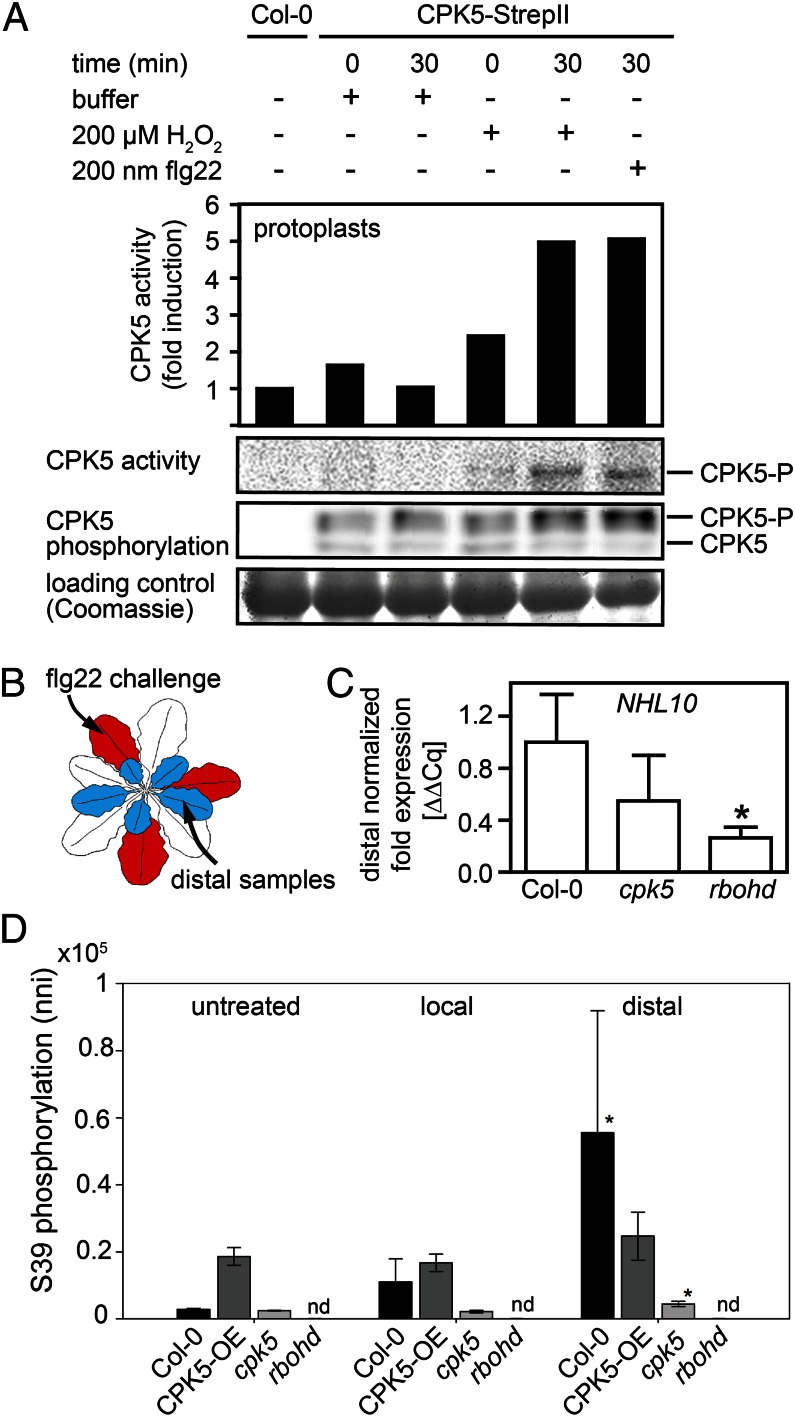
To confirm CPK5 involvement in RBOHD pS39 and pS148 phosphorylation induced by externally applied ROS in the form of H2O2 (Fig. 3 B and C), we investigated ROS-induced CPK5 modification and activation by assessing the in-gel protein kinase activity of CPK5 using the Arabidopsis protoplast system (Fig. 4A). As already observed for flg22 stimulation, H2O2 also induced rapid CPK5 phosphorylation and an increase in CPK5 kinase activity.
Fig. 4.
CPK5 and RBOHD are required for activation of distal defense responses. (A) Biochemical CPK5 kinase activity is induced by H2O2. Col-0 protoplasts expressing full-length CPK5 were treated for 30 min with buffer (−), 200 μM H2O2, or 200 nM flg22. Expression and CPK5 phosphorylation were monitored by Coomassie staining and immunoblot analysis. CPK5 kinase activity was determined by histone in-gel kinase assay, and fold induction of kinase activity was calculated by phosphoimaging. (B) Scheme of distal response study sampling. (C) Distal flg22-induced NHL10 expression is dependent on CPK5 and RBOHD. Forty-five minutes after 200 nM flg22 injection into a lower rosette leaf of 6-wk-old Col-0, cpk5, and rbohd plants, NHL10 gene expression was quantified by qRT-PCR in an upper leaf. Error bars, SEM (n ≥ 7); Student t test,*P < 0.05. (D) flg22-induced phosphorylation of RBOHD at S39 quantified via directed mass spectrometry in distal leaves harvested 15 min after treatment of lower leaves as under C. Error bars, SD (n = 3); pairwise t tests compared with untreated state, *P < 0.05.
CPK5 and RBOHD Are Required for Rapid Defense Response Activation at Distal Sites.
Our data are consistent with a proposed model for ROS-based cell-to-cell communication (27): ROS generated via NADPH oxidase RBOHD has been discussed as a possible component of a self-propagating mechanism for cell-to-cell communication, enabling long-distance signaling. Consistent with this hypothesis, rbohd plants were shown to lack the ability to propagate systemic signals rapidly. However, the function of plant NADPH oxidases depends on calcium-binding EF hands, as well as on phosphorylation at distinct amino acids in the RBOH N terminus. Thus, this model thus far fails to explain what drives activation of the NADPH oxidase. We postulate that CPK5 and RBOHD are part of a mutually activating circuit that facilitates signal autopropagation to distal sites, as a prerequisite for the induction of defense reactions. To test this hypothesis, we assessed the importance of CPK5 and RBOHD activities in long-distance signaling for the induction of biochemical and transcriptional defense responses at a distal plant site either 15 or 45 min after local application of flg22 as the triggering stimulus (Fig. 4B). Compared with Col-0, flg22-induced activation of NHL10 expression in distal leaves was reduced (to ∼50%) in cpk5 and strongly reduced (to ∼25%) in rbohd plants (Fig. 4C), although basal NHL10 expression in unstimulated rbohd plants was unaltered to Col-0 (Fig. 2B). Likewise, transcript amounts of PHI1, FRK1, and ZAT12 were decreased in distal tissues of cpk5 and rbohd (SI Appendix, Fig. S9). To further corroborate the requirement of CPK5 for rapid ROS-mediated signal propagation, we quantified biochemical the in vivo phosphorylation of distal RBOHD protein at S39 via SRM MS 15 min after flg22 stimulation in the same experimental setup (Fig. 4 B and D). Constitutive pS39 phosphorylation occurred in untreated, in local flg22-treated, and in distal leaves of the CPK5-overexpressing line. Consistent with the constitutive CPK5 kinase activity (SI Appendix, Fig. S3B), these data also explain the enhanced constitutive ROS levels accumulating in untreated plants over a prolonged time period of 18 h in these lines (SI Appendix, Fig. S3C). In Col-0, a flg22-induced RBOHD pS39 increase was observed within 15 min in local and in distal leaves, whereas in vivo phosphorylation was strongly reduced in cpk5 and absent in rbohd.
To further assess the requirement of RBOHD for CPK5-mediated pathogen resistance we generated a cross between 35S::CPK5-YFP#7 and rbohd. These plants showed an intermediate phenotype with respect to stunted growth and leaf chlorosis (SI Appendix, Fig. S9B) but retained CPK5-dependent enhanced pathogen resistance to Pst DC3000 (SI Appendix, Fig. S9C) even in the absence of RBOHD-mediated rapid ROS production (Fig. 2C). These data provide evidence for a dual role for CPK5 in rapid posttranslational regulation of ROS-mediated immune signal propagation, and, in its active form at distal plant sites, for sustained immune reactions involving transcriptional reprogramming and phytohormone signaling.
Discussion
In this study, we identified CPK5 of the Arabidopsis CDPK gene family as a positive regulator of innate immune signaling with a dual function in rapid signal propagation and in the induction of prolonged transcriptional and phytohormone-mediated defense responses mediating plant resistance to bacterial pathogens. Based on targeted MS (SRM) phosphopeptide analysis, we identified RBOHD as a direct in vivo phosphorylation target for CPK5. The use of targeted SRM in vivo phosphoproteomics enabled us to detect subtle differences in flg22-induced RBOHD phosphorylation in a single cpk mutant at the biochemical level. Particularly, by this method we could resolve these changes during signal propagation between local and distal plant tissues, demonstrating the power of targeted phosphoproteomics in signal transduction research.
Our analysis identified six stimulus-dependent serine residues in the N terminus of RBOHD, of which phosphorylation was increased in CPK5 overexpressing lines at S39, S148, S163, and S347, whereas it was unaltered or decreased at S162 and S343. Studies with coexpressed CPK5-VK and RBOHD in N. benthamiana confirmed a requirement of the identified phosphorylation sites for full ROS production (SI Appendix, Fig. S8), tallying with previous data, showing that a RBOHD S343A/S347A variant was unable to restore flg22-induced ROS production in an rbohd background (23). It was shown that no constitutive ROS production mediated by NADPH oxidases RBOHD or StRBOHB was achieved by phosphomimetic amino acid substitutions to aspartate (23, 33).
In CPK5-overexpression lines, constitutive RBOHD phosphorylation, detectable even in untreated plants, is consistent with sustained ROS accumulation in the entire plant over a prolonged period (SI Appendix, Fig. S3C). These overall ROS levels, detectable by nitroblue tetrazolium (NBT) staining, are neither significantly altered in rbohd nor cpk5. This result may be due to the low specificity and resolution of the NBT staining but also suggests the contribution of various enzymes to overall sustained ROS synthesis. In contrast, 60 min after PAMP stimulation by flg22, only residual flg22-induced in vivo phosphorylation of the RBOHD peptide was observed in cpk5 plants (Fig. 3 D and E), consistent with the reduced total ROS levels detected within 60 min after PAMP stimulation (Fig. 2C). This provides a mechanistic explanation for the incrementally reduced flg22-triggered ROS production in plants of double (cpk5,cpk6), triple, and quadruple (cpk5,cpk6,cpk11,cpk4VIGS) mutants, which has previously been reported (14), providing evidence that RBOHD is phosphorylated by several CPKs. In this context, it is interesting to note that rapid flg22-induced ROS production is not compromised in the cpk6 mutant, despite the observed biochemical activation of CPK6 on its ectopic expression in protoplasts (SI Appendix, Fig. S6). Guard cell–expressed CPK6 has been characterized in the regulation of anion channel activity controlling stomatal aperture (34, 35). Whereas an additional function in guard cell ROS signaling was suggested (36), CPK6 seems unlikely to be a key player in PAMP-triggered ROS production via RBOHD in leaf mesophyll cells.
Remarkably, RBOHD phosphorylation and activation at distal sites within the plant, analyzed 15 min after local PAMP stimulation, was almost abolished in cpk5 compared with the WT. In addition, reduced transcript levels of early CPK5-dependent genes of PHI1, NHL10, FRK1, or ZAT12 were observed in distal tissues 45 min after the triggering stimulus was applied in cpk5 and rbohd mutants. These data provide evidence for a role for mutual activation based on posttranslational modifications of CPK5 and RBOHD in rapid ROS production, which is required for signal propagation to distal plant sites as a prerequisite for distal defense response activation. A role for rapid, RBOHD-produced ROS, particularly during the early onset of the plant defense, is further supported by data showing enhanced bacterial pathogen growth in rbohd when analyzed at day 1 but not at day 3 (37).
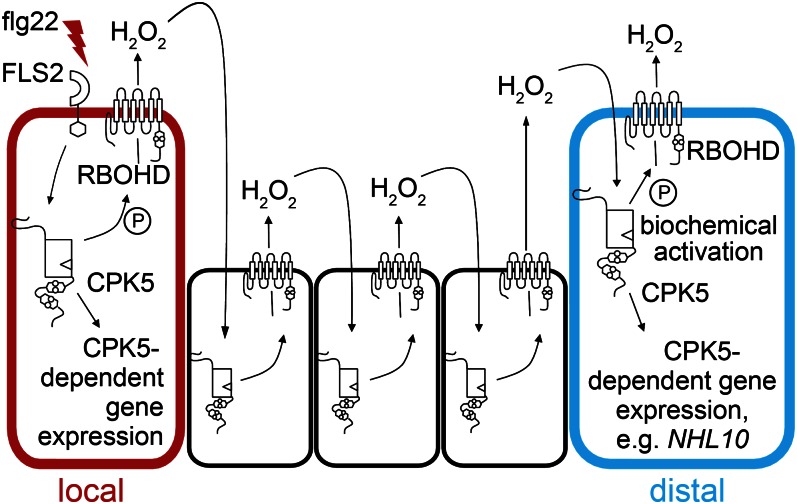
Based on our combined biochemical and functional data, we propose a dual function for CPK5 in signal propagation and defense response activation. In an extension of a preexisting model of extracellular signal propagation via an apoplastic ROS wave (38), we propose that CPK5 and RBOHD are key components of a self-propagating activation circuit mediating cell-to-cell communication (Fig. 5): perception of a (PAMP-) triggering stimulus initiates an immediate rise in intracellular calcium, causing the biochemical activation of CPK5, leading to the phosphorylation of RBOHD and other CPK5 substrates. Extracellular ROS generated by the NADPH oxidase, probably after dismutation of O2− to H2O2, represents the cell permeable signal to neighboring cells. Perception of H2O2 via an, as yet, unknown mechanism, serves as the stimulus for further reiterations of calcium-dependent CPK5 activation and RBOHD phosphorylation, resulting in a kinase/ROS-mediated activation relay (38, 39). As consequence of this relay, the signal is spread to distal plant cells, leading to the biochemical activation of CPK5 and thus to the induction of CPK5-mediated defense responses in these cells, ultimately driving pathogen resistance. This model is consistent with our observation that CPK5-overexpressing lines show enhanced pathogen resistance even in the absence of RBOHD enzyme. In these lines, ectopically CPK5 is present throughout the entire plant and has constitutively adopted its active form, triggering sustained innate immune responses. Thus, although the biochemical activation of CPK5 is rendered independent of an autopropagating ROS signal in these lines, sustained CPK5-controlled effects on transcriptional regulation and phytohormone-mediated responses lead to pathogen resistance.
Fig. 5.
Model for CPK5 and RBOHD activation circuit facilitating ROS-mediated cell-to-cell signal propagation.
Materials and Methods
DNA Constructs.
Coding regions of full-length CPK5 (At4g35310), truncated CPK5-VK, and kinase–deficient variants carrying amino acid substitution D221A were fused to StrepII-tag or YFP at the C terminus in plant expression vector pXCSG. YFP-tagged full-length RBOHD (At5g47910) constructs were cloned into pXNS2 (SI Appendix, SI Materials and Methods and Table S1).
Transient Protoplast Expression Assays.
Protoplast isolation and transient expression were conducted as described. Stress application was performed 14 h after transfection. Protein expression and CPK5 phosphorylation was monitored by immunoblot analysis, and protein kinase activity was determined by histone in-gel kinase assay or in vitro affinity-complex kinase assays with immobilized enzyme as described (SI Appendix).
Analysis of Transgenic CPK5 Overexpressing and Mutant Plants.
Transgenic plants carrying pXCS-CPK5-YFP and pXCS-CPK5m-YFP were generated following the Agrobacterium-based transformation via the floraldip method, and independent transformants were selected from seeds. ROS production was determined in CPK5 and CPK5m overexpressing, cpk5 (SAIL_657C06), cpk6 (SALK 025460C), and rbohd (SALK_070610) mutant lines using the luminol-based quantification. Bacterial growth assays were performed with 6-wk-old plants after syringe infiltration of Pst DC3000. Gene expression analysis by quantitative real-time RT-PCR was carried out using primers listed in SI Appendix, Table S1. Actin2 (At3g18780) was used as a control gene.
Targeted Analysis of Phosphorylation by Selected Reaction Monitoring.
Tryptic peptide mixtures resulting from rosette leaves of 4-wk-old plants or from protoplasts 6 h after transfection were analyzed by selected reaction monitoring using nanoflow HPLC and a triple quadrupole mass spectrometer. Ion intensities of target peptides GAF(pS)GPLGRPK and VF(pS)R originating from RBOHD were normalized based on ion intensities of coeluting stable-isotope labeled synthetic standard peptide and normalized ion intensities sums of fragment ions in each sample were calculated (SI Appendix).
Supplementary Material
Acknowledgments
We thank Karla Dünnbier and Ruth Lintermann for plant care and technical support, Birgit Kemmerling and Frederic Brunner for providing the fls2 mutant and 35S::NahG line, and Jane Parker for critical reading of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) within Priority Programme SPP1212 and Collaborative Research Centre CRC 973 (to T.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221294110/-/DCSupplemental.
References
- 1.Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 2.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 3.Schwessinger B, Zipfel C. News from the frontline: Recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol. 2008;11(4):389–395. doi: 10.1016/j.pbi.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell. 2009;35(1):48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005;17(3):385–394. doi: 10.1016/j.cellsig.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Harmon AC, Gribskov M, Harper JF. CDPKs: A kinase for every Ca2+ signal? Trends Plant Sci. 2000;5(4):154–159. doi: 10.1016/s1360-1385(00)01577-6. [DOI] [PubMed] [Google Scholar]
- 7.Harper JF, Harmon A. Plants, symbiosis and parasites: A calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6(7):555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 8.Kudla J, Batistic O, Hashimoto K. Calcium signals: The lead currency of plant information processing. Plant Cell. 2010;22(3):541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romeis T, Ludwig AA, Martin R, Jones JD. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 2001;20(20):5556–5567. doi: 10.1093/emboj/20.20.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig AA, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc Natl Acad Sci USA. 2005;102(30):10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi M, et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19(3):1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudsocq M, Sheen J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013;18(1):30–40. doi: 10.1016/j.tplants.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 63(3):526–540. [DOI] [PubMed]
- 14.Boudsocq M, et al. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature. 2010;464(7287):418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiger D, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal. 2011;4(173):ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- 16.Romeis T, Piedras P, Jones JD. Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell. 2000;12(5):803–816. doi: 10.1105/tpc.12.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tena G, Boudsocq M, Sheen J. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol. 2011;14(5):519–529. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138(4):414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara Y, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283(14):8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 20.Oda T, et al. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem. 2010;285(2):1435–1445. doi: 10.1074/jbc.M109.058909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki N, et al. Respiratory burst oxidases: The engines of ROS signaling. Curr Opin Plant Biol. 2011;14(6):691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Kimura S, et al. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta. 2012;1823(2):398–405. doi: 10.1016/j.bbamcr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 23. Nuhse TS, Bottrill AR, Jones AM, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51(5):931–940. [DOI] [PMC free article] [PubMed]
- 24.Benschop JJ, et al. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics. 2007;6(7):1198–1214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8(4):397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99(1):517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller G, et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal. 2009;2(84):ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 28.Cheng SH, Willmann MR, Chen HC, Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129(2):469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte CP, et al. Tobacco calcium-dependent protein kinases are differentially phosphorylated in vivo as part of a kinase cascade that regulates stress response. J Biol Chem. 2010;285(13):9740–9748. doi: 10.1074/jbc.M109.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witte CP, Noël LD, Gielbert J, Parker JE, Romeis T. Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol Biol. 2004;55(1):135–147. doi: 10.1007/s11103-004-0501-y. [DOI] [PubMed] [Google Scholar]
- 31.Du L, et al. Ca(2+)/calmodulin regulates salicylic-acid-mediated plant immunity. Nature. 2009;457(7233):1154–1158. doi: 10.1038/nature07612. [DOI] [PubMed] [Google Scholar]
- 32. Curran A, et al. (2011) Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci 2:36. [DOI] [PMC free article] [PubMed]
- 33.Kobayashi M, et al. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 2012;196(1):223–237. doi: 10.1111/j.1469-8137.2012.04226.x. [DOI] [PubMed] [Google Scholar]
- 34.Brandt B, et al. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA. 2012;109(26):10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scherzer S, Maierhofer T, Al-Rasheid KA, Geiger D, Hedrich R (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Molec Plant 5(6):1409–1412. [DOI] [PubMed]
- 36.Mori IC, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol. 2006;4(10):e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mersmann S, Bourdais G, Rietz S, Robatzek S. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 2010;154(1):391–400. doi: 10.1104/pp.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittler R, et al. ROS signaling: The new wave? Trends Plant Sci. 2011;16(6):300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol Plant. 2010;138(4):405–413. doi: 10.1111/j.1399-3054.2009.01321.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.