Significance
Patient E.P. developed profound amnesia from encephalitis and then was studied for 14 years before his death in 2008. He had no capacity for acquiring new knowledge about facts and events and had retrograde amnesia covering several decades. This report presents detailed neurohistological findings for this case and relates these findings to his neuropsychological data. The damage included all the structures of the medial temporal lobe bilaterally. In addition, the lateral temporal cortex was shrunken and gliotic. The findings illuminate a number of issues about memory, perception, and cognition and about the functions of medial and lateral temporal lobe.
Abstract
We present neurohistological information for a case of bilateral, symmetrical damage to the medial temporal lobe and well-documented memory impairment. E.P. developed profound memory impairment at age 70 y and then was studied for 14 y He had no capacity for learning facts and events and had retrograde amnesia covering several decades. He also had a modest impairment of semantic knowledge. Neurohistological analysis revealed bilaterally symmetrical lesions of the medial temporal lobe that eliminated the temporal pole, the amygdala, the entorhinal cortex, the hippocampus, the perirhinal cortex, and rostral parahippocampal cortex. The lesion also extended laterally to involve the fusiform gyrus substantially. Last, the superior, inferior, and middle temporal gyri were atrophic, and subjacent white matter was gliotic. Several considerations indicate that E.P.’s severe memory impairment was caused by his medial temporal lesions, whereas his impaired semantic knowledge was caused by lateral temporal damage. His lateral temporal damage also may have contributed to his extensive retrograde amnesia. The findings illuminate the anatomical relationship between memory, perception, and semantic knowledge.
The medial temporal lobe has been associated with memory function for more than a century (1–3). The development of an animal model of human memory impairment in the nonhuman primate (4) led to the identification of the specific structures that are important: the hippocampus [including the cornu ammonis (CA) fields, dentate gyrus, and subicular complex] and the adjacent entorhinal, perirhinal, and parahippocampal cortices (5). This work demonstrated further that memory impairment is less severe when bilateral removal is limited to the hippocampus than when the lesion is enlarged to include the adjacent cortical structures (6).
Studies of memory-impaired patients demonstrated this same point. For example, the noted patient H.M (1), who sustained a large bilateral medial temporal lobe resection to relieve epilepsy, had very severe memory impairment. In contrast, patients with damage limited to the hippocampus are less severely impaired (7).
The most useful information about the functional neuroanatomy of human memory comes from cases in which there are opportunities to carry out extensive neuropsychological testing as well as postmortem neurohistological analysis. This testing has been accomplished for patients with damage limited to the hippocampus proper (8–11). However, less information is available for patients with larger medial temporal lobe lesions. Patient P.B. developed memory impairment following surgical resection of the right medial temporal lobe and, as discovered later in postmortem examination, a sclerotic lesion of the left hippocampus (12, 13). P.B. was less severely impaired than H.M. (14). Patient N.T. developed memory impairment following right temporal lobectomy and, as discovered later, a lesion of the left hippocampus (15, 16). In both these cases, the lesions were asymmetrical, and it is difficult to know to what extent the right extrahippocampal damage contributed to the neuropsychological findings, particularly for N.T., for whom both medial and lateral right temporal cortex was removed.
Here we present neuropsychological and neurohistological findings for patient E.P. (17). E.P. developed profound memory impairment in 1992 after viral encephalitis, an impairment that proved to be even more severe than that in patient H.M. Structural MRI revealed bilateral and symmetrical damage to his medial temporal lobe (Fig. 1). Beginning in 1994, E.P. was studied for 14 y before he died in 2008. Neurohistological information in cases of large, bilaterally symmetrical medial temporal lobe lesions and well-documented, severe memory impairment is quite rare. This information makes it possible to address two fundamental questions: What anatomical damage is sufficient to cause profound, nearly absolute impairment in memory? Can damage to the medial temporal lobe also impair perception and semantic knowledge along with memory, as suggested in recent studies (18, 19)?
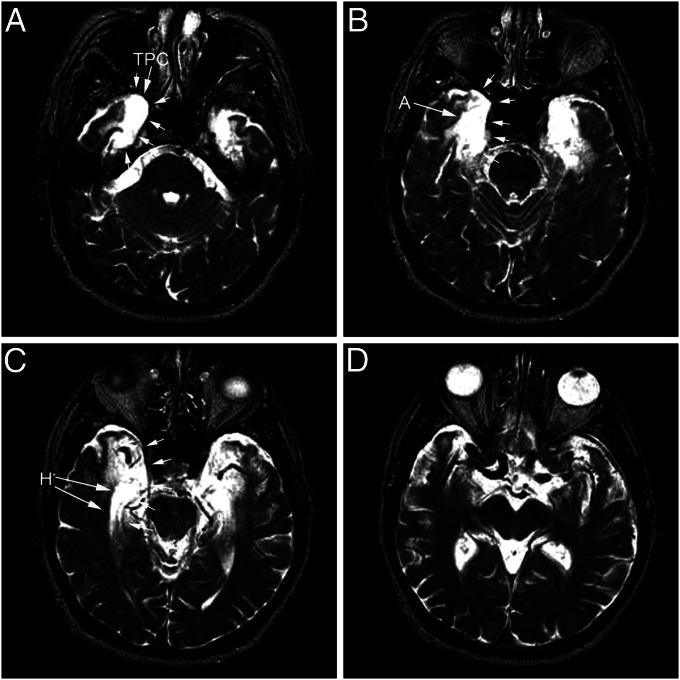
Fig. 1.
T2-weighted MRI images of E.P.’s brain from 1994. Axial sections are arranged from ventral to dorsal (A–D). Note areas of hyperintensity in the medial temporal lobe indicated by white arrows. Regions included within the abnormal hyperintensity include the temporopolar cortex (TPC), amygdaloid complex (A), and hippocampal formation bilaterally (H). The left side of each image illustrates the left side of the brain.
Results
E.P.’s case history is presented in Case History.
Intellectual and Perceptual Functions.
In 1994, at age 71 y, E.P. obtained a full-scale IQ score of 103 on the Wechsler Adult Intelligence Scale-Revised (WAIS-R). His performance was low on the Vocabulary subtest (33 for E.P. vs. 56 for four controls) (20). His intellectual function remained stable during the 14 y that we tested him (98 on the WAIS-III in 1999). He also scored 118 on the Dementia Rating Scale (DRS; maximum score = 144) (21), with most points (15) lost on the memory subportion of the test. Eleven healthy volunteers averaged 139.7 on this test (22).
E.P. performed well on tests of frontal lobe function. He scored 36 (maximum score = 37) on the Initiation – Perseveration subportion of the DRS and 94 (population average score = 100, SD =15) on the Attention-Concentration index of the Wechsler Memory Scale-Revised (WMS-R). Both these tests are sensitive to frontal lobe dysfunction (22). E.P. also was given the Wisconsin Card Sorting Test in 1994 and again in 1998. He was unable to sort any categories and averaged 62% perseverative errors. Poor performance on this test has not always been found to be diagnostic of frontal lobe dysfunction (23). Note, too, that E.P. has no evidence of damage to dorsolateral frontal cortex, a region often linked to poor card-sorting performance (24).
E.P.’s primary perceptual impairment was anosmia. In light of suggestions that medial temporal lobe structures, especially perirhinal cortex, are important for certain kinds of visual perception (see ref. 18 for review), E.P. was given a number of tests of visual perception, including difficult tests of object discrimination (25), face discrimination (25, 26), discrimination of blended, feature-ambiguous images (26), and discrimination of morphed, feature-ambiguous objects, faces, and scenes (27). Across 13 different tests from these three studies, E.P. scored close to the control mean (E.P.’s mean = −0.14 SD). His only low scores came from two tests of face discrimination. The task was to select the odd face when presented with five different orientations of one face and a sixth orientation of another face (25, 26). On these two tasks, E.P. scored more than two SDs below the control mean, an impairment possibly related to his difficulty in discriminating emotion in faces (see below).
In one other instance, E.P. was moderately impaired on a complex test of visual perception. E.P. saw 80 line drawings that represented either “possible objects” that potentially could exist in three dimensions or “impossible objects” that could not exist in three dimensions (28, 29). E.P. identified 77.5% of the objects correctly. Controls (n = 5; mean age = 80.8 y) scored 88.8% (SD = 5.6).
Semantic Knowledge.
Naming.
The Boston Naming Test (30) asks participants to provide the names for 60 drawings of common and uncommon objects (e.g., pencil, broom, abacus, trellis). Controls (n = 9) and patients with restricted hippocampal lesions (n = 4) scored 55.7 and 54.5 items correct, respectively. E.P. was markedly impaired, with a score of 42 items correct (20). We also gave E.P. the 84-item version of this same test, which also had been given to patient H.M. H.M. scored 83% and within 1 SD of controls (controls = 90% correct, estimated from fig. 2 of ref. 31); E.P. scored 63% correct (17).
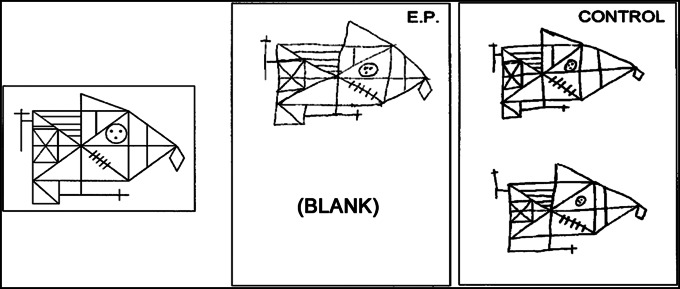
Fig. 2.
The Rey–Osterrieth figure. E.P. and a control were asked to copy the figure shown at the left (Upper) and then 10–15 min later, without forewarning, to reproduce the figure from memory (Lower). E.P. did not recall copying a figure and declined to make a guess.
Detection and explanation of ambiguity in sentences.
E.P. read 65 ambiguous sentences (e.g., “The man spoke to the woman in tears”), together with 25 unambiguous sentences and attempted to identify which sentences were ambiguous and also (for all 65 ambiguous sentences) to explain the ambiguity (32). Controls (n = 9) detected 78.6% of the ambiguous sentences and correctly explained 68.5% of them; E.P. performed poorly (31% detected and 41.5% explained). A mixed group of patients with hippocampal or diencephalic lesions (n = 4) performed as well as controls (71.6% of ambiguous sentences detected, and 66.2% explained).
Semantic test battery.
E.P. and controls (n = 8) were given nine tests (33) modified from the Semantic Test Battery as originally introduced by Hodges et al. (34). All the tests were based on the same 48 line drawings of 24 animals and 24 objects. Fig. S1 (Upper) shows the performance of controls (n = 8) and E.P. on the six tests that yielded a percent correct score. Scores for the noted patient H.M. on the same tests are included for comparison. These six tests asked participants to express their knowledge about the test items by pointing to the correct drawing (from among eight in the same category) when given its name or its description, naming the item when given either the drawing or its definition, answering yes/no questions about an item, and sorting the items into categories or subcategories. E.P. was modestly, albeit significantly, impaired on the tests involving animals (P < 0.02) and was marginally impaired on the tests involving objects (P < 0.08). He performed well only on the test of category sorting. H.M.’s scores were marginally better overall than E.P.’s scores (P < 0.08 for both animals and objects). Nonetheless, H.M. was marginally impaired relative to controls on the tests involving animals (P < 0.09).
Fig. S1 (Lower) shows performance on the seventh and eighth tests, which asked participants to provide definitions for the items in the semantic test battery when given either the item’s name or a drawing of the item. E.P. and H.M. both performed poorly, substantially outside the range of control scores. The last of the nine tests (category fluency) asked participants to name as many examples as possible from each of four categories of animals (animals, birds, water creatures, dog breeds) and four categories of objects (household items, vehicles, musical instruments, and types of boat). For each category, participants were given 1 min to respond. On this test controls generated 58.5 responses for living things and 70.4 responses for nonliving things. E.P. scored substantially outside the control range (44 living things and 46 nonliving things). H.M. scored more poorly than E.P. (21 living things and 22 nonliving things).
Perception of Emotion.
E.P. rated 60 color pictures for their emotional intensity and pleasantness. The pictures varied widely in subject matter and emotional content. Despite his amygdala damage, E.P. gave emotionality ratings very similar to those given by his comparison groups (35). However, E.P. had difficulty identifying emotion in facial expressions (36). He consistently misidentified expressions of sadness and fear, although he performed as well as his comparison group in identifying expressions of anger, disgust, happiness, or surprise.
Immediate Memory and Working Memory.
E.P. performed normally on verbal and nonverbal tests of immediate memory. He was given the digit span test on 12 different occasions (17), averaging 6.6 digits [controls in that study averaged 7.3 digits; in another study (37), controls averaged 6.9 digits]. Patient H.M. scored 6 digits (38). On the spatial span task from the WMS-III, E.P. averaged 5.5 locations across four administrations. Controls averaged 5.9 locations.
E.P. also performed well on tests of working memory (39). He retained three names across a 14-s delay (92.2% vs. 94.5% for controls), he retained single faces for 2 s (96.9% vs. 98.8% for controls), and he retained three different object–location associations for 8 s (87.5% vs. 93.2% for controls). All these scores were within one SD of the control mean. E.P. and other memory-impaired patients performed poorly in more challenging conditions of the same kind of tests (e.g., single faces after 14 s, six object–location associations after 8 s). Independent evidence suggested that performance under these conditions depends substantially on long-term memory (39).
Spatial Cognition.
E.P. took two tests that assessed his capacity for spatial cognition. In one test he was blindfolded and led along paths (up to 15 m in length and involving two turns) while being asked to hold his start location actively in mind (40). At the end of the path, E.P. was able to point accurately to his start location (trial times about 30 s). However, several minutes later E.P could remember nothing of what he had been doing and suggested that he had been “in conversation.” His ability to keep track of a reference location for a time as he moved through space (sometimes termed “path integration”) presumably depended on his intact working memory.
In a second test, E.P. performed well when asked to navigate mentally through the environment where he grew up (41). He also could find a new route when the route he first chose was made unavailable, and he was able to point accurately toward landmarks in his childhood environment when imagining himself in a particular location and facing a particular direction. His good performance on this test presumably relates to his generally good performance on tests of remote memory that asked about his early life (see Retrograde Memory).
Declarative Memory (New Learning).
Despite E.P.’s intact performance on tests of immediate memory and working memory, his declarative memory was profoundly impaired, as documented by every test of recall and recognition that he was given. His deficit was apparent across all types of material (e.g., scenes, words, dot patterns, synthetic sounds). Table S1 and Fig. 2 illustrate E.P.’s performance on standard memory tests. He exhibited no capacity for new learning. For example, in two earlier studies (25, 42), he took 42 different recognition memory tests (20 or 24 words tested after a 5- to10-min delay by yes–no recognition or forced-choice recognition). His average score across all the tests was 49.3% correct (chance = 50.0%). It also is notable that, like other patients with medial temporal lobe damage, E.P. was unable to acquire trace eyeblink conditioning (43).
Unlike many memory-impaired patients, including H.M. (44), E.P.’s recognition memory did not improve after extended exposure to study material (45). For example E.P. viewed 40 scenes for a total presentation time of 20 s each and then took a yes–no recognition test (10-min delay). Across three separate study-test sessions, E.P. scored at chance (50.6% correct). E.P.’s performance also remained at chance levels when he was tested in a forced-choice format and when the tests involved shorter lists of items. In contrast, with similar tests H.M. benefited from extended exposure time (120 scenes presented for a total of 20 s each), obtaining a score of 78.8% correct across four tests (44).
Additional tests explored the possibility that E.P. might have been capable of identifying items based on a simple, nonspecific sense of familiarity, even if an item could not be recalled or associated with its original context (46). Accordingly, in three similarly designed tests E.P. was asked to identify a word, name, or face that could have become familiar only after his illness in 1992. For example, he was asked to select the one real item when it was presented together with eight plausible foils (Prozac, Flozac, Flozam, Prozam, Grosam, Grozac, Grodaz, Prodaz, Flodaz). Across 74 test items, E.P. scored 16.2% correct (chance = 11.0%, P = 0.11). E.P.’s only hint of new learning came from an eight-item test of household objects that had been acquired after the onset of his amnesia. Photos of each item were presented together with five other exemplars of the same object (e.g., a different lamp, car, or table). E.P’s spouse scored 100% correct, indicating that the objects were readily recognizable. E.P. scored 33% correct across three tests (chance = 16.6%, P = 0.057). Although these data suggest a limited ability to learn postmorbidly when items have been exposed (presumably) hundreds or thousands of times, it also is possible that E.P. based his selections in part on longstanding preferences for certain types of objects and that the objects purchased by the family after 1992 conformed to these preferences.
E.P. moved to the San Diego area in 1993 after he became amnesic. His poor knowledge of his house and neighborhood further illustrated his anterograde memory impairment. He was unable to name any streets in his neighborhood or describe how he would travel to places that he visited with his wife (e.g., the supermarket, the post office). In addition, he was not able to draw a floor plan of his house, nor was he able to point in the direction of the Pacific Ocean (although he lived within 2 miles of the coast).
Retrograde Memory.
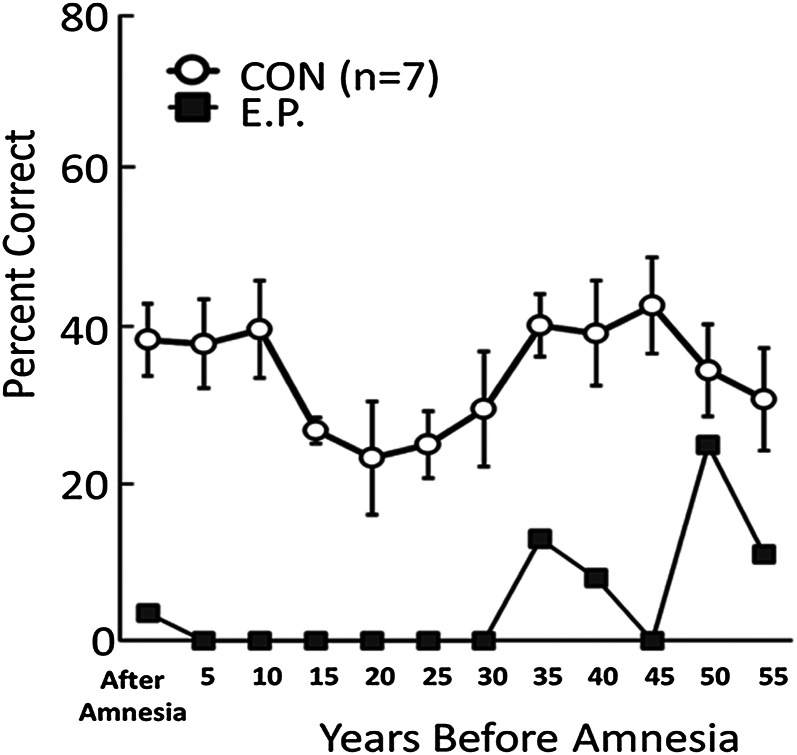
E.P. had severe and extensive retrograde amnesia for facts and events but nonetheless was able to retrieve memories from his early life. Specifically, he was markedly impaired on tests of recall and recognition for public events, famous faces, and famous names that came into the news after 1950 (20). His performance was good on tests that queried his memory from before the age of about 25 y, i.e., before the late 1940s. For example, on one test of news events that occurred from 1938 to 2005 (47), E.P.’s retrograde amnesia covered at least 30–40 y (Fig. 3). Nonetheless, his performance improved somewhat when questions concerned events that had occurred >30 y before his amnesia and reached normal levels for the period 46–50 y before amnesia when he was 20–24 y old.
Fig. 3.
Recall performance on a test of news events that occurred from 1938 to 2005. The data point at 5 represents 1–5 y before the onset of E.P.’s amnesia, i.e., 1986–1991, the point at 10 represents 6–10 y before his amnesia, and so on. Error bars indicate SEM. Data are from ref. 47.
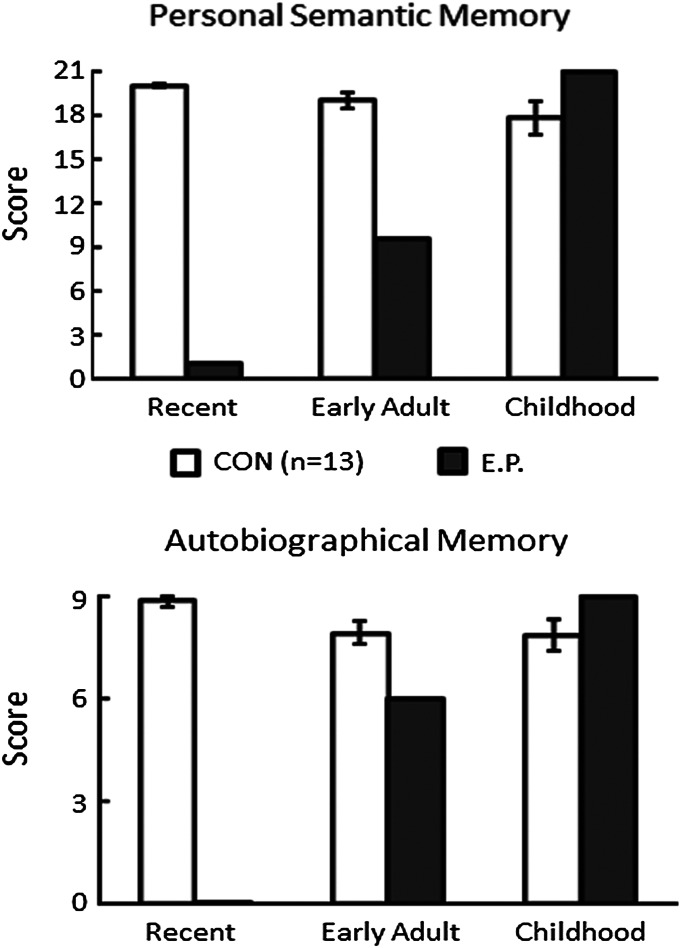
The same pattern was apparent on a standardized test of autobiographical memory (Fig. 4). This testing method (48) quantifies the recall of autobiographical incidents and personal semantic facts using a structured interview. E.P. was unable to answer any questions about his recent life, he was moderately impaired in answering questions about early adulthood, and he was fully intact in answering questions about his childhood (until age 18 y) (47).
Fig. 4.
Performance of E.P. and controls on the Autobiographical Memory Interview (48). (Upper) Items that assessed factual knowledge about an individual’s past (maximum score = 21). (Lower) Items that assessed memory for autobiographical events (maximum score = 9). Error bars indicate SEM. Data are from ref. 47.
In other tests (49), E.P. and controls recollected events from early life, and transcripts of the recollections were then analyzed for narrative content. E.P. produced as many details in each narrative as his controls (Fig. S2, Left). Despite his success in remembering remote events, E.P. tended to repeat details within his narrative recollections (Fig. S2, Right), presumably because he had difficulty remembering what he already had said. Additional measures suggested that E.P.’s early memories were as vivid as the controls’ memories and were normal in other respects as well (50). Still other tests were designed to increase the sensitivity of autobiographical memory tests by eliciting narrative recollections about a small number of prominent episodes (51). In this case, an average of 50 or more details were elicited for each memory. E.P. was impaired when memories were drawn from the recent past but was fully intact when memories were drawn from the remote past.
Nondeclarative Memory.
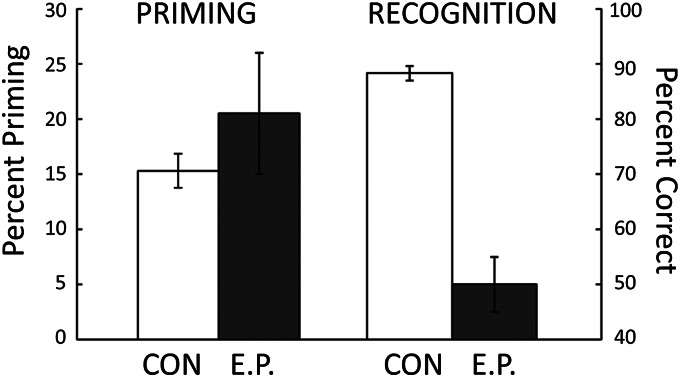
E.P. exhibited intact nondeclarative memory on a number of different tests, including perceptual priming (25, 42, 52), conceptual priming (53), category learning (54, 55), visuomotor skill learning (56), and delay eyeblink conditioning (43). For example, on 12 separate tests of perceptual identification priming (42), E.P. and controls studied 24 words and after 5 min tried to identify 48 briefly presented words (about 28 ms exposure time). Words that had been presented earlier were identified more frequently than newly presented words (E.P. 55% vs. 34%; controls: 48% vs. 32%) (Fig. 5). However, in six parallel tests of declarative memory, E.P. could not recognize the words he had read 5 min earlier (E.P., 50% correct; controls, 88% correct; chance performance = 50%) (Fig. 5). The fact that E.P. consistently exhibited chance performance on tests of recognition memory, while nevertheless exhibiting intact priming, indicates that recognition judgments do not benefit from the information expressed in priming phenomena. These findings also provide difficulty for the idea (e.g., ref. 57) that priming and recognition depend on the operation of a single memory system.
Fig. 5.
After studying 24 words, E.P. and seven controls were given 12 tests of perceptual identification priming and six parallel tests of recognition memory. Priming scores were calculated as percent correct identification of 24 briefly presented old words minus percent correct identification of 24 briefly presented new words. Recognition scores were calculated as percent correct on a two-choice test of 24 word pairs (one old word and one new word). For controls, brackets indicate SEM. For E.P., brackets show SEM across all the tests. Data are from ref. 42.
E.P.’s capacity for nondeclarative memory also likely accounts for other instances in which he exhibited a considerable capacity for learning, although he performed much worse than controls. For example, in one study, E.P. studied 48 three-word sentences (e.g., “Fire warmed tepee”) during 24 study sessions across 12 wk (58). Although he fell far short of controls, E.P. demonstrated gradual and unmistakable improvement in learning the sentences (e.g. answering the question, “fire warmed __?”). Notably, his learning was not accompanied by conscious knowledge about which answers were correct. Specifically, his response times and his confidence ratings were the same for correct and incorrect answers. Furthermore, unlike controls, his performance collapsed when the second word of the sentence was replaced by a synonym (e.g., fire heated __?”). Thus, what E.P. learned was rigidly organized, unavailable as conscious knowledge, and in this sense exhibited the characteristics of nondeclarative memory.
In another study, E.P. gradually learned eight object discriminations concurrently during 36 sessions across 18 wk, eventually achieving better than 85% correct performance (59). Controls achieved this score after two sessions. However, at the start of each session E.P. could not describe the task, the instructions, or the objects. Furthermore, unlike controls, his performance fell to chance levels when the task format was altered. Thus, E.P. sometimes was able to acquire the kinds of tasks that ordinarily are learned rapidly as declarative memory. However, for E.P. the learning was extremely slow, and what was acquired was rigidly organized and altogether different from declarative memory.
We also noted three ways in which E.P.’s behavior changed during the time that we knew him, changes that likely reflect his capacity for nondeclarative memory. First, in our earliest visits to his house, he was hesitant and puzzled that we wished to talk with him and administer tests. Eventually, with encouragement from his wife, he would consent to testing. During the subsequent years, he gradually came to greet us warmly, even moving spontaneously to the testing table without specific direction. This change occurred without any recognition of who we were, although the same persons had visited him more than 200 times.
Second, E.P.’s response to some of the test materials changed. One test, which E.P. was given frequently, required him to touch the computer screen with the eraser end of a pencil (54). After many repetitions of this procedure, E.P. began to pick up the pencil and orient the eraser end toward the screen before he was instructed to use the pencil. However, he consistently denied having taken the test before. Last, in another test, E.P. worked with pairs of objects placed in front of him on a table, one pair at a time (59). During multiple sessions across many weeks he worked at learning which object in each pair had been designated “correct.” The procedure on each trial was to pick up one of the objects, turn it over, and discover whether the word “correct” was printed underneath. When he was later given a variant of the same test that asked him simply to point to the correct object, he said, “But I’d like to turn them over. I love to turn them over.”
We suppose that these examples of behavioral change are instances of habit learning, which E.P. was able to accomplish gradually and which allowed him to modify his behavior in response to regularities in his environment.
Neuropathological Findings.
Synopsis.
Before describing E.P.’s neuropathological findings in detail, we present here a synopsis of the most salient findings in relation to his profound memory impairment. As already appreciated from earlier MRI findings (17), the major brain damage in E.P. involved his medial temporal lobes. Starting at the rostral pole of the temporal lobe, the dorsomedial aspect of the temporopolar cortex was damaged substantially and bilaterally. Caudal to this level, the amygdaloid complex was entirely eliminated bilaterally. Below and behind the amygdala, the entorhinal cortex was almost entirely eliminated bilaterally, and there was only a hint of the characteristic layer II islands at its caudal extreme. Similarly, almost all of the other fields of the hippocampal formation (dentate gyrus, hippocampus, subiculum, presubiculum, and parasubiculum) were eliminated bilaterally. Only small remnants of the dentate gyrus, subiculum, and presubiculum were present at the most caudal levels. Because of this massive loss of the substance of the hippocampal formation, the fimbria and fornix were markedly shrunken. In addition, the entire perirhinal cortex (Brodmann areas 35 and 36) was eliminated bilaterally. Approximately 70–80% of the rostrocaudal extent of the parahippocampal cortex (areas TF and TH) was preserved but showed a poorly defined laminar organization. This feature also was observed more caudally in the retrosplenial cortex.
There was gliosis in the white matter of the temporal stem that extended into the orbitofrontal cortex. The rostral temporal stem, which carries fibers of the uncinate fasciculus between the temporal and frontal lobes, was invaded by the cystic formations that replaced the amygdala and hippocampal formation. The rostral fusiform gyrus was directly damaged. However, direct damage to the medial temporal lobe did not extend laterally beyond the medial bank of the inferior temporal sulcus. Aside from the direct damage, there was substantial atrophy of the temporal neocortex along with heavy gliosis within the subcortical white matter. There were no areas of clear cell loss in the frontal lobe, but gliosis was apparent in the underlying white matter, particularly in the orbital regions. Beyond the temporal and frontal lobes, the anterior commissure was atrophic and gliotic, and there was substantial cell loss in the claustrum, the medial septal nucleus, and the medial mammillary nucleus. There was patchy cell loss in the thalamus, particularly in the anterior nucleus and the pulvinar. Last, there was a mass in the fourth ventricle below the cerebellum that invaded the substance of the dorsal medulla.
Gross appearance of the brain.
The ventral surface of E.P.’s brain (Fig. 6) contained large, brownish-tinged cysts that occupied the medial aspect of both temporal lobes (Fig. 6, arrows). The cysts had a semitranslucent appearance because of a thin layer of pia mater covering a series of cavities that extended deeply into the temporal lobe tissue. The cystic formation was about 3 cm in rostrocaudal extent on the right hemisphere and was about 2.5 cm in extent on the left hemisphere. The cysts occupied portions of the medial temporal lobe that originally included the amygdala rostrally and the hippocampal formation more caudally. The cysts also involved the region normally occupied by the entorhinal cortex and extended laterally to involve the perirhinal cortex along both the medial and lateral banks of the collateral sulcus. The maximal lateral extent of the damage was the medial bank of the inferior temporal sulcus. The cysts did not continue into the caudal portion of the parahippocampal gyrus, and thus much of the posterior parahippocampal cortex (areas TF and TH) appeared to be intact. The lateral extent of the grossly apparent damage was greater in the right hemisphere than in the left. Rostrally, the fusiform gyrus was occupied by the cyst. However, it was largely intact more posteriorly (Fig. 6). The lateral portion of the temporal lobe did not exhibit any gross abnormalities.
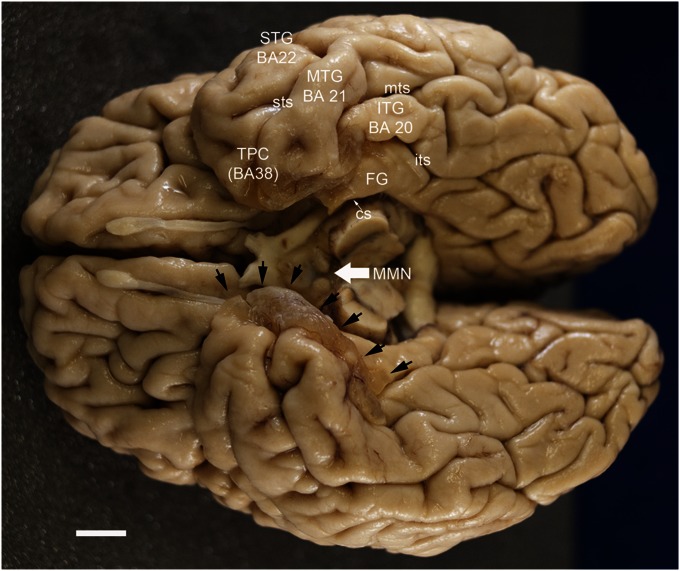
Fig. 6.
Photograph of the ventral surface of E.P.’s brain. Note the brownish-tinged cysts that occupy the anterior, medial temporal lobe (cysts on left side of brain are indicated by black arrows). See the text for a complete description. The medial mammillary nuclei (MMN) are shrunken, particularly on the left side of the brain. (Scale bar: 1cm.) cs, collateral sulcus; FG, fusiform gyrus; ITG, inferior temporal gyrus; its, inferior temporal sulcus; MTG, middle temporal gyrus; mts, middle temporal sulcus; STG, superior temporal gyrus; sts, superior temporal sulcus; TPC, temporopolar cortex.
The remaining cortical surfaces of the brain had a normal appearance. In particular, the cystic lesions did not extend into the orbital regions of the frontal lobe or into the insular cortex. However, there were other abnormalities on the ventral surface. The mammillary bodies, for example, were shrunken, especially on the left side (Fig. 6).
Microscopic appearance of the brain.
The description of the microscopic features of E.P.’s neuropathology is based on an analysis of Nissl-stained sections through the entire rostrocaudal extent of the brain. Our description begins with a presentation of the hippocampal formation and amygdala. We then move to the cerebral cortex and then to other portions of the brain.
Hippocampal formation.
Consistent with the cystic formations visible on the surface of the brain, the various fields that make up the hippocampal formation were eliminated nearly completely and bilaterally in E.P. This damage is quite apparent if one examines the low-magnification series of coronal sections (Figs. S3–S6) that illustrate medial temporal lobe neuroanatomy in E.P. in comparison with an age-matched control (H.T.). The extent of tissue loss in the hippocampal formation can be appreciated better from the higher-power photomicrographs that make up Fig. 7B.
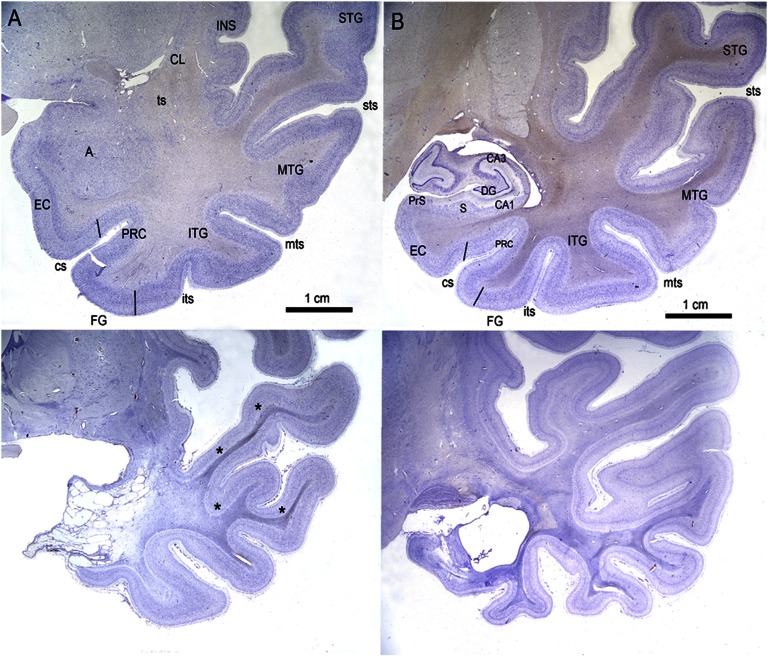
Fig. 7.
(A and B) Higher-magnification photomicrographs of Nissl-stained coronal sections through the medial temporal lobe of E.P. (Lower) and control H.T. (Upper). A is through the level of the amygdaloid complex, and B is through the level of the rostral hippocampal formation. The gliosis and shrinkage of the white matter of the temporal stem and white matter of the superior, middle, inferior, and fusiform gyri are also evident (asterisks). A, amygdala; CA1, CA1 field; CA3, CA3 field; CL, claustrum; cs, collateral sulcus; DG, dentate gyrus; EC, entorhinal cortex; FG, fusiform gyrus; INS, insular cortex; ITG, inferior temporal gyrus; its, inferior temporal sulcus; MTG, middle temporal gyrus; mts, middle temporal sulcus; PRC, perirhinal cortex; PrS, presubiculum; S, subiculum; STG, superior temporal gyrus; sts, superior temporal sulcus; ts, temporal stem.
The granule cell layer of the dentate gyrus was eliminated almost completely. Small patches of granule cells were visible in the right hemisphere at caudal levels (Fig. 7B and Fig. S5). The polymorphic cell layer of the dentate gyrus was devoid of neurons, and the molecular layer (the termination zone for entorhinal and associational connections) was markedly shrunken.
Within the hippocampus proper, neurons were totally absent in fields CA3, CA2, and CA1. The stratum radiatum and lacunosum-moleculare were atrophic and invested with a dense glial cell population. The fimbria was extremely atrophic, as was the fornix along its entire trajectory (Fig. S5). Only small patches of neurons could be observed in the distal-most portion of the subiculum. Islands of presubicular and parasubicular cells also were observed occasionally in the caudal-most portion of the hippocampal formation.
In the entorhinal cortex, there was a nearly complete loss of neurons bilaterally throughout its rostrocaudal extent. The rostral portion of the entorhinal cortex, which underlies the amygdala, was eliminated and replaced by the large cystic cavities described above (Fig. S4). Only the most medial part of the caudal extreme of the entorhinal cortex contained a few identifiable islands of layer II neurons (Fig. S5).
Perirhinal cortex.
It was not possible to identify any tissue belonging to the perirhinal cortex in E.P.’s brain.
Parahippocampal cortex.
The rostral border of the parahippocampal cortex is at approximately the same level as the lateral geniculate nucleus. The cystic formations ended at this level on both sides, and thus most (perhaps 70–80%) of the parahippocampal cortex was preserved (Fig. S6). Although present, the parahippocampal cortex in E.P. appeared shrunken and had an abnormal appearance because of the lack of lamination. There was also substantial gliosis of the white matter subjacent to the parahippocampal cortex.
Amygdaloid complex.
The amygdaloid complex was almost entirely eliminated bilaterally in E.P and was replaced by cystic cavities (Fig. 7A and Fig. S4). In the caudal extreme of the amygdala, there were remnants of the hippocampal–amygdaloid transitional area. The fiber tracts associated with the amygdala, the stria terminalis, and the ventral amygdalofugal pathway, were atrophic and gliotic.
Piriform cortex.
The piriform cortex appeared to be entirely eliminated bilaterally.
Cerebral cortex.
Temporal lobe.
Damage to the temporal cortex started at its anterior pole bilaterally. We noted an area of damage to the dorsomedial aspect of the temporopolar cortex (Fig. S3) in lateral temporopolar cortex (TPCl) and medial temporopolar cortex (TPCm) as defined by Blaizot et al. (60). There was a rather abrupt boundary between the damaged temporopolar cortex and the preserved cortex located dorsally and laterally in the superior temporal gyrus (or A22 of ref. 61). The region of damage was continuous caudally with the medial temporal lobe damage described above.
The gray matter of the lateral aspect of the temporal cortex was intact, although it was markedly atrophic (Fig. 7A and Figs. S3 and S4). It is apparent in Fig. 7 that the cortex of the temporal lobe is substantially thinner in E.P. than in the control H.T. Besides the shrinkage of the gray matter, the white matter of both the inferior temporal gyrus and the middle temporal gyrus was both atrophic and highly gliotic (Fig. 7A, asterisks). The superior temporal gyrus, particularly at caudal levels of the temporal lobe, had a more normal appearance (although it was shrunken), and the transverse gyrus of Heschl (auditory cortex) had a normal cytoarchitectonic appearance.
Fusiform gyrus.
The fusiform gyrus is bounded medially by the collateral sulcus and laterally by the inferior temporal sulcus (occipito-temporal sulcus). The fusiform gyrus lies lateral to the perirhinal cortex rostrally and the parahippocampal cortex more posteriorly. The cysts in the rostral temporal lobe of E.P. encroached on the fusiform gyrus from approximately the mid rostrocaudal level of the entorhinal cortex to the level of the lateral geniculate nucleus on the right side; damage extended a little less caudally on the left side. Although the remainder of the fusiform gyrus was visible, a substantial portion of the cortex had a disrupted laminar organization and was associated with marked gliosis of the white matter. The unfolded maps (Fig. 8) indicate that virtually all of the fusiform gyrus (gray profile) that lies within the temporal lobe was removed. As indicated in Table 1, for the portion of the fusiform gyrus that lies within the temporal lobe, only 5% of the left and only about 1% of the right fusiform gyrus could be measured in E.P.
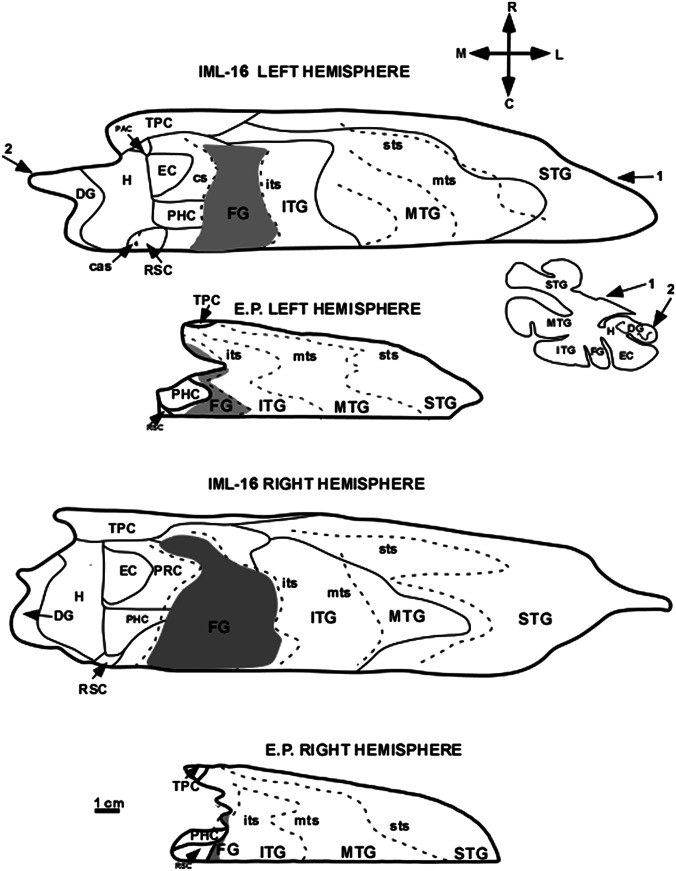
Fig. 8.
Unfolded 2D maps of the left and right temporal lobes at the same scale in E.P. and control case IML-16. The unfolded maps provide accurate representations of the surface areas of regions in the medial and lateral temporal lobes. For a description of how the maps were constructed, see Materials and Methods. It is clear that many regions of the medial temporal lobe are entirely missing in E.P. and that the entire temporal lobe is substantially shrunken compared with the control case. An outline of one of the coronal sections used to produce the unfolded temporal lobe map of “IML-16 LEFT HEMISPHERE” is shown below and to the right of that map. Numbers 1 and 2 on the map indicate the rostrocaudal level at which this coronal section is located. Both sets of numbers indicate that the outline of the coronal section begins at the inferior limiting sulcus (indicated by the number 1) and ends at the dentate gyrus (indicated by the number 2). The shaded area represents the fusiform gyrus (FG), located between the collateral and inferior temporal sulci; only the portion of the fusiform gyrus located within the temporal lobe is illustrated on the unfolded map. cas, calcarine sulcus; H, hippocampus; PAC, periamygdaloid cortex; PHC, parahippocampal cortex; RSC, retrosplenial cortex. For other abbreviations, see Fig. 7.
Table 1.
Areal measurements derived from 2D unfolded maps
| Area, cm2 |
||||
| Structure | Control IML-16 left | E.P. left | Control IML-16 right | E.P. right |
| Superior temporal gyrus, Brodmann area 22+41+42 | 11 | 4.7 | 20.1 | 5.1 |
| Middle temporal gyrus, Brodmann area 21 | 13.7 | 5.0 | 12.1 | 4.8 |
| Inferior temporal sulcus, Brodmann area 20 | 7.7 | 2.9 | 7.7 | 2.4 |
| Fusiform gyrus, Brodmann area 20 | 4.2 | 0.2 | 8.1 | 0.1 |
| Temporopolar cortex | 2.2 | 0.05 | 2.7 | 0.1 |
| Perirhinal cortex | 2.9 | 0 | 3.2 | 0 |
| Parahippocampal cortex | 1.3 | 0.6 | 1.3 | 0.3 |
| Hippocampus | 3.4 | 0 | 3.8 | 0 |
| Dentate gyrus | 1.6 | 0 | 1.6 | 0 |
| Entorhinal cortex | 0.9 | 0 | 1.3 | 0 |
Areal measurements that approximate the surface area of regions in the temporal lobe of patient E.P. and control subject IML-16. The calculations for fusiform gyrus Brodmann area 20 were based on the portion of fusiform gyrus that lies within the temporal lobe.
Temporal stem.
The temporal stem is defined as the white matter located between the inferior limiting sulcus of the insula and the lateral margin of the temporal horn of the lateral ventricle. It carries a series of projections, including the uncinate fasciculus, between medial and lateral structures of the temporal lobe and between the temporal and frontal lobes. At rostral levels (Fig. S3) of the temporal lobe, the temporal stem was included within the cystic region that occupied the amygdaloid complex. Presumably, fibers in this region were transected. At more caudal levels (Figs. S4 and S5), the temporal stem was visible but highly gliotic. The gliosis became less apparent at progressively more caudal levels of the temporal lobes (Figs. S4 and S5).
Unfolded Maps.
Fig. 8 illustrates unfolded 2D maps of the temporal lobe of E.P. and an age-matched control, IML-16. These maps represent the surface area of the cortex, and Table 1 presents areal measurements of the temporal lobe gyri and medial temporal lobe structures (the parahippocampal region and hippocampus, comprising the subicular complex, the fields CA1, CA2, and CA3, and the dentate gyrus). What is immediately apparent is that the entire temporal lobe in E.P. is markedly atrophic, because the surface area is much smaller than in IML-16.
In the parahippocampal region, there was complete destruction of the perirhinal cortex (Brodmann areas 35 and 36). The temporopolar cortex was reduced bilaterally to 2–3% of the control value, the perirhinal cortex was entirely missing, and the parahippocampal cortex (areas TF and TH of ref. 62) was reduced by about 50% on the left side and 75% on the right.
The fusiform gyrus was described previously. The superior, middle, and inferior temporal gyri were all reduced in surface area by at least 50% compared with the age-matched control.
Insular cortex.
The rostral (agranular and dysgranular) portions of the insular cortex and the subjacent parainsular cortex had patches of cell loss. These were most noticeable around the inferior limiting sulcus. This portion of the insula also appeared to have a somewhat shrunken and disorganized appearance (Fig. 7A and Figs. S3 and S4). At progressively more caudal levels of the insular cortex, it took on a more normal appearance.
Frontal lobe.
The frontal cortex generally had an overall normal cytoarchitectonic appearance. However, there was substantial gliosis of the white matter, particularly in the orbitofrontal and ventromedial cortex. A small infarct, limited to the upper three layers, was located in the lower bank of the right inferior frontal sulcus.
Cingulate cortex.
The subcallosal gyrus (Brodmann area 25) had patchy cell loss, mostly of layer III. The major portions of the cingulate gyrus (Brodmann areas 24 and 23) had a relatively normal appearance. The induseum griseum, the thin extension of several fields of the hippocampal formation over the corpus callosum, was absent.
Parietal and occipital cortices.
The parietal and occipital cortex had a normal appearance.
Subcortical Structures.
Claustrum.
The claustrum demonstrated substantial, bilateral neuronal loss, particularly of its ventral half.
Hypothalamus.
The most noticeable alteration of the hypothalamus occurred in the medial mammillary nucleus; it was shrunken and appeared to have severe cell loss throughout (Fig. S4). There were darkly stained cells dorsal to the medial mammillary nucleus that appeared to be portions of the supramammillary nucleus, and these cells were normal in E.P. There also appeared to be focal areas of cell loss in the anterior hypothalamic area and in the paraventricular nucleus, although the patchy, distributed pattern of neurons in the normal human hypothalamus makes these observations uncertain.
Striatum.
The caudate and putamen had a generally normal appearance except for the presence of a high level of glial cells, many of which were aligned with fiber bundles traversing the region.
Basal forebrain.
As expected after a large hippocampal lesion, there was substantial retrograde cell degeneration in the medial septal nucleus. The septal nuclei had a generally atrophic appearance. The basal nucleus of Meynert and nucleus of the diagonal band had a normal appearance.
Cerebellum and brainstem.
The general cytoarchitecture of the cerebellum was preserved, and there did not appear to be loss of Purkinje cells or any other cerebellar neurons. However, a prominent mass was partially located within the fourth ventricle. This irregularly shaped abnormal mass (about 2 cm in its longest dimension) seemed to be related to the choroid plexus and was likely a benign papilloma of the choroid plexus. It was highly vascularized, and it displayed necrotic and hemorrhagic foci. It did not enter the substance of the cerebellum, but there was a focal invasion of the dorsal medulla by the mass.
The organization of the mesencephalic, pontine, and medullary nuclear groups appeared to be normal.
Discussion
We have documented the performance of a patient (E.P.) on tests of memory and other cognitive functions, which were given during a period of 14 y. We also have described the findings from a detailed neurohistological analysis of E.P.’s brain carried out after he died in 2008. E.P. had no capacity for learning new facts and events (declarative memory). His severe disability occurred against a background of largely intact intellectual functions and intact perceptual functions, including the ability to discriminate among objects with high feature ambiguity. He also had intact immediate memory and working memory and intact performance on a variety of tests of skill learning, priming, and classical conditioning (nondeclarative memory). In addition to his severely impaired new-learning ability, E.P. was also markedly impaired in remembering facts and events that would have been learned before the onset of his amnesia. His memory loss for facts and autobiographical events appeared to extend at least three to four decades into the past. Nonetheless, he was able to recollect both facts and personal events from before about age 25 y. In addition, he was able to navigate mentally along the routes and among the locations of his childhood environment. The fact that memory was available for facts and events from before age 25 y may reflect the salience or special status of early memories. In any case, as discussed previously (47), E.P.’s retrograde memory loss seems too extensive to attribute to an impaired consolidation process.
In addition to these findings, which describe a severe instance of human memory impairment, E.P. also exhibited modest but unequivocal difficulty in certain other domains. He had difficulty discriminating among faces, he had difficulty detecting the emotions of fear and sadness in faces, and he had modest difficulty on tests of semantic knowledge (tests that assess long-established knowledge about word meanings and the identity and function of common objects). He also did poorly in identifying and explaining ambiguous sentences and in distinguishing possible from impossible objects (drawings of objects that could or could not exist in three dimensions).
E.P.’s performance on cognitive tests is considerably illuminated by the neuropathological findings. It is notable that his impairment was as selective as it was, given the wide variety of neuropathological change that could be detected. The severity of his memory impairment can be understood by the damage bilaterally to the hippocampal formation, the entorhinal cortex, the perirhinal cortex, and some rostral parahippocampal cortex. Earlier work with nonhuman primates established that such large lesions of the medial temporal lobe severely impaired memory and did so to a greater degree than lesions limited to the hippocampus itself (4–6). Subsequent neurohistological findings from four patients (R.B., L.M., W.H., G.D.; refs. 8 and 10) confirmed that the memory impairment associated with limited hippocampal lesions is only moderately severe. MRI findings for the noted patient H.M. emphasized this same point (63). That is, lesions of the hippocampal formation, together with damage to adjacent cortical structures, exacerbate the effects of more selective hippocampal lesions.
Interestingly, E.P.’s memory impairment was even more severe than H.M.’s impairment. First, H.M.’s memory performance benefited from extended exposure to study material (44), but the same procedures did not benefit E.P (45). Second, H.M. did acquire significant amounts of new factual information after the onset of his amnesia (64), but E.P. did not acquire new declarative memories (46, 47, 65). The latter finding suggests that H.M.’s residual fact learning was not due to some special mechanism or to direct learning into neocortex, as had seemed possible (64), but by his memory impairment not being as severe as it might have been. These differences between E.P. and H.M. most likely are attributable to the sparing of portions of the ventral perirhinal cortex and almost all the parahippocampal cortex in H.M. (63). In contrast, in E.P. the perirhinal cortex was entirely missing bilaterally, and a portion of rostral parahippocampal cortex was damaged as well. It is also true that H.M.’s lesion involved only about half of the rostrocaudal extent of the dentate gyrus, hippocampus, and subiculum. However, because the lesion also involved all of entorhinal cortex (the major source of afferent input from cortex to hippocampus), it is unclear whether posterior sparing of the hippocampal formation in H.M. had functional significance.
It has been suggested that the afferent and efferent connections of temporal stem white matter might be important in understanding memory impairment (66, 67). Although E.P. sustained damage to the temporal stem, H.M. did not (63). Accordingly, severe memory impairment can occur without damage to the temporal stem. It also seems unlikely that temporal stem damage could have contributed to the severity of E.P.’s memory impairment. Specifically, temporal stem damage in monkeys spares performance on a number of tasks sensitive to hippocampal lesions (68, 69).
The deficits exhibited by E.P. in addition to his severe memory impairment also are illuminated by the neuropathology. His inability to identify smells presumably resulted from the loss of piriform cortex. Impaired ability to identify emotions in faces likely resulted from the elimination of the amygdala (70). E.P. also had some difficulty in discriminating among faces, possibly reflecting the involvement of the fusiform gyrus (71).
It was particularly notable that E.P. had some difficulty on a number of tests of semantic knowledge that assessed long-established information about word meanings and objects (Fig. S1), including knowledge about their 3D structure. We suggest that these impairments (in vocabulary, sentence disambiguation, and object knowledge) reflect the significant damage that E.P. sustained to the lateral temporal cortex in the fusiform gyrus and in the superior, inferior, and middle temporal gyri. In particular, this tissue was shrunken, and the white matter was markedly atrophic and gliotic, presumably reflecting loss of afferent input (e.g., from amygdala and perirhinal cortex) as well as intracortical afferents that funnel through the temporal stem. Loss of efferents from lateral temporal cortex through retrograde degeneration also could be a factor. We suggest that some shrinkage and gliosis within lateral temporal cortex may be a typical (albeit indirect) consequence of large medial temporal lobe lesions, given the substantial connectivity between medial and lateral portions of the temporal lobe. If so, such changes need to be considered when evaluating the pattern of sparing and loss in memory-impaired patients.
Useful information about impaired semantic knowledge comes from patients with semantic dementia, also known as the temporal variant of fronto-temporal dementia (34, 72). These patients have progressive atrophy, prominently involving the fusiform gyrus and inferior and middle temporal gyri (as well as some involvement within the medial temporal lobe itself), and they have severe loss of conceptual knowledge about objects, facts, and word meanings. Direct comparisons between E.P. and patients with semantic dementia, using the same tests, revealed a sharp distinction: E.P. performed worse than the patients with semantic dementia on tests of anterograde memory and better on tests of semantic knowledge (73). These findings correspond with E.P.’s medial temporal lobe damage being considerably more extensive than typically reported in semantic dementia (74, 75). Furthermore, the critical region damaged in semantic dementia is thought to be the inferolateral temporal neocortex (34, 76). It also is notable that three memory-impaired patients (E.P., G.P., and G.T.) all had complete damage to the perirhinal and entorhinal cortices. However, G.T., who had more extensive lateral temporal damage, performed worse than the other two patients on most of the tests of semantic knowledge (73). It also is relevant that patient H.M. performed marginally better than E.P. on tests of semantic knowledge (Fig. S1, Upper) (33) and on the basis of MRI findings appeared to have less involvement of lateral temporal cortex (63). These findings all emphasize the importance of structures lateral to the medial temporal lobe for understanding impaired semantic knowledge.
In agreement with this conclusion, impaired semantic knowledge has been correlated with reduced volume of lateral temporal cortex but not with reduced volume of the parahippocampal gyrus (75). It is true that in one study the extent of atrophy in perirhinal cortex was correlated with the severity of impaired semantic knowledge (77). However, the importance of the perirhinal cortex in semantic dementia appears doubtful, given that E.P.’s perirhinal cortex was missing altogether but his impairment on tests of semantic knowledge was quite modest in comparison with the losses found in semantic dementia (73). Taken together, these considerations count against proposals that the perirhinal cortex is important for semantic knowledge about objects. Similar considerations (78, 79) count against proposals that the perirhinal cortex is important for visual perception.
In summary, the neuropsychological and neurohistological findings for E.P. describe a patient with profound memory impairment and additional, more modest impairments in semantic knowledge. The findings emphasize the importance of medial temporal lobe structures for memory functions and the importance of lateral temporal cortex for long-established knowledge about the world. In light of this role of lateral temporal cortex, it is possible that the changes detected in E.P.’s lateral temporal cortex contributed to the extent and severity of his retrograde memory loss. Our findings also highlight the importance of evaluating the status of lateral temporal cortex when interpreting neuropsychological findings from memory-impaired patients with large medial temporal lobe lesions.
Materials and Methods
Acquisition of Postmortem Brain.
Plans to obtain the brain upon E.P.’s death were approved by the Institutional Review Board at the University of California, San Diego, and consent for brain donation was obtained from E.P. and his family. Approximately 4 h after death, the brain was removed and suspended by vessels of the circle of Willis in a cold solution of 4% paraformaldehyde with 0.02% picric acid in 0.1 M phosphate buffer (pH 7.2). About 9 h later, the brain was transferred to fresh 4% paraformaldehyde in 0.1 M phosphate buffer and then was placed in fresh solution every 2 d. The brain was maintained at 4 °C in the fixative solution until it was transferred to J.A.’s laboratory on March 14, 2008. Preparation and analysis of brain tissue are described in SI Materials and Methods.
E.P.’s brain was compared with two brains from age-matched individuals with no known neurological or psychiatric disorders. Control case H.T. was a white woman who died of cardiac arrest at age 88 y. This brain was processed exactly as E.P.’s brain. Images of this brain were used to prepare Fig. 7 and Figs. S3–S6. To have a more comparable brain to produce the unfolded maps in Fig. 8 and make areal measurements for Table 1, a second control brain, IML-16, was prepared. This brain was from an 88-y-old man who died suddenly of a cardiac arrest. Only the temporal lobe was available from this case, and thus it was not appropriate for the low-power photomicrographs in Figs. S3–S6.
2D Reconstruction of the Temporal Lobe.
A 2D unfolded map (80) of the whole temporal lobe of E.P. was created as described previously (60, 81) to illustrate and estimate the extent of temporal lobe tissue loss. The control brain IML-16 was used to create an unfolded map of a normal brain for comparison. Areal measurements of the unfolded maps were made using Canvas X (Deneba) software.
Supplementary Material
Acknowledgments
We thank Nicola Broadbent, Trinidad Fernández, Jennifer Frascino, Joaquin Garijo, Ashley Knutson, Paul Maechler, Christine Smith, and Joyce Zouzounis for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs, The Kavli Institute for Brain and Mind, National Institute of Mental Health (NIMH) Grant 24600, NIMH Grant 84756, National Eye Institute (NEI) Grant 18359, National Institute of Neurological Disorders and Stroke Grant 16980, and Grants TSI-020110-2009-362, BFU 2009-14705, and PR 2010-0434 (to R.I.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306244110/-/DCSupplemental.
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victor M, Angevine JB, Jr, Mancall EL, Fisher CM. [Memory loss with lesions of hippocampal formation. Report of a case with some remarks on the anatomical basis of memory] Arch Neurol. 1961;5:244–263. doi: 10.1001/archneur.1961.00450150010002. [DOI] [PubMed] [Google Scholar]
- 3.Bekhterev VW. Demonstration eines Gehirns mit Zerstörung der vorderen und inneren Theile der Hirnrinde beider Schläfenlappen. Neurol Zentralbl. 1900;19:990–991. [Google Scholar]
- 4.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 6.Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4(4):483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]
- 7.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 8.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victor M, Agamanolis D. Amnesia due to lesions confined to the hippocampus: A clinical-pathologic study. J Cogn Neurosci. 1990;2(9):246–257. doi: 10.1162/jocn.1990.2.3.246. [DOI] [PubMed] [Google Scholar]
- 10.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold JJ, Squire LR. The anatomy of amnesia: Neurohistological analysis of three new cases. Learn Mem. 2006;13(6):699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958;79(5):475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- 13.Penfield W, Mathieson G. Memory. Autopsy findings and comments on the role of hippocampus in experiential recall. Arch Neurol. 1974;31(3):145–154. doi: 10.1001/archneur.1974.00490390027001. [DOI] [PubMed] [Google Scholar]
- 14.Teuber HL, Milner B, Vaughn HG. Persistent anterograde amnesia after stab wound of the basal brain. Neuropsychologia. 1968;6(3):267–282. [Google Scholar]
- 15.Warrington EK, Duchen LW. A re-appraisal of a case of persistent global amnesia following right temporal lobectomy: A clinico-pathological study. Neuropsychologia. 1992;30(5):437–450. doi: 10.1016/0028-3932(92)90091-y. [DOI] [PubMed] [Google Scholar]
- 16.Chan D, Revesz T, Rudge P. Hippocampal, but not parahippocampal, damage in a case of dense retrograde amnesia: A pathological study. Neurosci Lett. 2002;329(1):61–64. doi: 10.1016/j.neulet.2002.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: A neuroanatomical and neuropsychological profile of patient E. P. J Neurosci. 2000;20(18):7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48(4):831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Barense MD, Rogers TT, Bussey TJ, Saksida LM, Graham KS. Influence of conceptual knowledge on visual object discrimination: Insights from semantic dementia and MTL amnesia. Cereb Cortex. 2010;20(11):2568–2582. doi: 10.1093/cercor/bhq004. [DOI] [PubMed] [Google Scholar]
- 20.Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. J Neurosci. 1998;18(10):3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattis S. Dementia Rating Scale. In: Bellack R, Keraso B, editors. Geriatric Psychiatry X. New York: Grune and Stratton; 1976. pp. 77–121. [Google Scholar]
- 22.Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989;103(3):548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol. 1991;13(6):909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- 24.Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9(1):90–100. [Google Scholar]
- 25.Stark CEL, Squire LR. Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behav Neurosci. 2000;114(3):459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- 26.Levy DA, Shrager Y, Squire LR. Intact visual discrimination of complex and feature-ambiguous stimuli in the absence of perirhinal cortex. Learn Mem. 2005;12(1):61–66. doi: 10.1101/lm.84405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26(8):2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacter DL, Cooper LA, Delaney SM. Implicit memory for unfamiliar objects depends on access to structural descriptions. J Exp Psychol Gen. 1990;119(1):5–24. doi: 10.1037/0096-3445.119.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Williams P, Tarr MJ. Structural processing and implicit memory for possible and impossible figures. J Exp Psychol Learn Mem Cogn. 1997;23(6):1344–1361. doi: 10.1037//0278-7393.23.6.1344. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea Febiger; 1983. [Google Scholar]
- 31.Kensinger EA, Ullman MT, Corkin S. Bilateral medial temporal lobe damage does not affect lexical or grammatical processing: Evidence from amnesic patient H.M. Hippocampus. 2001;11(4):347–360. doi: 10.1002/hipo.1049. [DOI] [PubMed] [Google Scholar]
- 32.Schmolck H, Stefanacci L, Squire LR. Detection and explanation of sentence ambiguity are unaffected by hippocampal lesions but are impaired by larger temporal lobe lesions. Hippocampus. 2000;10(6):759–770. doi: 10.1002/1098-1063(2000)10:6<759::AID-HIPO1013>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H.M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002;12(4):520–533. doi: 10.1002/hipo.10039. [DOI] [PubMed] [Google Scholar]
- 34.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 35.Hamann SB, Cahill L, Squire LR. Emotional perception and memory in amnesia. Neuropsychology. 1997;11(1):104–113. doi: 10.1037//0894-4105.11.1.104. [DOI] [PubMed] [Google Scholar]
- 36.Schmolck H, Squire LR. Impaired perception of facial emotions following bilateral damage to the anterior temporal lobe. Neuropsychology. 2001;15(1):30–38. [PubMed] [Google Scholar]
- 37.Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2(2):151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- 38.Keane MM, Gabrieli JD, Mapstone HC, Johnson KA, Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995;118(Pt 5):1129–1148. doi: 10.1093/brain/118.5.1129. [DOI] [PubMed] [Google Scholar]
- 39.Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28(18):4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: Path integration does not require hippocampus or entorhinal cortex. Proc Natl Acad Sci USA. 2008;105(33):12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400(6745):675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- 42.Hamann SB, Squire LR. Intact perceptual memory in the absence of conscious memory. Behav Neurosci. 1997;111(4):850–854. doi: 10.1037//0735-7044.111.4.850. [DOI] [PubMed] [Google Scholar]
- 43.Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280(5360):77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 44.Freed DM, Corkin S, Cohen NJ. Forgetting in H.M.: A second look. Neuropsychologia. 1987;25(3):461–471. doi: 10.1016/0028-3932(87)90071-6. [DOI] [PubMed] [Google Scholar]
- 45.Reed JM, Hamann SB, Stefanacci L, Squire LR. When amnesic patients perform well on recognition memory tests. Behav Neurosci. 1997;111(6):1163–1170. doi: 10.1037//0735-7044.111.6.1163. [DOI] [PubMed] [Google Scholar]
- 46.Bayley PJ, O’Reilly RC, Curran T, Squire LR. New semantic learning in patients with large medial temporal lobe lesions. Hippocampus. 2008;18(6):575–583. doi: 10.1002/hipo.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. 1989;11(5):724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- 49.Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;37:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 50.Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46(5):799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirwan CB, Bayley PJ, Galván VV, Squire LR. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proc Natl Acad Sci USA. 2008;105(7):2676–2680. doi: 10.1073/pnas.0712155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conroy MA, Hopkins RO, Squire LR. On the contribution of perceptual fluency and priming to recognition memory. Cogn Affect Behav Neurosci. 2005;5(1):14–20. doi: 10.3758/cabn.5.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy DA, Stark CEL, Squire LR. Intact conceptual priming in the absence of declarative memory. Psychol Sci. 2004;15(10):680–686. doi: 10.1111/j.0956-7976.2004.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Squire LR, Knowlton BJ. Learning about categories in the absence of memory. Proc Natl Acad Sci USA. 1995;92(26):12470–12474. doi: 10.1073/pnas.92.26.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reed JM, Squire LR, Patalano AL, Smith EE, Jonides J. Learning about categories that are defined by object-like stimuli despite impaired declarative memory. Behav Neurosci. 1999;113(3):411–419. doi: 10.1037//0735-7044.113.3.411. [DOI] [PubMed] [Google Scholar]
- 56.Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. J Cogn Neurosci. 1998;10(2):248–263. doi: 10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- 57.Berry CJ, Shanks DR, Speekenbrink M, Henson RN. Models of recognition, repetition priming, and fluency: Exploring a new framework. Psychol Rev. 2012;119(1):40–79. doi: 10.1037/a0025464. [DOI] [PubMed] [Google Scholar]
- 58.Bayley PJ, Squire LR. Medial temporal lobe amnesia: Gradual acquisition of factual information by nondeclarative memory. J Neurosci. 2002;22(13):5741–5748. doi: 10.1523/JNEUROSCI.22-13-05741.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436(7050):550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaizot X, et al. The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cereb Cortex. 2010;20(9):2198–2212. doi: 10.1093/cercor/bhp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brodmann K. 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues [Comparative Localization Studies in the Brain Cortex, its Fundamentals Represented on the Basis of its Cellular Architecture] (Barth, Leipzig) in German.
- 62.Bailey P, von Bonin G. The Isocortex of Man. Urbana, IL: University of Illinois Press; 1951. [Google Scholar]
- 63.Corkin S, Amaral DG, González RG, Johnson KA, Hyman BT. H. M.’s medial temporal lobe lesion: Findings from magnetic resonance imaging. J Neurosci. 1997;17(10):3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Kane G, Kensinger EA, Corkin S. Evidence for semantic learning in profound amnesia: An investigation with patient H.M. Hippocampus. 2004;14(4):417–425. doi: 10.1002/hipo.20005. [DOI] [PubMed] [Google Scholar]
- 65.Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005;15(2):273–280. doi: 10.1002/hipo.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horel JA. The neuroanatomy of amnesia. A critique of the hippocampal memory hypothesis. Brain. 1978;101(3):403–445. doi: 10.1093/brain/101.3.403. [DOI] [PubMed] [Google Scholar]
- 67.Gaffan D. Against memory systems. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1111–1121. doi: 10.1098/rstb.2002.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: Amygdala-hippocampus versus temporal stem. Science. 1982;218(4579):1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- 69.Zola-Morgan S, Squire LR. Preserved learning in monkeys with medial temporal lesions: Sparing of motor and cognitive skills. J Neurosci. 1984;4(4):1072–1085. doi: 10.1523/JNEUROSCI.04-04-01072.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adolphs R. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 2008;18(2):166–172. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanwisher N, Yovel G. The fusiform face area: A cortical region specialized for the perception of faces. Philos Trans Roy Soc Lond Series B. 2006;361(1476):2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodges JR, Graham KS. 2001. Episodic memory: Insights from semantic dementia. Philos Trans Roy Soc Lond Series B 356(17):1423–1434.
- 73.Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: Medial vs. lateral temporal lobe. Proc Natl Acad Sci USA. 2004;101(17):6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mummery CJ, et al. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. [PubMed] [Google Scholar]
- 75.Galton CJ, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57(2):216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 76.Hodges JR, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: A comparative neuropsychological study. Neuropsychology. 1999;13(1):31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- 77.Davies RR, Xuereb JH, Hodges JR. The human perirhinal cortex in semantic memory: An in vivo and postmortem volumetric magnetic resonance imaging study in semantic dementia, Alzheimer's disease and matched controls. Neuropathol Appl Neurobiol. 2002;28(5):167–168. [Google Scholar]
- 78.Suzuki WA. Perception and the medial temporal lobe: Evaluating the current evidence. Neuron. 2009;61(5):657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 79.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Essen DC, Maunsell JHR. Two-dimensional maps of the cerebral cortex. J Comp Neurol. 1980;191(2):255–281. doi: 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- 81.Insausti R, et al. MRI-based volumetric analyses of the human entorhinal, perirhinal and temporopolar cortices. AJNR Am J Neuroradiol. 1998;16:659–671. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.