Abstract
Stress and glucocorticoid hormones regulate hippocampal neurogenesis, but the molecular mechanisms mediating these effects are poorly understood. Here we identify the glucocorticoid receptor (GR) target gene, serum- and glucocorticoid-inducible kinase 1 (SGK1), as one such mechanism. Using a human hippocampal progenitor cell line, we found that a small molecule inhibitor for SGK1, GSK650394, counteracted the cortisol-induced reduction in neurogenesis. Moreover, gene expression and pathway analysis showed that inhibition of the neurogenic Hedgehog pathway by cortisol was SGK1-dependent. SGK1 also potentiated and maintained GR activation in the presence of cortisol, and even after cortisol withdrawal, by increasing GR phosphorylation and GR nuclear translocation. Experiments combining the inhibitor for SGK1, GSK650394, with the GR antagonist, RU486, demonstrated that SGK1 was involved in the cortisol-induced reduction in progenitor proliferation both downstream of GR, by regulating relevant target genes, and upstream of GR, by increasing GR function. Corroborating the relevance of these findings in clinical and rodent settings, we also observed a significant increase of SGK1 mRNA in peripheral blood of drug-free depressed patients, as well as in the hippocampus of rats subjected to either unpredictable chronic mild stress or prenatal stress. Our findings identify SGK1 as a mediator for the effects of cortisol on neurogenesis and GR function, with particular relevance to stress and depression.
Keywords: antidepressants, hypothalamus–pituitary–adrenal axis, stem cells, neuroplasticity
Glucocorticoid hormones are consistently increased in severely depressed patients and in rodent stress models (1). A large body of evidence has demonstrated that chronically high concentrations of glucocorticoids impair hippocampal neurogenesis, that is, the development of new neurons from stem cells in the dentate gyrus, by activating the glucocorticoid receptor (GR) (1–6). However, the mechanisms by which GR activation reduces neurogenesis are not fully understood. Here we explore the role of the GR target gene, serum- and glucocorticoid-regulated kinase 1 (SGK1), in these effects.
SGK1 is a serine/threonine kinase implicated in the cellular stress response and neuronal function (7). We and others have previously shown that glucocorticoids increase SGK1 expression in human neural stem cells and in rodent neurons (3, 8). Importantly, SGK1 mediates some glucocorticoid effects on brain function; for example, acute stress enhances neuronal excitability and working memory in the rodent prefrontal cortex via glucocorticoid-induced activation of SGK1 (9), and chronic stress causes morphological oligodendrocyte abnormalities by up-regulating SGK1 in the murine corpus callosum (10). These data suggest that SGK1 may be a downstream mediator of glucocorticoid effects on the brain. However, recent evidence has also suggested that enhanced SGK1 expression is associated with greater GR-mediated gene expression in response to glucocorticoids (11). Therefore, another, nonmutually exclusive possibility is that SGK1 also directly enhances GR function and potentiates glucocorticoid effects. Here we examined both these possibilities.
We have previously used a human hippocampal progenitor cell line to examine the effects of glucocorticoids and antidepressants on neurogenesis (3, 6, 12), and we have shown that inhibition of Hedgehog signaling critically contributes to the reduction in neuronal differentiation upon treatment with the human glucocorticoid hormone, cortisol (6). Here we investigate the role of SGK1 in these effects of cortisol in vitro. Moreover, we analyze SGK1 gene expression in the peripheral blood of drug-free depressed patients, and in the hippocampus of rats exposed to unpredictable chronic mild stress (UCMS) or prenatal stress (PNS), two in vivo stress models known to precipitate depressive behavior and to decrease hippocampal neurogenesis (13–15). Our findings identify SGK1 as a key enzyme involved in the downstream mechanisms by which glucocorticoids reduce neurogenesis and in the upstream potentiation and maintenance of GR function, even after glucocorticoid withdrawal.
Results
SGK1 Mediates the Cortisol-Induced Decrease in Neurogenesis.
We have previously reported that GR activation increases SGK1 expression, and that GR-mediated concentrations of cortisol (CORT) decrease proliferation and neuronal differentiation of human hippocampal progenitor cells (3, 6). In particular, we have shown that the concentration of 100 µM CORT exerts consistent GR-mediated effects on cell proliferation and neuronal differentiation, and induces GR transactivation without saturating the receptor response (6). This concentration was thus used for the present study.
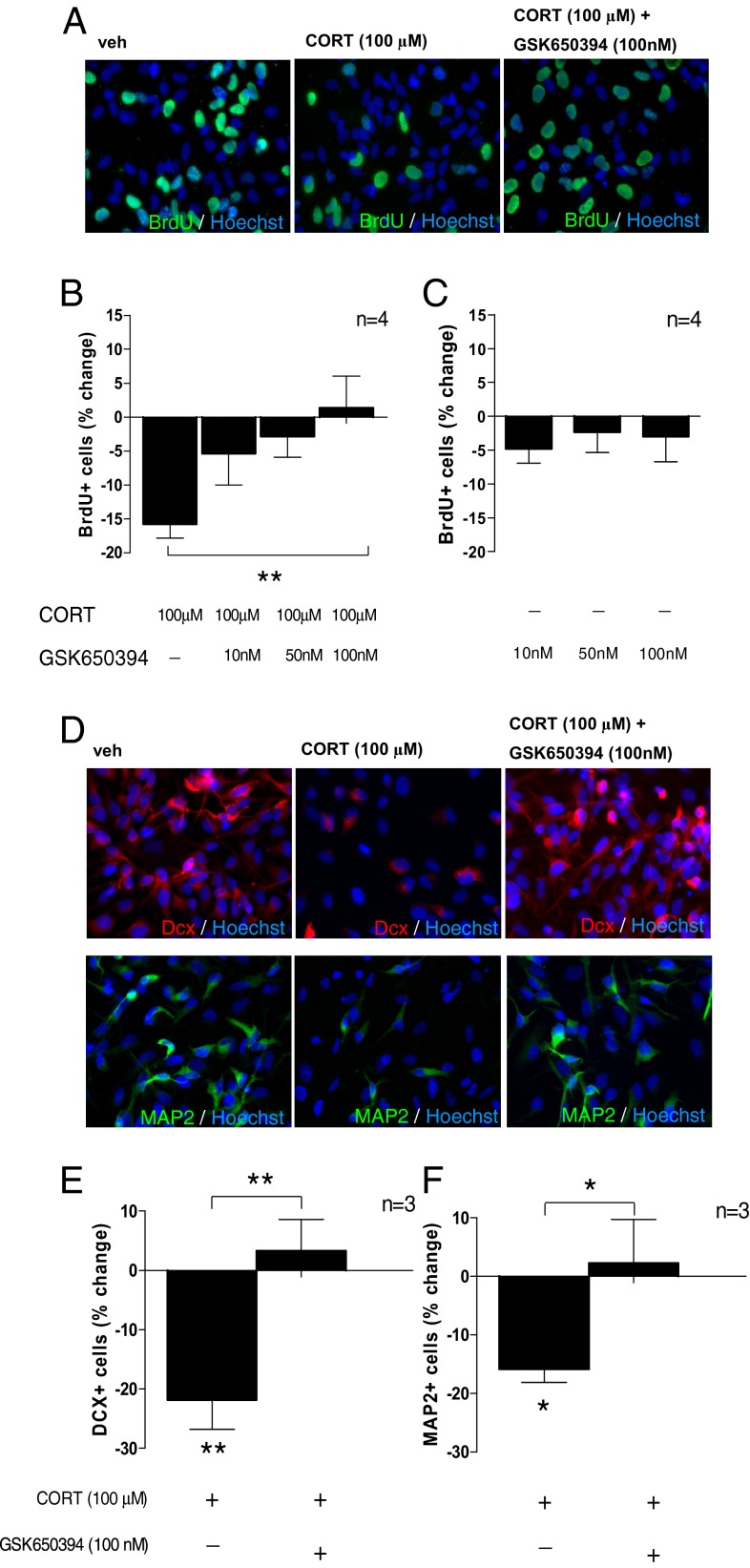
Here we used the small molecule inhibitor for SGK1, GSK650394 (16), to test whether SGK1 is mechanistically involved in the effects of CORT on neurogenesis. As hypothesized, GSK650394 (at 10 nM, 50 nM, and 100 nM) dose-dependently counteracted the CORT-induced reduction in BrdU-positive, proliferating progenitor cells (one-way ANOVA, P = 0.01, F1,4 = 4.17; Fig. 1 A and B). GSK650394 alone did not exert any effects on proliferation at these concentrations (one-way ANOVA, P = 0.84, F1,3 = 0.178; Fig. 1C), indicating that SGK1 indeed mediates the effect of CORT, but does not eliciting any effect by itself.
Fig. 1.
CORT (100 µM) decreases neurogenesis via an SGK1-dependent effect. (A) BrdU immunocytochemistry (ICC) was used to assess proliferation. (B) The SGK1 inhibitor, GSK650394 (10 nM, 50 nM, 100 nM) dose-dependently counteracted the CORT-induced reduction in proliferation (n = 4). (C) GSK650394 alone did not change proliferation (n = 4). (D) ICC for doublecortin (Dcx) and microtubule-associated protein 2 (MAP2) was used to assess neuronal differentiation. (E and F) GSK650394 (100 nM) counteracted the CORT-induced decrease in Dcx-positive neuroblasts (n = 3; E) and in MAP2-positive neurons (n = 3; F). Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001.
We also examined differentiation into Dcx-positive neuroblasts and into microtubule-associated protein 2 (MAP2)-positive neurons (Fig. 1D). In line with our findings on proliferation, the dose of GSK650394 that most effectively counteracted the effects of CORT on proliferation (100 nM, as shown in Fig. 1B) also counteracted the CORT-induced reduction in Dcx-positive (P = 0.003; Fig. 1E) and in MAP2-positive cells (P = 0.03; Fig. 1F). Taken together, these data demonstrate that CORT decreases hippocampal progenitor cell proliferation and neuronal differentiation via an SGK1-dependent mechanism.
Cortisol Increases SGK1 Expression.
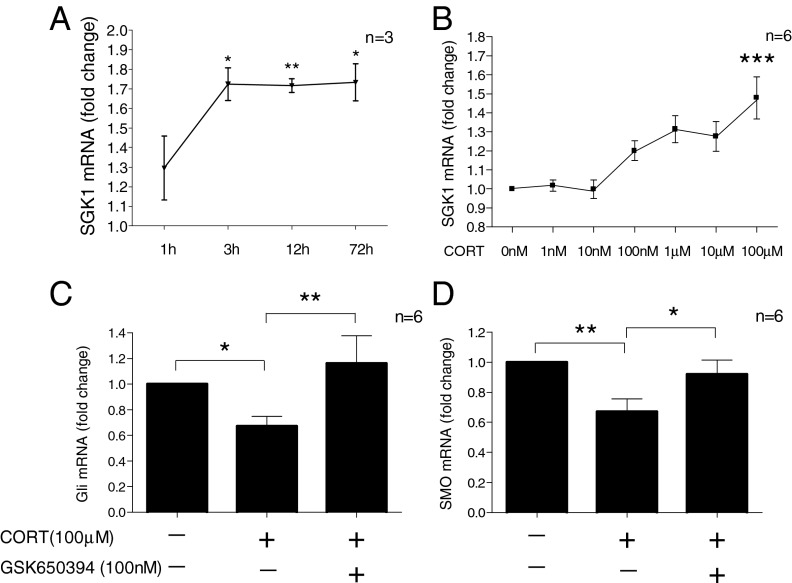
We then further characterized the time course of CORT-induced SGK1 expression. SGK1 mRNA was marginally elevated after 1 h (1.3 ± 0.2 fold, P = 0.11), but significantly increased after 3 h (1.7 ± 0.08 fold, P = 0.01), 12 h (1.7 ± 0.03, P = 0.002) and 72 h (1.7 ± 0.09 fold, P = 0.02) of treatment (Fig. 2A). Moreover, the GR-antagonist, RU486 (50 nM), abolished the CORT-induced increase in SGK1 mRNA (Fig. S1A), confirming that this effect is GR-dependent. In line with our findings on the mRNA level, no changes in SGK1 protein were observed after 1 h of CORT treatment (1.4 ± 0.5 fold, P = 0.5), whereas treatment for 12 h significantly increased SGK1 protein levels (by 4.4 ± 1.1 fold, P = 0.02) (Fig. S1B). Finally, after 12 h of treatment, SGK1 expression was significantly increased only by high concentrations of CORT (100 µM), but not by lower concentrations (1–100 nM) (Fig. 2B). This result is in line with our previous data, showing that 100 µM CORT predominantly affects the GR, whereas 1–100 nM CORT predominantly affects the MR in the same cells (6).
Fig. 2.
CORT increases SGK1 expression and inhibits Hedgehog signaling. (A) CORT (100 µM) increases SGK1 mRNA expression after 3, 12, and 72 h (n = 3). (B) CORT (1 nM to 100 µM for 12 h) dose-dependently increases SGK1 mRNA expression (n = 6). (C and D) GSK650394 (100 nM) counteracts the CORT (100 µM)-induced decrease of the Hedgehog pathway genes Gli (n = 6; C) and SMO (n = 6; D). Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001.
SGK1 Is Involved in Cortisol-Induced Changes in Molecular Signaling Pathways.
We have previously shown that cortisol decreases neuronal differentiation by inhibiting Hedgehog signaling (6). Here we conducted Affymetrix gene expression microarray and pathway analysis on mRNA samples of cells treated with CORT (100 µM) and GSK650394 (100 nM). Using this approach, we confirm and extend our previous findings, by showing that CORT significantly decreases Hedgehog signaling (vehicle vs. 100 µM CORT; P = 0.04, n = 5) and that this effect is counteracted by GSK650394 (vehicle vs. 100 µM CORT + 100 nM GSK650394; P = 0.22, n = 5). Accordingly, quantitative real-time PCR (qRT-PCR) analysis confirmed that the CORT-induced decrease in expression of the key Hedgehog genes, glioma-associated oncogene (Gli) and Smoothened (Smo) was counteracted by GSK650394 (one-way ANOVA for Gli: P = 0.005, F1,3 = 6.63, Fig. 2C; for Smo: P = 0.006, F1,3 = 6.9, Fig. 2D).
SGK1 Mediates the Cortisol-Induced Increase in GR Phosphorylation.
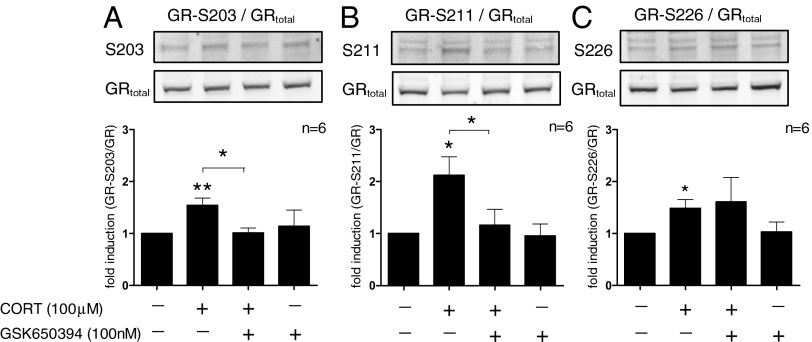
Next, we wanted to test whether SGK1 may also act as an “upstream” regulator of the GR. Therefore, we investigated a critical component of GR function: phosphorylation at the serine residues S203, S211, and S226. CORT treatment for 12 h induced phosphorylation at the phospho-sites S203 (by 1.5 ± 0.1 fold, P = 0.007), S211 (by 2.1 ± 0.4 fold, P = 0.01), and S226 (by 1.5 ± 0.2 fold, P = 0.03). Interestingly, the SGK1-inhibitor, GSK650394 (100 nM), blocked the CORT-induced phosphorylation at S203 (P = 0.01) and S211 (P = 0.04), but not at S226 (P = 0.4) (Fig. 3 A–C). Importantly, SGK1 was involved in GR phosphorylation only when SGK1 expression was increased (that is, after 12 h of cortisol treatment), but not at low, basal levels. In fact, after 1 h of CORT treatment, when SGK1 levels are not yet elevated (as shown in Fig. 2A and Fig. S1B), GR phosphorylation was already increased at S203 (by 2.5 ± 0.5-fold, P = 0.04), S211 (by 6.9 ± 2.4-fold, P = 0.04), and S226 (by 2.8 ± 0.9-fold, P = 0.07), but these effects were not counteracted by GSK650394 (Fig. S2 A–C). Kinases involved in this early, SGK1-independent GR phosphorylation are presented in SI Results and Fig. S3.
Fig. 3.
SGK1 mediates CORT effects on GR phosphorylation. After 12 h, CORT (100 µM) increases GR phosphorylation at S203 (n = 6; A), S211 (n = 6; B), and S226 (n = 6; C). GSK650394 (100 nM) counteracted the increase in S203 and S211 phosphorylation. Data are mean ± SEM *P < 0.05, **P < 0.01.
SGK1 Mediates Cortisol-Induced GR Nuclear Translocation.
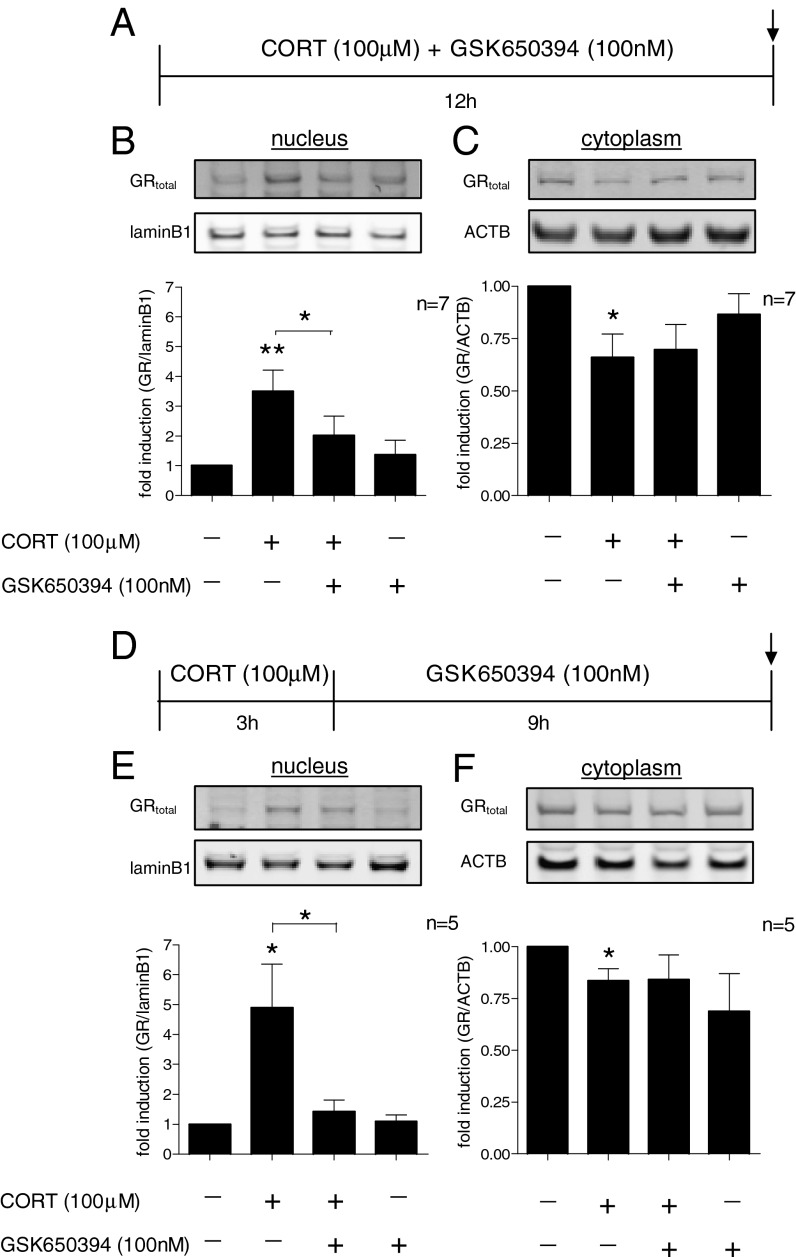
Having demonstrated that SGK1 mediates the cortisol-induced increase in GR phosphorylation at the S203 and S211 phospho-sites, which enhance GR nuclear translocation, but not at the S226 site, which inhibits nuclear translocation, we then directly assessed SGK1-dependent changes in GR nuclear translocation upon cortisol treatment. As expected, after 12 h of treatment, CORT induced GR nuclear translocation as indicated by a 3.5-fold increase in GR nuclear protein (P = 0.008; Fig. 4 A and B). Cotreatment with GSK650394 counteracted this effect (P = 0.02; Fig. 4B), demonstrating that CORT induces GR translocation via an SGK1-dependent mechanism. In contrast, cytoplasmic levels of GR were decreased upon CORT treatment (by 44%, P = 0.02), but this decrease was not counteracted by GSK650394 (Fig. 4C), suggesting that this effect may rather reflect an SGK1-independent decrease in overall GR expression. This finding was confirmed by a separate set of experiments in which we found that CORT decreases GR protein expression in whole cell lysates (by 20%, P = 0.04), and that this effect was also not counteracted by GSK650394 (Fig. S4). Taken together, these data demonstrate that SGK1 is also involved in regulating GR activation and translocation during glucocorticoid exposure.
Fig. 4.
SGK1 mediates and maintains CORT effects on GR nuclear translocation. (A) Cotreatment with CORT (100 µM) and GSK650394 (100 nM) for 12 h. (B) GSK650394 counteracted the CORT-induced increase in nuclear GR (n = 7). (C) The CORT-induced decrease in cytoplasmic GR was not counteracted by GSK650394 (n = 7). (D) CORT treatment for the first 3 h and GSK650394 treatment during the subsequent 9 h in the absence of any CORT. (E) CORT (for only 3 h) was sufficient to induce GR nuclear translocation after a total incubation of 12 h. GSK650394 (during the 9-h period after cortisol) abolished the CORT-induced increase in nuclear GR (n = 5). (F) CORT (for only 3 h) decreased cytoplasmic GR. This effect was not counteracted by subsequent GSK650394 treatment (n = 5). Data are mean ± SEM *P < 0.05, **P < 0.01.
SGK1 Maintains GR Activation in the Absence of Cortisol.
Having shown that CORT increases SGK1 levels, and that these increased SGK1 levels mediate the CORT-induced GR phosphorylation and nuclear translocation, we then tested the hypothesis that SGK1 may maintain GR activation even after CORT has been removed. Therefore, we treated cells with CORT for 3 h, a time long enough to significantly induce SGK1 mRNA expression (as shown in Fig. 2A). We then extensively washed cells in CORT-free media and investigated GR nuclear translocation after a total incubation time of 12 h, that is, after further 9 h of incubation in the absence of CORT (Fig. 4D). At 12 h, even though CORT had not been present for the last 9 h, SGK1 protein expression was still increased (by ∼2-fold, P = 0.04) and the GR was still translocated to the nucleus (by ∼4.5-fold, P = 0.04; Fig. 4E). Indeed, this persistent GR translocation in the absence of CORT was maintained by SGK1, because it was counteracted when SGK1 was blocked during the 9-h period after the end of the initial 3-h CORT treatment (P = 0.02; Fig. 4E, third column). Again, and in line with our data on continuous CORT treatment for 12 h, cytoplasmic GR was decreased even after CORT removal (by 17%, P = 0.02), and this effect was not counteracted by the SGK1 inhibitor (Fig. 4F), likely reflecting an SGK1-independent reduction in overall GR expression, as discussed above.
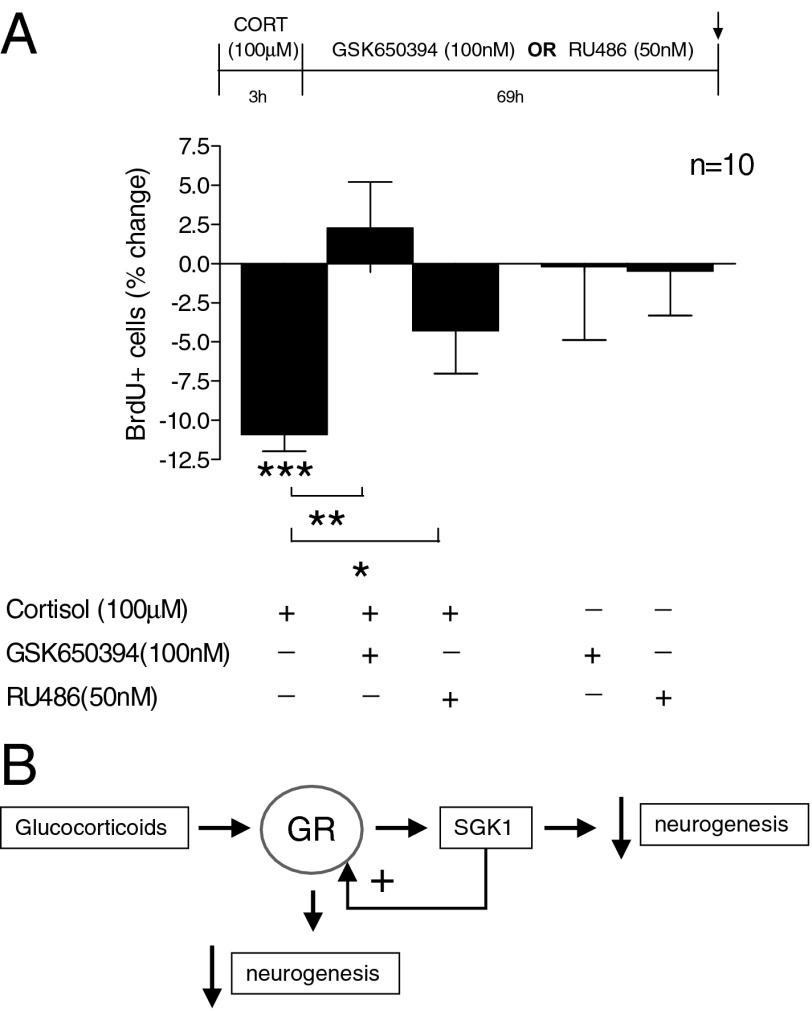
SGK1 Mediates the Decrease In Progenitor Proliferation After Cortisol Removal.
In light of our finding that SGK1 maintains GR translocation even after CORT removal, we next tested whether progenitor cell proliferation was also decreased under these conditions. We thus treated cells with CORT for 3 h, washed them extensively in CORT-free media, and then investigated proliferation 69 h later (that is, after a total incubation time of 72 h). Even though CORT was only present for the first 3 h of incubation, it was sufficient to reduce proliferation to levels comparable to those after continuous 72-h treatment (∼11%, P = 0.0009; Fig. 5A, first column). We then incubated cells with the SGK1 inhibitor, GSK650394, during the 69-h period after the initial 3-h CORT stimulus, that is, in the absence of any CORT. As hypothesized, this treatment completely abolished the CORT-induced reduction in proliferation (P = 0.0012; Fig. 5A, second column). These data thus suggest that the CORT-induced SGK1 expression maintains GR activation even after CORT removal, and that this SGK1-dependent GR activation (in the absence of CORT) is functionally relevant for prolonging the effects of CORT on hippocampal progenitor proliferation.
Fig. 5.
SGK1 effects on proliferation occur upstream and downstream of the GR. (A) CORT (100 µM) for only the first 3 h of a 72-h incubation period was sufficient to reduce the number of BrdU-positive cells (first column). Treatment with GSK650394 (100 nM) during the 69-h period after the initial 3-h CORT stimulus abolished the CORT-induced reduction in proliferation (second column), whereas the GR antagonist RU486 (50 nM) partially counteracted the effect of CORT (third column). n = 10. Data are mean ± SEM *P < 0.05, **P < 0.01; ***P < 0.001. (B) Diagram depicting the proposed model of SGK1-dependent regulation of neurogenesis and GR function.
SGK1 Effects on Proliferation Occur both Upstream and Downstream of the GR.
In the above-described experiments, we have demonstrated a role for SGK1 in mediating cortisol effects on human hippocampal progenitor cells. We now wanted to clarify whether these effects of SGK1 were predominantly downstream or upstream of GR activation. Therefore, we treated cells as above (CORT for only 3 h, then investigated proliferation 69 h later), but in the presence of the GR antagonist, RU486 (50 nM), during the 69-h period after CORT treatment. We had previously demonstrated that coincubation with RU486 blocks the CORT-induced reduction in proliferation after 72 h (6). In these novel experimental conditions, if the effects of the 3-h CORT treatment on proliferation were blocked by RU486 (during the 69 h after the initial CORT stimulus), it would indicate that the decrease in proliferation was dependent on the ability of SGK1 to maintain GR activation; in contrast, if the effects of the 3-h CORT treatment were not blocked by RU486 (during the 69 h after CORT), it would indicate that increased levels of SGK1 were sufficient to exert all downstream effects leading to decreased proliferation. Interestingly, treatment with RU486 only partially counteracted the reduction in proliferation (by ∼60%, P = 0.04; Fig. 5A, third column). These data thus suggest that SGK1 mediates the CORT-induced reduction in hippocampal progenitor proliferation by acting both upstream of GR, by enhancing GR function, as well as downstream of GR, by regulating neurogenesis-relevant molecular targets (Fig. 5B).
Increased SGK1 mRNA Expression in Depressed Patients.
To corroborate the clinical relevance of our cellular and molecular findings, we measured SGK1 mRNA expression in peripheral blood of patients with major depression, a condition in which both hypercortisolemia and reduced neurogenesis are considered relevant pathophysiological mechanisms. We have previously described candidate gene expression profiles associated with GR function and neuroplasticity in the Genome-based Therapeutic Drugs for Depression (GENDEP) study (17). Here we analyzed SGK1 expression in a subpopulation of this cohort. All patients (n = 25) were drug-free for at least 2 wk before assessment, and 18 of these 25 patients were drug naïve. Controls (n = 14) had no present or past psychiatric disorder and did not take any hormonal treatment. There were no significant differences in age and sex between patients and controls (P = 0.97 for age, and P = 0.74 for sex). The full patients’ clinical characteristics and inclusion criteria are described in the SI Materials and Methods.
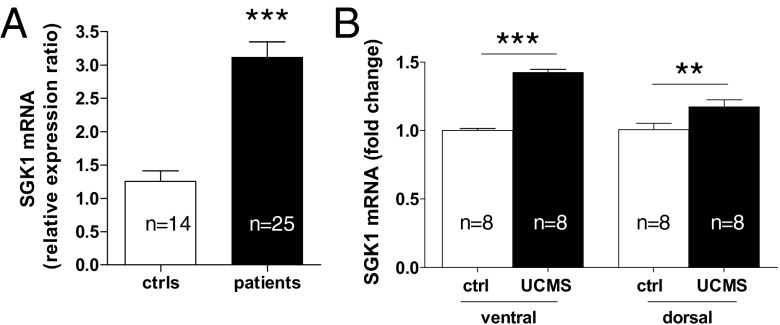
In line with our data on human hippocampal progenitor cells, we found that depressed patients had significantly higher SGK1 mRNA levels (by ∼2.5-fold; controls: 1.26 ± 0.16, patients: 3.11 ± 0.24, P < 0.0001, Fig. 6A). Even though our sample included more male than female patients (n = 15 males, n = 10 females), there was no effect of sex on SGK1 mRNA expression in controls (female controls: 1.27 ± 0.3, n = 7, male controls: 1.24 ± 0.13, n = 7; P = 0.9) or depressed patients (female depressed: 3.14 ± 0.3, n = 10, male depressed: 3.09 ± 0.3, n = 15; P = 0.9). Moreover, we correlated SGK1 expression with the expression of GR-relevant genes that we had previously analyzed in the same individuals (17). Consistent with our in vitro data showing decreased GR in the presence of increased SGK1 expression, SGK1 mRNA levels correlated negatively with GR levels in our clinical sample (r = −0.32, P = 0.046). Moreover, SGK1 correlated positively with mRNA levels of the GR-target gene, FK506 binding protein 5 (FKBP5) (r = 0.45, P = 0.004), and negatively with genes involved in neuroplasticity [brain-derived neurotrophic factor (BDNF): r = −0.32, P = 0.05; VGF: r = −0.33, P = 0.037; p11: r = −0.43, P = 0.007].
Fig. 6.
SGK1 mRNA expression is increased in drug-free depressed patients (ctrls, n = 14; patients, n = 25) (A) and the ventral and dorsal hippocampus of rats upon unpredictable chronic mild stress (UCMS, n = 8; B). Data are mean ± SEM; **P < 0.01; ***P < 0.001.
Increased Hippocampal SGK1 Expression in Rodent Stress Models.
Finally, we analyzed SGK1 mRNA expression in the hippocampus of adult rats exposed to two well established rodent models of depression, characterized by both increased corticosterone levels and reduced neurogenesis (13–15): unpredictable chronic mild stress (UCMS) and prenatal stress (PNS). UCMS has been shown to reduce hippocampal neurogenesis in the ventral and in the dorsal hippocampus (18). Accordingly, in our experiments, UCMS significantly increased SGK1 expression both in the ventral hippocampus (by ∼1.4-fold, P < 0.001, n = 8) and in the dorsal hippocampus (by ∼1.2-fold, P < 0.01, n = 8) (Fig. 6B). In addition, we had previously shown that PNS causes increased blood corticosterone levels and molecular signaling changes in the rat hippocampus, including a reduction in Hedgehog signaling (6). For the current study, we analyzed SGK1 mRNA expression in the whole hippocampus of the same rats and found an increase of ∼1.4-fold (P = 0.02, n = 5, Fig. S5). These data in rodent stress models thus further support an important role for SGK1 in stress and glucocorticoid effects on the hippocampus.
Discussion
Here we demonstrate that the GR target gene, SGK1, mediates the cortisol-induced decrease in proliferation and neuronal differentiation of human hippocampal progenitor cells, by acting both downstream of GR activation (via SGK1-dependent inhibition of the Hedgehog pathway) and upstream of GR activation (via SGK1-dependent GR phosphorylation and nuclear translocation). Additionally, we find that SGK1 mRNA is increased in the peripheral blood of drug-free depressed patients and in the hippocampus of rats after unpredictable chronic mild stress and prenatal stress. Our findings identify SGK1 as a mechanism for mediating glucocorticoid effects on neurogenesis and for enhancing GR activation, which may be of particular relevance for stress-induced mental disorders, such as depression.
Although SGK1 was initially described for its role in regulating sodium transport in renal collecting duct cells (19), recent studies have provided evidence for a novel role of this kinase in stress and glucocorticoid actions on the brain (9, 10). Our study extends these previous findings by showing that SGK1 also mediates the inhibitory effects of cortisol on proliferation and neuronal differentiation of human hippocampal progenitor cells. Moreover, we find that inhibition of Hedgehog signaling may be one of the molecular mechanisms resulting from the increased SGK1 expression by cortisol.
Importantly, we demonstrate that SGK1 is not only a mechanism “downstream” of GR activation, but is in fact also involved in regulating GR function. Specifically, the GR-mediated increase in SGK1 expression upon cortisol treatment further enhances GR phosphorylation at the serine residues S203 and S211, which are phosphorylation sites known to facilitate nuclear translocation of the receptor (20, 21). Accordingly, SGK1 enhances and maintains GR nuclear translocation even after cortisol withdrawal. This sustained SGK1-mediated GR activation in the absence of cortisol has functional consequences and results in a reduction in progenitor proliferation that is abolished by inhibiting SGK1 even after cortisol has been removed. This interpretation is consistent with studies showing that a single-nucleotide polymorphism in the SGK1 promoter region, with a putative effect on increasing SGK1 expression upon glucocorticoid treatment, enhances glucocorticoid-mediated gene transcription (11). Therefore, we propose that GR-dependent SGK1 up-regulation may be a mechanism for maintaining and prolonging GR activation after exposure to increased glucocorticoid levels, such as during stress, and even after glucocorticoid levels have normalized.
Our human in vitro data appear to be relevant also in vivo both in rodents and in humans, as we have identified increased SGK1 mRNA expression in the hippocampus of rats exposed to two different types of stress, and in the blood of drug-free depressed patients. Specifically, UCMS increased SGK1 expression both in the ventral and in the dorsal hippocampus, which is in line with studies showing that UCMS also decreases neurogenesis in both of these regions (18). Considering that the ventral hippocampus has been implicated in mood, whereas the dorsal hippocampus has been implicated predominantly in cognition (22), the role of SGK1 in the effects of glucocorticoids on neurogenesis and behavior in rodent stress models will be an interesting line of future investigation.
The negative correlation between SGK1 and levels of three genes involved in neuroplasticity in our clinical sample (BDNF, VGF, and p11), may offer an indication that this mechanism may also potentially be involved in regulating neurogenesis in depression. However, although adult hippocampal neurogenesis has repeatedly been implicated in stress-induced depressive symptoms and antidepressant treatment response in rodents (23, 24), the functional role of neurogenesis in humans remains elusive. Indeed, compared with rodents, relatively few neurons are being added to the human brain (25), and human postmortem brain studies have delivered conflicting results with regards to changes in hippocampal cell proliferation in depression and upon antidepressant treatment (26–28). It is also important to mention that one previous report has examined SGK1 mRNA expression in depressed patients, and found no difference compared with controls (29); this may likely be due to the fact that in their study, 29 of the 40 patients were on antidepressants, and not drug-free as in our study.
Interestingly, under certain conditions stress may also lead to an increase in neurogenesis in the presence of elevated glucocorticoids, particularly when the stress has hedonic value, for example, during mating (30), environmental enrichment (31) or physical exercise (32). Indeed, some studies have suggested that concomitant regulation of protective factors, such as oxytocin, may contribute to the neurogenesis increase in these situations (33). Whether SGK1 also contributes to such “protective” effects of glucocorticoids remains to be elucidated.
Some limitations of our study need to be considered: First, although we have determined that the cortisol effects in our in vitro experiments are influenced by albumin in the cell culture media (SI Results and Fig S6), it is difficult to estimate how the cortisol concentrations in our cell culture experiments compare with physiological cortisol concentrations in the human hippocampus in vivo. Second, the sample size of our clinical population is small and the changes in SGK1 mRNA expression should thus be replicated in a second cohort of drug-free depressed patients. Although all depressed patients included in this study were drug-free for at least 2 wk at the time of assessment, and ∼70% were in fact drug-naïve, we cannot fully exclude an influence of previous antidepressant medication on SGK1 expression in some of our patients.
In conclusion, and in light of recent findings demonstrating that neurogenesis is important for antidepressant treatment response (4, 23), depression pathogenesis (24), and HPA axis regulation (24, 34), we propose that SGK1 may represent an antidepressant treatment strategy to counteract stress-induced impairments in neurogenesis and associated biological abnormalities in depression.
Materials and Methods
The complete description can be found in SI Materials and Methods.
Cell Culture and Neurogenesis Assay.
The immortalized, multipotent human fetal hippocampal progenitor cell line, HPC03A/07 (propriety of ReNeuron), was used for all cellular and molecular analyses (3, 6, 12, 35). Details of this cell line, the differentiation protocol and immunocytochemistry can be found in SI Materials and Methods.
Western Blot Analysis.
Protein lysates were obtained using whole-cell protein extraction buffer [20 mM Tris⋅HCl/150 mM NaCl/1 mM EDTA/1 mM EGTA/1% (vol/vol) Triton X-100/2 nM calyculin A/1×protease and phosphatase inhibitors (Pierce, Thermo Fisher Scientific)] and commercially available Nuclear Extraction kits (Active Motif). Samples were processed using NuPAGE system (Invitrogen) and analyzed by Western Blot.
Gene Expression Analysis.
RNA was isolated from human cells and rat tissue using RNeasy mini kit (Qiagen). Human blood was collected in PaxGene Tubes (Qiagen) and RNA was isolated using the PAXGene Blood RNA kit (Qiagen). RNA was reverse-transcribed with SuperScript III (Invitrogen) before quantitative real-time PCR (qRT-PCR). Microarray was performed using Human GenomeU219 Array on the Affymetrix GeneAtlas platform. Partek software (Ariadne) was used for quality control and to identify signaling pathways.
Recruitment and Assessment of Depressed Patients.
We selected 25 depressed patients (10 females, 15 males) from the Genome-based Therapeutic Drugs for Depression (GENDEP) study who were drug-naïve or had been drug-free for at least 2 wk before assessment (17, 36). Controls (7 females, 7 males) were recruited in London through local advertisement and volunteer databases, as part of the GENDEP study. The study was approved by the Institute of Psychiatry Research Ethical Committee (#292/03) and by the ethics boards in all participating centers. All participants provided written consent. Subject characteristics and exclusion criteria are detailed in the SI Materials and Methods.
Rodent Stress Procedures.
For UCMS, male Sprague–Dawley rats were exposed to a variable sequence of mild to moderate, unpredictable stressors once or twice daily for a total duration of 6 wk. For PNS, pregnant dams were restraint in Plexiglas cylinders under bright light during the last week of gestation and male offspring were assessed at postnatal day 62. All procedures were in accordance with the European Economic Communities Council Directive 86/609 1987, Decreto Legislativo 116/92, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Milan Animals Research Committee.
Statistical Analysis.
Multiple comparisons were conducted using one-way ANOVA with Newman–Keuls post hoc test. Means of two treatment groups were analyzed using Student t test or Mann–Whitney test. Pathway regulation was assessed using Robust MultiChip Average ANOVA and Mann–Whitney U test. Pearson correlation was used to correlate gene expression changes. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
C.A. was funded by the National Institute of Health Research Biomedical Research Centre for Mental Health, Institute of Psychiatry and South London and Maudsley National Health Service Foundation Trust. P.A.Z. was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award. M.G. was supported by the Italian Ministry of Health (Ricerca Corrente) and Regione Lombardia (ID:17387Sal-13). S.T. was supported by Research Councils UK. R.U. was supported by the Canada Research Chairs program and the European Commission Innovative Medicine Initiative Joint Undertaking Grant 115008. M.A.R. was funded by Regione Lombardia (Accordo Quadro 2010). C.M.P. was funded by the Medical Research Council (G108/603) and the Commission of European Communities Seventh Framework Programme Collaborative Project Grant Agreement 22963 (Mood Inflame).
Footnotes
Conflict of interest statement: J.P. acted as a consultant and received payment from ReNeuron Group within the last 2 y. C.M.P. has received fees as a speaker or as a member of advisory board, as well as research funding, from pharmaceutical companies that commercialize or are developing antidepressants in the last 3 y, such as Lilly, Servier, and Janssen. M.A.R. has received compensation as speaker/consultant for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Servier, and Takeda. P.A.Z. has received speaker fees from Servier.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300886110/-/DCSupplemental.
References
- 1.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12(9):3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anacker C, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16(7):738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer JL, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18(8):629–631. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 6.Anacker C, et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology. 2013;38(5):872–883. doi: 10.1038/npp.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588(Pt 18):3349–3354. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato H, et al. Large-scale analysis of glucocorticoid target genes in rat hypothalamus. J Neurochem. 2008;106(2):805–814. doi: 10.1111/j.1471-4159.2008.05489.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuen EY, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16(2):156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyata S, et al. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS ONE. 2011;6(5):e19859. doi: 10.1371/journal.pone.0019859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luca F, et al. Adaptive variation regulates the expression of the human SGK1 gene in response to stress. PLoS Genet. 2009;5(5):e1000489. doi: 10.1371/journal.pgen.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surget A, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16(12):1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanti A, Rainer Q, Minier F, Surget A, Belzung C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63(3):374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Sherk AB, et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68(18):7475–7483. doi: 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattaneo A, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: Differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38(2):376. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nollet M, et al. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37(10):2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang F, Huang DY, Vallon V. SGK, renal function and hypertension. J Nephrol. 2010;23(Suppl 16):S124–S129. [PMC free article] [PubMed] [Google Scholar]
- 20.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti G, et al. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: Modulation by antidepressant treatment. Neuropsychopharmacology. 2013;38(4):616–627. doi: 10.1038/npp.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 24.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakic P. Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 26.Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58(6):940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 29.Frodl T, et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: High IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transcult Psychiatry. 2012;2:e88. doi: 10.1038/tp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS ONE. 2010;5(7):e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown J, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 32.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 33.Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22(4):861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anacker C, Pariante CM. Can adult neurogenesis buffer stress responses and depressive behaviour? Mol Psychiatry. 2012;17(1):9–10. doi: 10.1038/mp.2011.133. [DOI] [PubMed] [Google Scholar]
- 35.Zunszain PA, et al. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uher R, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167(5):555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.