Abstract
It is well known that ocean acidification can have profound impacts on marine organisms. However, we know little about the direct and indirect effects of ocean acidification and also how these effects interact with other features of environmental change such as warming and declining consumer pressure. In this study, we tested whether the presence of consumers (invertebrate mesograzers) influenced the interactive effects of ocean acidification and warming on benthic microalgae in a seagrass community mesocosm experiment. Net effects of acidification and warming on benthic microalgal biomass and production, as assessed by analysis of variance, were relatively weak regardless of grazer presence. However, partitioning these net effects into direct and indirect effects using structural equation modeling revealed several strong relationships. In the absence of grazers, benthic microalgae were negatively and indirectly affected by sediment-associated microalgal grazers and macroalgal shading, but directly and positively affected by acidification and warming. Combining indirect and direct effects yielded no or weak net effects. In the presence of grazers, almost all direct and indirect climate effects were nonsignificant. Our analyses highlight that (i) indirect effects of climate change may be at least as strong as direct effects, (ii) grazers are crucial in mediating these effects, and (iii) effects of ocean acidification may be apparent only through indirect effects and in combination with other variables (e.g., warming). These findings highlight the importance of experimental designs and statistical analyses that allow us to separate and quantify the direct and indirect effects of multiple climate variables on natural communities.
Keywords: food web, global warming, herbivory, species interaction, top-down
Across biomes, ecosystems are simultaneously affected by changing climate (1, 2) and consumer populations (3–6). Increasingly, evidence is showing that consumers have not only direct impacts on processes such as biomass production, but also indirect impacts by mediating the effects of climate change on lower trophic levels (7–9). For example, efficient herbivores such as muskoxen and caribou dampen the effects of warming on plant communities on the arctic tundra (10). In contrast, predation on herbivores augments the effects of warming on plants by shifting the control of shallow-water aquatic food webs from top-down to bottom-up (11–13).
Although warming is an important driver of ecosystem change, it is far from the only one. Ecosystems are often exposed to several stressors simultaneously, and multiple stressors can often influence ecosystem functioning by interacting in a nonadditive way, either synergistically or antagonistically (14, 15). In marine ecosystems, increasing concentrations of CO2 are reducing ocean pH, leading to ocean acidification—a process that is already having impacts and may have far-reaching ecological consequences (16, 17). Higher CO2 concentrations can stimulate plant growth, leading to increased bottom-up control (18–20). At the same time, lower pH can negatively impact plants as well as herbivores (20, 21), thereby reducing top-down control. Moreover, warming and acidification have frequently been found to interact to augment the effects of CO2 enrichment (18, 22, 23). Consequently, there is a pressing need to include multiple climate factors in climate change studies and to evaluate responses at the level of communities with interacting organisms rather than single species (15). Importantly, indirect climate effects (those mediated by species interactions such as consumption or competition) have been acknowledged as potentially as important as direct effects, yet they have rarely been quantified and contrasted with direct effects (15).
Here, we experimentally tested whether consumers can mediate the effects of warming and acidification on primary producers in seagrass ecosystems, one of the most productive, and threatened, coastal ecosystems on earth (24). In addition to seagrass itself, these systems also include other primary producers, such as filamentous macroalgae and sediment-associated microalgae. As focal organisms we chose sediment-growing microalgae, an often neglected but highly important group of primary producers in shallow aquatic ecosystems (25, 26).
Most climate change experiments have used factorial statistical methods, such as analysis of variance (ANOVA), which estimate net effects—the sum of direct and indirect effects (27, 28). ANOVA is a powerful tool for analyzing complex ecological interactions between predictor variables (29). However, ANOVA is not able to partition net effects into direct and indirect effects (30), nor can it detect counteracting indirect effects, which often dominate in ecosystems (27). Consequently, relying solely on ANOVA can mislead our interpretations of results, which ultimately hampers our understanding and hence our efforts to mitigate climate change. Partitioning net effects into direct and indirect effects, and estimating their relative importance, is readily achieved using structural equation modeling (SEM) (31), a framework for understanding causal processes (32). Consequently, SEM is being increasingly used to disentangle complex community- or ecosystem-level effects of environmental and climate change (33–35).
To understand if and how invertebrate consumers (from here on “mesograzers”) mediate the effects of ocean acidification and warming on benthic microalgae, we used data from a factorial mesocosm experiment on shallow marine ecosystems dominated by the habitat-forming eelgrass Zostera marina L. (from here on “Zostera”). We hypothesized that the effects of acidification and warming on benthic microalgae would be regulated by mesograzers (the amphipod Gammarus locusta and the gastropods Littorina littorea and Rissoa sp.) because mesograzer respiration is more temperature-dependent than plant photosynthesis (7, 36, 37). Therefore, we expected top-down control to strengthen with warming. Second, we hypothesized that indirect effects of acidification and warming would be at least as strong as direct effects (we define indirect effects as those mediated by predation, herbivores, and shading of the sediment surface by macroalgal growth). To estimate the relative importance of direct and indirect effects on benthic microalgae, we first assessed the single and joint effects of acidification, warming, and consumers on benthic microalgae, macroalgae, eelgrass, and sediment fauna, using ANOVA techniques (estimating net single and joint effects of acidification, warming, and mesograzer presence). We then used multigroup SEM (see Materials and Methods for a detailed description of the statistical method) to assess the relative importance of direct vs. indirect effects of the same three factors on biomass and production of benthic microalgae.
Results
ANOVA Analysis.
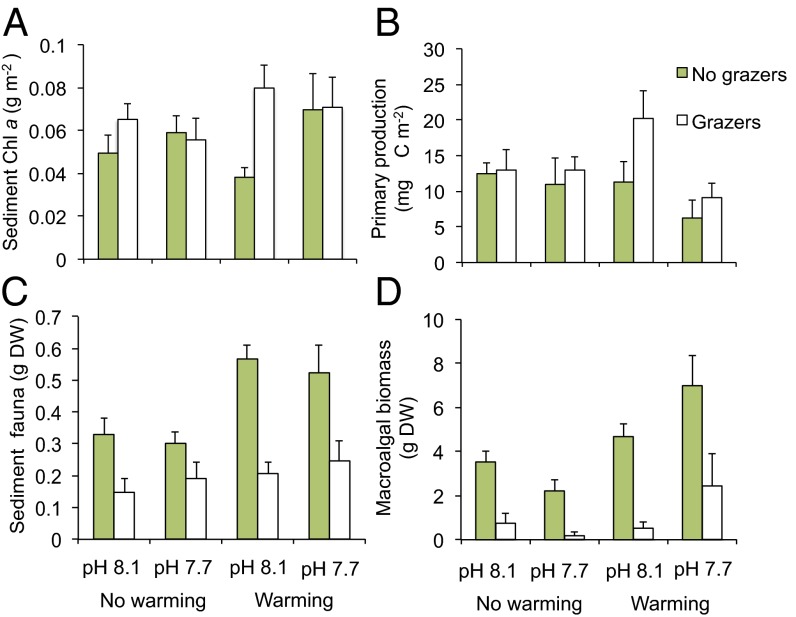
Neither acidification (F = 0.57, P = 0.46) nor warming (F = 0.98, P = 0.33) had single effects on benthic microalgal biomass (Table S1). In the presence of mesograzers, acidification weakly increased benthic microalgal biomass (mesograzer × acidification interaction; F = 3.90, P = 0.057) (Fig. 1A). Benthic microalgal production, however, was reduced by acidification (F = 4.81, P = 0.036), but remained unaffected by warming (F = 0.05, P = 0.82), by mesograzers (F = 3.06, P = 0.09) (Fig. 1B), and by the interaction between warming and acidification (warming × acidification, F = 3.24, P = 0.082) (Fig. 1B and Table S1).
Fig. 1.
Effects of experimental warming, acidification, and mesograzers on (A) sediment chlorophyll a (proxy for benthic microalgae biomass), (B) primary production by benthic microalgae, (C) total biomass of sediment-associated macrofauna, and (D) total biomass of macroalgae. Data are means +SE; n = 5.
Warming increased the total biomass of sediment-associated fauna (F = 14.08, P < 0.001), whereas acidification had no effect (F = 0.01, P = 0.937) (Table S1). The presence of the three mesograzers decreased the biomass of sediment-associated fauna (F = 36.88, P < 0.001) and also removed the positive effect of warming (warming × mesograzer, F = 5.08, P = 0.031) (Fig. 1C and Table S1). Macroalgal biomass was strongly reduced by the presence of mesograzers (F = 16.09, P < 0.001), independently of acidification and warming (Fig. 1D) (warming × mesograzer, warming × acidification, and warming × mesograzer × acidification interactions; all P > 0.25) (Table S1). Meanwhile, macroalgal biomass was stimulated by warming (F = 9.92, P = 0.003), particularly in combination with acidification (warming × acidification, F = 7.10, P = 0.012) (Table S1). The biomass of Zostera was not stimulated by warming (F = 0.23, P = 0.62) but nearly so by acidification (F = 3.30, P = 0.07). When mesograzers were present, Zostera biomass was higher than in the absence of mesograzers (F = 48.61, P < 0.001), but warming decreased the biomass of Zostera during the presence of mesograzers (warm × mesograzers, F = 4.62, P = 0.039). Complete ANOVA tables for measured response variables and mesograzer biomass are provided in Tables S1 and S2.
SEM Analyses.
Individual and multigroup SEM models were statistically similar to the observed data (Table 1). SEM analyses revealed strong indirect effects of acidification and warming on benthic microalgae that were at least as strong as direct effects—but only in the absence of mesograzers (Figs. 2 and 3). In the presence of mesograzers, the direct and indirect effects of acidification and warming were weak or absent. The strength and pattern of effects also differed with respect to the response variables benthic microalgal biomass and benthic microalgal production (compare Figs. 2 and 3).
Table 1.
SEM statistics
| Models | χ2 | df | P |
| Individual group models | |||
| Mesograzers present (BMA biomass) | 5.58 | 5 | 0.34 |
| Mesograzers absent (BMA biomass) | 7.35 | 5 | 0.19 |
| Mesograzers present (BMA primary production) | 5.58 | 5 | 0.34 |
| Mesograzers absent (BMA primary production) | 7.35 | 5 | 0.19 |
| Multigroup models | |||
| Model A (BMA biomass) | 16.67 | 19 | 0.61 |
| Model B (BMA primary production) | 17.24 | 19 | 0.57 |
Chi-square (χ2) likelihood tests for the individual and multigroup SEM models for benthic microalgae (BMA) biomass and production. When P ≥ 0.05, fitted models are not significantly different from observed data.
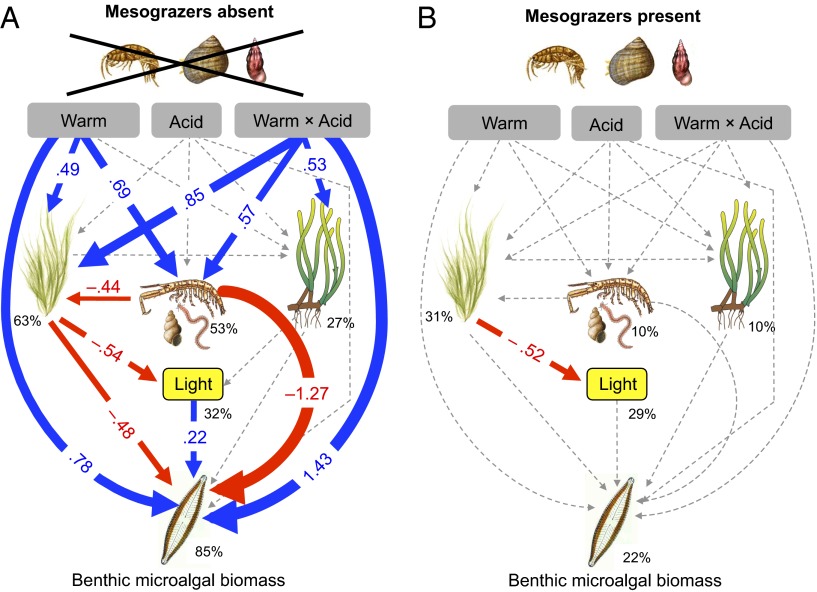
Fig. 2.
Path diagrams showing how experimental warming (Warm), acidification (Acid), and the interaction between warming and acidification (Warm × Acid) affect the macroalgae, sediment-associated fauna, Z. marina, light, and benthic microalgal biomass. Path diagram represents (A) seagrass meadows with mesograzers (G. locusta, L. littorea, and Rissoa sp.) absent and (B) seagrass meadows with mesograzers present. Solid paths (blue positive and red negative effects) are statistically significant (P < 0.05) whereas the dashed lines are not. At each significant path the standardized coefficients are represented and interpreted as follows: If, for example, Warm × Acid goes up by 1 SD, the benthic microalgae biomass goes up by 1.43 SD. Standardized coefficients are therefore used to compare the strength of direct vs. indirect effects. Percentages indicate the variance explained by the model.
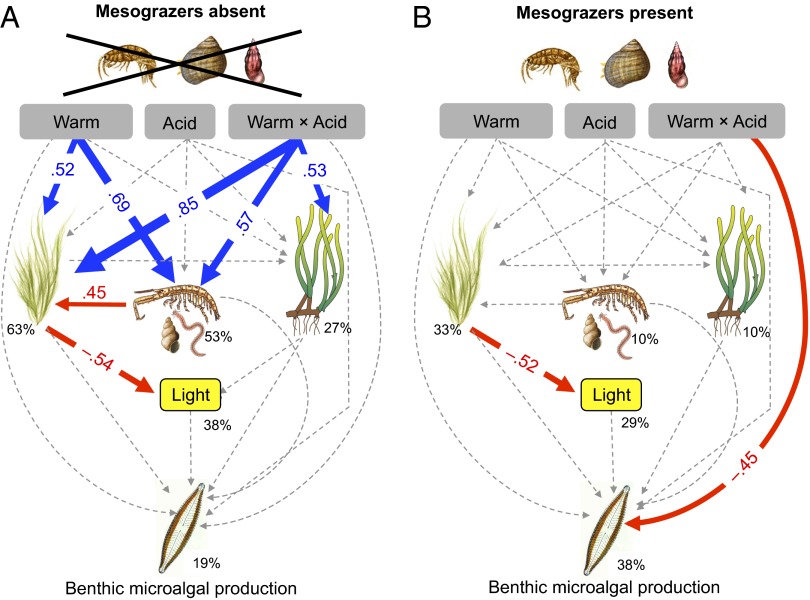
Fig. 3.
Path diagrams showing how experimental warming (Warm), acidification (Acid), and the interaction between warming and acidification (Warm x Acid) affect the macroalgae, sediment-associated fauna, Z. marina, light, and benthic microalgal production. Path diagram represents (A) seagrass meadows with mesograzers (G. locusta, L. littorea, and Rissoa sp.) absent and (B) seagrass meadows with mesograzers present. Solid paths (blue positive and red negative effects) are statistically significant (P < 0.05) whereas the dashed lines are not. At each significant path the standardized coefficients are represented and interpreted as follows: If, for example, Warm × Acid goes up by 1 SD, the benthic microalgae primary production goes down by 0.45 SD. Standardized coefficients are therefore used to compare the strength of direct vs. indirect effects. Percentages indicate the variance explained by the model.
Effects on Benthic Microalgal Biomass.
In the absence of mesograzers, both warming alone and warming together with acidification had strong direct positive effects on benthic microalgal biomass (path coefficients: 0.78 and 1.43, respectively) (Fig. 2A). Simultaneously, warming alone, and the interaction between warming and acidification, positively affected biomass of both macroalgae and sediment-associated macrofauna, two variables that in turn negatively affected benthic microalgal biomass (Fig. 2A). As a consequence, warming and the warming × acidification interaction also had strong indirect and negative effects on the benthic microalgal biomass (−1.27 and −0.48, respectively), which counteracted the direct positive effects mentioned above. This explains why the ANOVA analyses reported only weak (net) effects of warming or acidification. The biomass of Zostera was positively affected by the warming × acidification interaction, but had no effect on other variables (Fig. 2 A and B). Likewise, acidification alone had no direct or indirect effects.
In stark contrast to the intricate network of effects that we observed in the absence of mesograzers (Fig. 2A), the presence of mesograzers resulted in no effects of warming, acidification, or their interaction (Fig. 2B). Moreover, the inclusion of mesograzers in our model with microalgal biomass reduced the amount of percentages explained by the model from 85% to 22% (Fig. 2).
Comparisons of intercepts for each measured variable between the two groups (mesograzers present vs. mesograzers absent) (Table S3) showed that the model changed significantly when macroalgae and light, sediment fauna, and Zostera were set as equal across the two groups. Thus, mesograzer presence directly affected these variables (as also shown by ANOVA) (Fig. 1 A–D). However, the same type of mesograzer effect was not observed for benthic microalgal biomass and light, suggesting that these variables were unaffected by mesograzer presence (Table S3).
Effects on Benthic Microalgal Production.
SEM analyses on benthic microalgal primary production showed no direct effects of acidification, warming, or their interaction in the absence of the mesograzers (Fig. 3A). Although there were strong direct effects of warming on both macroalgae and sediment-associated fauna (Fig. 3A), these did not translate into indirect effects on benthic microalgal production. In the presence of mesograzers, all of these effects were absent and were replaced by a strong negative direct effect of the warming × acidification interaction (Fig. 3B). The effect of this same interaction term was also marginally significant in the ANOVA analysis (warming × acidification, F = 3.24, P = 0.082) (Fig. 1B and Table S1).
Standardized total direct and indirect effects for the groups “mesograzers absent” and “mesograzers present” are given in Tables S4–S7.
Discussion
Results from our multifactorial mesocosm experiment show clearly that the presence of effective consumers, such as algal-feeding invertebrate mesograzers, mediate the effects of climate change on primary producer biomass. The strong direct and indirect effects of ocean acidification and warming that we observed in the absence of the mesograzers were almost completely absent when mesograzers were present (Fig. 2). A rapidly growing body of literature shows similar effects of consumers on the responses of plant communities to climate change (10, 18, 38). These effects and their strength have been demonstrated in response to warming and ocean acidification, two environmental changes that co-occur in most ocean areas.
The almost complete absence of direct and indirect effects of climate factors in the presence of mesograzers is striking (Figs. 2 and 3). In most vegetated coastal ecosystems, algal-feeding mesograzers play a key role in controlling ephemeral algae biomass, thereby facilitating habitat-forming perennial macrovegetation, such as the eelgrass Z. marina (39, 40). Suppression or removal of mesograzers, for example, by trophic cascades induced by overfishing of large predators, can flip these ecosystems to bottom-up stimulation (11, 12, 39, 40). We observed this same pattern; mesograzers affected the balance between bottom-up and top-down regulation by (i) feeding on epiphytic and floating macroalgae, leading to increased light penetration; and (ii) preying upon smaller sediment-associated fauna that in turn fed on the benthic microalgae. One of the mesograzers, G. locusta, is known to feed on other invertebrates (and even conspecifics) if populations reach high density and/or if the preferred food (green macroalgae) is scarce (41). In our experiment, G. locusta became food-limited after having grazed down all preferred macroalgae (23), leading to increased predation on sediment-associated fauna and, consequently, to reduced grazing pressure on benthic microalgae. When G. locusta was absent, sediment-associated fauna were released from this top-down control, resulting in increased grazing on benthic microalgae. In the absence of all mesograzers, the observed strong indirect effects (Figs. 2A and 3A) cancelled each other out to generate little or no net effect on benthic microalgae biomass or production (Table S1).
Indirect effects have been found to be at least as important as direct effects in structuring communities (27, 42), yet identifying these effects has not been a priority in climate change research. Comparing the results of ANOVA (estimating net effects) and SEM (partitioning net effects into direct and indirect effects) provides insights on the importance of the indirect effects in our experiment. For example, in the absence of mesograzers, acidification and warming stimulated benthic microalgal biomass directly, but this was counteracted by indirect effects of grazing from sediment-associated fauna and by competition with macroalgae for light (Fig. 2A). On the other hand, there were only direct negative effects of acidification and warming on benthic microalgal production (Fig. 3A). This illustrates the value of using statistical methods that can partition direct and indirect effects, such as SEM, if we want to estimate, explain, and (potentially) predict the effects of climate change within ecosystems, rather than target the effects on individual species or processes.
Our finding that macroalgae, the preferred food of several of the manipulated mesograzers, were strongly impacted by all three factors (acidification, warming, and mesograzer presence) (Figs. 2 and 3; Table S1) reflects recent studies that have found similar responses of primary producers to CO2, warming, and mesograzers (1, 6, 23). For benthic microalgae, however, the effects of acidification and warming (in the absence of mesograzers) were weak—both in terms of biomass (which integrates effects over the whole experiment) and production (a short-term response). Similar weak responses of benthic microalgae to acidification and warming have recently been reported from a range of other systems (43–46). Consequently, for shallow coastal sediments, benthic microalgae may be relatively resilient to the changes in pH and water temperature expected over the next century, regardless of mesograzer presence.
Most marine climate change experiments—and especially those involving ocean acidification—have assessed direct effects of single factors and on single species (15). In our study, acidification alone had no direct or indirect effects on either primary producers or consumers (Table S1; Figs. 2 and 3). However, acidification in combination with warming had strong, positive direct and indirect effects on many components of the system (Figs. 2A and 3A). Macroalgae, sediment-associated fauna, and Zostera were all positively influenced by the combination of acidification and warming. Benthic microalgal biomass was also directly augmented by this interaction (although note the negative effect on benthic microalgal production) (Figs. 2A and 3A). We believe that the different responses of benthic microalgal biomass and production were caused by changed light conditions, which in turn depended on treatment effects on macroalgae. Both light and CO2 are primary resources for benthic microalgae; however, changes in these two resources can interact so that high CO2 (i.e., lower pH) can have a negative effect on autotrophic production at high light intensities, but a positive effect at low light intensities (47, 48). These results were completely obscured when we investigated net responses using ANOVA techniques (Table S1), the traditional approach in ocean acidification and climate change research (49). This result also illustrates the importance of investigating the effects of ocean acidification in conjunction with other co-occurring climate variables (15, 17) and highlights the risk that simpler statistical analyses (even of multitrophic level and multiple-response designs) may overlook ecologically important information. Relying on only ANOVA techniques would have led us to very different (and nonsignificant) conclusions about the effects of ocean acidification.
In summary, we showed that, although net effects of ocean acidification and warming on benthic microalgae were generally weak regardless of mesograzer presence/absence, these weak net effects in fact comprised strong direct and indirect climate effects on different components of the community. Thus, although benthic microalgae appear to be resilient to simulated climate change, our understanding of the community-level processes that lead to this resilience is critically dependent on our analytical methods. We suggest that the use of only standard analyses of net effects (such as ANOVA) has limited our understanding of the combined effects of ocean acidification and warming on multivariable ecosystems. The effects of climate change need to be addressed from a multitrophic and multifactor perspective. This means that we need to manipulate factors separately and jointly and to apply statistical analyses that can separate net effects into direct and indirect effects. Only then will we be able to begin to understand—and make ecologically relevant predictions of—the effects of near-future climate change.
Materials and Methods
Study System.
Z. marina is a clonal angiosperm that forms large underwater meadows, typically at depths of 2–4 m on the Swedish western coast (50). Seagrass meadows support a wide array of trophic interactions between herbivores feeding on epiphytic and free-floating macroalgae, but also function as nursery and feeding grounds for ecologically and commercially important fish (40, 51). In these vegetated sediment systems, benthic microalgae are an important food source for crustacean and gastropod herbivores, which in turn are eaten by fish and crustaceans (52). Through the production of oxygen via photosynthesis, microphytobenthos also “indirectly” control key biogeochemical processes in the sediment (53, 54) and, in theory, could counteract effects of expected global warming by sustaining net benthic autotrophy (46).
Experimental Design.
The experiment consisted of three treatments: (i) ocean warming (two levels: ambient and warming), (ii) ocean acidification (two levels: ambient and acidified), and (iii) mesograzers (two levels: presence vs. absence of G. locusta, L. littorea, and Rissoa sp). The treatments were allocated in an orthogonal design, resulting in eight treatment combinations, each of which was replicated five times (n = 5). The experiment was run for 5 wk during July and August 2010. For further details regarding experimental design, see Eklöf et al. (23).
Construction of Mesocosms.
Natural sediment collected from a Z. marina meadow was mixed with cleaned beach sand (to a total volume of 8 L) and placed in 30-L semitransparent buckets. On top of the sand–sediment mix, a layer of natural surface sediment (<0.5 cm) was placed to initiate the growth of a diatom biofilm. One week before the start of the experiment, 28 cleaned Z. marina shoots were planted in each mesocosm and allowed to acclimatize in water with ambient temperature and pH for 1 wk. The resulting shoot density (350 shoots m−2) was within the range of that in nearby shallow areas (40). On the day that the experiment started, we added 2 g wet weight (WW) of green algae (Cladophora spp.) into each mesocosm to simulate a loose and drifting macroalgal community. We then added the mesograzers in the mesograzer treatment—20 adult G. locusta, 7 adult L. littorea [1.44 ± 0.18 g wet weight per individual (WW⋅ind−1)], and 5 adult Rissoa sp. (0.22 ± 0.02 g WW⋅ind−1)—before imposing the climate treatments (see below).
Climate Manipulations.
Each mesocosm was connected to a flow-through system in a semiopen greenhouse at the Sven Lovén Center for Marine Sciences–Kristineberg on the western coast of Sweden. Incoming seawater from the fjord (salinity ∼24) was manipulated to simulate changes in seawater temperature and pH expected by the year 2100 for Intergovernmental Panel on Climate Change scenario A1FI (55). Seawater was pumped into two 1-m3 tanks, one of which (warming) was heated to 4 °C above ambient levels using an immersion heater controlled by a computerized thermostat. The heated and nonheated seawater was then pumped into smaller (80 L) tanks, in which water was bubbled with pure air (ambient, pH = ∼8.1) or air enriched with CO2 (acidified, pH = ∼7.7) using a computerized pH-stat (Aqua-Medic). The manipulation of water temperature and pH in header tanks ensured that actual levels in the mesocosms fluctuated temporally just as in natural seagrass beds while maintaining fixed differences between treatment means (∆T = 3.2 °C, ∆pH = −0.35 units) [see Eklöf et al. (23)]. Seawater pH was logged throughout the experiment (Beckman-Coulter pHi460). For details regarding properties of the carbonate system, see Table S8.
Benthic Microalgae.
At termination of the experiment, 0.5-cm deep sediment samples were collected using a 2-mL cut-off plastic syringe (area 0.594 cm2) and placed in 20-mL scintillation vials. The samples for sediment Chlorophyll a (Chl a) (used as a proxy for benthic microalgal biomass) were immediately frozen (−20 °C) until analysis. Chl a was extracted with acetone [90% (acetone/vol)] for 24 h at 8 °C. Samples were ultrasonicated while cooling on ice, centrifuged, and measured spectrophotometrically (UV-2401PC; Shimadzu). Chl a and phaeopigment concentrations were calculated using standard methods and equations (56). Sediment organic content (loss on ignition) was calculated based on estimates of sediment dry weight and ash-free dry weight. Potential benthic microalgal primary production was measured by incubating sediment slurries with 14C-sodium bicarbonate. First, 150 μL of 14C-solution in 2 mL seawater was added, giving a final concentration of 3.6 μCi per vial. The vials were then incubated at temperature and light conditions representative for the respective treatment for 1.5–2 h. Incubations were terminated by carefully removing water and adding 30 μL 1% HCl. Samples were treated further as described by Sundbäck et al. (57). During counting (TRI-CARB 2900TR software; Quanta Smart V 1.1), curves for corrections of quenching were applied using static controller (neutralizing the charged ions on the surface of interest). Total inorganic carbon concentration in the water and carbon assimilation were calculated using equations in Aertebjerg and Bresta (58). All samples were corrected for dark fixation.
Macroalgae, Eelgrass, and Sediment Fauna.
After sediment sampling, all macroalgae, Z. marina, and G. locusta were gently removed and frozen. Finally, the remaining contents (sediment, gastropod mesograzers, and sediment-associated fauna) were sieved out and frozen. Following thawing, macroalgae were identified as to genus, dried [60 °C until constant dry weight (DW)], and weighed. Zostera leaves, roots, and rhizomes were cleaned, dried, and weighed. Sediment-associated fauna (Corophium spp., Hydrobia spp., and Nereis spp. were sorted, counted, dried, and weighed (DW). All mesograzers were counted, dried, and weighed [for details, see Eklöf et al. (23)].
Statistical Analyses.
ANOVA.
Effects of warming, acidification, and their interaction on the biomass of the three mesograzers G. locusta, L. littorea, and Rissoa sp. were tested using type I ANOVA with warming (fixed: no warming vs. warming) and acidification (fixed: ambient vs. acidified) as orthogonal factors. Single and joint effects of warming, acidification, and mesograzers on Z. marina biomass, macroalgal biomass, benthic microalgae biomass and production, and the total biomass of sediment fauna were tested using a type I ANOVA with warming, acidification, and mesograzers (fixed: absence vs. presence). Significance levels were set at α = 0.05. Before analysis, all response variables were checked for normality and homogeneity of variances using Box, residual, and Q-Q plots. All analyses were performed using R (59).
Structural equation modeling.
To partition the net effects of the three experimental treatments on benthic microalgal biomass and production into direct and indirect effects, we constructed two separate structural equation models, one including benthic microalgal biomass and one including benthic microalgal production. Because the presence of mesograzers induced two different ecosystem states [a seagrass-dominated state in the presence of mesograzers and an macroalgal biomass dominated in their absence (23)], data were separated into two groups—mesograzers absent and mesograzers present—and analyzed with a multigroup SEM (60). This allowed us to test whether the paths of climate effects through the community differed between the two ecosystem states.
In the multigroup SEM, we assessed the effects of three binary predictor variables—(i) ocean warming (0/1), (ii) ocean acidification (0/1), and (iii) warming × acidification (0/1)—and modeled their direct and/or indirect effects on six continuous response variables: (i) macroalgal biomass (total biomass of all epiphytic and floating macroalgae), (ii) eelgrass biomass (total biomass of Zostera leaves, rhizomes, and roots), (iii) biomass of sediment-associated fauna (total biomass of Corophium spp., Hydrobia sp., and Nereis spp.), (iv) light penetration (percentage of surface light at the sediment surface), (v) benthic microalgal biomass, and (vi) benthic microalgal production. We hypothesized that (i) ocean warming and ocean acidification and their interaction would positively influence benthic microalgae, macroalgae, Zostera, and sediment fauna (8, 24, 49); (ii) macroalgae would negatively affect benthic microalgae, Zostera, and light availability (24, 61, 62); (iii) Zostera would negatively affect light availability; (iv) sediment fauna would negatively affect benthic microalgae (63, 64); and (v) macroalgae and decreased light availability (shading) would negatively affect benthic microalgal biomass and production (65, 66).
First, we analyzed the data in each group to ensure that the basic structure of the model was consistent with the data, following Grace (67). Data were analyzed by comparing models with the observed covariance matrix, using maximum likelihood and χ2 as goodness-of-fit measures. Data were considered significantly different from the model when P < 0.05. Because data from the individual groups fit the model (P > 0.05), we deemed it legitimate to perform a multigroup SEM analysis. All variables in the model were initially constrained to vary equally across both groups. Standardized residual covariances, which display the difference between sample covariance and implied covariance, were then examined to locate variable inequalities between groups. Any inequalities that differed between the two groups by >2 in absolute values were relaxed (or allowed to vary freely across groups), and the analysis was run again. This stepwise procedure was performed until the model χ2 no longer changed (60, 67). Intercepts of the regression equations were also investigated to test differences for each endogenous variable between the two groups (Table S3). Significance levels for individual paths between variables were set at α = 0.05. Structural equation models were run in AMOS (version 20).
Supplementary Material
Acknowledgments
We thank V. Telemo, L. Ulvestad, J. Egardt, E. Norin, C. Magyar, and P.-O. Moksnes for assistance in the field and the laboratory; H. Wood for measuring and calculating properties of the carbonate system; and two anonymous reviewers for comments that improved a previous version of the paper. Funding was provided by Forskningsrådet för miljö, areella näringa och samhällsbyggande (Formas) Grant 2009-4007 (to L.G. and J.N.H.) and Grant 2007-401 (to K.S.), VR Grant 2009-5457 (to L.G.), and the Memorial Fund of Birgit and Birger Wåhlström (K.S.). This work was partly performed at the Linnaeus Centre for Marine Evolutionary Biology (www.cemeb.science.gu.se) and supported by a Linnaeus grant from Vetenskapsrådet (VR) and Formas.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303797110/-/DCSupplemental.
References
- 1.Beaugrand G, Edwards M, Legendre L. Marine biodiversity, ecosystem functioning, and carbon cycles. Proc Natl Acad Sci USA. 2010;107(22):10120–10124. doi: 10.1073/pnas.0913855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauli H, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336(6079):353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- 3.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100(910):65–75. [Google Scholar]
- 4.Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265(5178):1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 5.Polis GA. Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos. 1999;86(1):3–15. [Google Scholar]
- 6.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor MI. Warming strengthens an herbivore-plant interaction. Ecology. 2009;90(2):388–398. doi: 10.1890/08-0034.1. [DOI] [PubMed] [Google Scholar]
- 8.Walther GR. Plants in a warmer world. Perspect Plant Ecol Evol Syst. 2004;6(3):169–185. [Google Scholar]
- 9.O’Connor MI, Gilbert B, Brown CJ. Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am Nat. 2011;178(5):626–638. doi: 10.1086/662171. [DOI] [PubMed] [Google Scholar]
- 10.Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shurin JB, Clasen JL, Greig HS, Kratina P, Thompson PL. Warming shifts top-down and bottom-up control of pond food web structure and function. Philos Trans R Soc Lond B Biol Sci. 2012;367(1605):3008–3017. doi: 10.1098/rstb.2012.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werner FJ, Matthiessen B. Temperature indirectly affects benthic microalgal diversity by altering effects of top–down but not bottom–up control. Oikos. 2013;122(1):52–63. [Google Scholar]
- 13.Hansson L, et al. Food-chain length alters community responses to global change in aquatic systems. Nat Clim Chang. 2013;3:228–233. [Google Scholar]
- 14.Darling ES, Côté IM. Quantifying the evidence for ecological synergies. Ecol Lett. 2008;11(12):1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 15.Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Glob Change Biol. 2012;18(5):1491–1498. [Google Scholar]
- 16.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 17.Doney SC, et al. Climate change impacts on marine ecosystems. Annu Rev Mar Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 18.Connell SD, Russell BD. The direct effects of increasing CO2 and temperature on non-calcifying organisms: Increasing the potential for phase shifts in kelp forests. Proc Biol Sci. 2010;277(1686):1409–1415. doi: 10.1098/rspb.2009.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett. 2010;13(11):1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 20.Harley CDG, et al. Effects of climate change on global seaweed communities. J Phycol. 2012;48(5):1064–1078. doi: 10.1111/j.1529-8817.2012.01224.x. [DOI] [PubMed] [Google Scholar]
- 21.Hurd CL, et al. Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: A mechanism for differential susceptibility? Glob Change Biol. 2011;17(10):3254–3262. [Google Scholar]
- 22.Harvey BP, Gwynn-Jones D, Moore PJ. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol. 2013 doi: 10.1002/ece3.516. 10.1002/ece3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eklöf JS, et al. Experimental climate change weakens the insurance effect of biodiversity. Ecol Lett. 2012;15(8):864–872. doi: 10.1111/j.1461-0248.2012.01810.x. [DOI] [PubMed] [Google Scholar]
- 24.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA. 2009;106(30):12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Underwood GJC, Kromkamp J. 1999. Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecological Research, Vol 29: Estuaries, eds Nedwell DB, Raffaelli DG (Academic press, Waltham, MA), pp 93–153. [Google Scholar]
- 26.Haese RR, Pronk GJ. Intra-annual variability in primary producer groups and nitrogen dynamics in an intermittently closed estuary exposed to Mediterranean climate. Estuaries Coasts. 2011;34:557–568. [Google Scholar]
- 27.Wootton JT. Indirect effects in complex ecosystems: Recent progress and future challenges. J Sea Res. 2002;48(2):157–172. [Google Scholar]
- 28.Harley CDG. Climate change, keystone predation, and biodiversity loss. Science. 2011;334(6059):1124–1127. doi: 10.1126/science.1210199. [DOI] [PubMed] [Google Scholar]
- 29.Underwood AJ. Experiments in Ecology. Their Logical Design and Interpretation Using Analysis of Variance. Cambridge, UK: Cambridge Univ Press; 1997. pp. 1–484. [Google Scholar]
- 30.Wootton JT. Predicting direct and indirect effects: An integrated approach using experiments and path-analysis. Ecology. 1994;75(1):151–165. [Google Scholar]
- 31.Grace JB. Structural Equation Modeling and Natural Systems. Cambridge, UK: Cambridge Univ Press; 2006. pp. 1–365. [Google Scholar]
- 32.Grace JB, et al. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere. 2012;3(8):1–44. [Google Scholar]
- 33.Byrnes JE, et al. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob Change Biol. 2011;17(8):2513–2524. [Google Scholar]
- 34.Blake RE, Duffy JE. Changes in biodiversity and environmental stressors influence community structure of an experimental eelgrass Zostera marina system. Mar Ecol Prog Ser. 2012;470:41–54. [Google Scholar]
- 35.Whalen AM, Duffy JE, Grace JB. Temporal shifts in top-down versus bottom-up control of epiphytic algae in a seagrass ecosystem. Ecology. 2013;94(2):510–520. doi: 10.1890/12-0156.1. [DOI] [PubMed] [Google Scholar]
- 36.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85(7):1771–1789. [Google Scholar]
- 37.Allen AP, Gillooly JF, Brown JH. Linking the global carbon cycle to individual metabolism. Funct Ecol. 2005;19:202–213. [Google Scholar]
- 38.Anthony KRN, et al. Ocean acidification and warming will lower coral reef resilience. Glob Change Biol. 2011;17:1798–1808. [Google Scholar]
- 39.Moksnes PO, Gullström M, Tryman K, Baden S. Trophic cascades in a temperate seagrass community. Oikos. 2008;117:763–777. [Google Scholar]
- 40.Eriksson BK, et al. Effects of altered offshore food webs on coastal ecosystems emphasize the need for cross-ecosystem management. Ambio. 2011;40(7):786–797. doi: 10.1007/s13280-011-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson S, Persson M, Moksnes P-O, Baden S. The role of the amphipod Gammarus locusta as a grazer on macroalgae in Swedish seagrass meadows. Mar Biol. 2009;156(5):969–981. [Google Scholar]
- 42.Kordas RL, Harley CDG, O’Connor MI. Community ecology in a warming world: The influence of temperature on interspecific interactions in marine systems. J Exp Mar Biol Ecol. 2011;400(1–2):218–226. [Google Scholar]
- 43.Alsterberg C, Hulth S, Sundbäck K. Response of a shallow-water sediment system to warming. Limnol Oceanogr. 2011;56(6):2147–2160. [Google Scholar]
- 44.Alsterberg C, Sundbäck K, Hulth S. Functioning of a shallow-water sediment system during experimental warming and nutrient enrichment. PLoS ONE. 2012;7(12):e51503. doi: 10.1371/journal.pone.0051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson VR, et al. Responses of marine benthic microalgae to elevated CO2. Mar Biol. 2011 10.1007/s00227-011-1840-2. [Google Scholar]
- 46.Hicks N, et al. Impact of biodiversity-climate futures on primary production and metabolism in a model benthic estuarine system. BCM Ecol. 2011;11:7. doi: 10.1186/1472-6785-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hein M, Sand-Jensen K. CO2 increases oceanic primary production. Nature. 1997;388:526–527. [Google Scholar]
- 48.Gao K, et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat Clim Chang. 2012;2:519–523. [Google Scholar]
- 49.Havenhand JN, Dupont S, Quinn GP. Designing ocean acidification experiments to maximize inference. In: Riebsell U, Fabry VJ, Hansson L, Gattuso J-P, editors. Guide to Best Practices for Ocean Acidification Research and Data Reporting. Luxembourg: Publications Office of the European Union; 2010. pp. 67–80. [Google Scholar]
- 50.Baden S, Boström C, Tobiasson S, Arponen H, Moksnes PO. Relative importance of trophic interactions and nutrient enrichment in seagrass ecosystems: A broad-scale field experiment in the Baltic-Skagerrak area. Limnol Oceanogr. 2010;55(3):1435–1448. [Google Scholar]
- 51.Baden S, Emanuelsson A, Pihl L, Svensson C-J, Åberg P. Shift in seagrass food web structure over decades is linked to overfishing. Mar Ecol Prog Ser. 2012;451:61–73. [Google Scholar]
- 52.Jaschinski S, Brepohl DC, Sommer U. Seasonal variation in carbon sources of mesograzers and small predators in an eelgrass community: Stable isotope and fatty acid analyses. Mar Ecol Prog Ser. 2011;431:69–82. [Google Scholar]
- 53.Sundbäck K, Miles A, Göransson E. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: An annual study. Mar Ecol Prog Ser. 2000;200:59–76. [Google Scholar]
- 54.Dalsgaard T. Benthic primary production and nutrient cycling in sediments with benthic microalgae and transient accumulation of macroalgae. Limnol Oceanogr. 2003;48(6):2138–2150. [Google Scholar]
- 55.Intergovernmental Panel on Climate Change . Intergovernmental panel on climate change. 2007: Synthesis report. In: Pachauri RK, Risinger A, editors. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva; 2007. pp. 45–47. [Google Scholar]
- 56.Lorenzen CJ. Determination of chlorophyll and pheo-pigments: Spectrophotometeric equations. Limnol Oceanogr. 1967;12(2):343–346. [Google Scholar]
- 57.Sundbäck K, Jönsson B, Nilsson P, Lindström I. Impact of accumulating drifting macroalgae on a shallow-water sediment system: An experimental study. Mar Ecol Prog Ser. 1990;58:261–274. [Google Scholar]
- 58.Aertebjerg NG, Bresta AM. 1984. Guidelines for the Measurements of Phytoplankton Primary Production. Baltic Marine Biologists Publication, Vol 1 (National Agency of Environmental Protection, Charlottenlund, Denmark), 2nd ed. [Google Scholar]
- 59.Development Core Team R . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 60.Grace JB, Jutila H. The relationship between species density and community biomass in grazed and ungrazed coastal meadows. Oikos. 1999;85:398–408. [Google Scholar]
- 61.Cloern JE. Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser. 2001;210:223–253. [Google Scholar]
- 62.McGlathery KJ, Sundbäck K, Anderson IC. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar Ecol Prog Ser. 2007;348:1–18. [Google Scholar]
- 63.Coull BC. Role of meiofauna in estuarine soft-bottom habitats. Aust J Ecol. 1999;24(4):327–343. [Google Scholar]
- 64.Blanchard GF. Measurment of meiofauna grazing rates on microphytobenthos: Is primary production a limiting factor. J Exp Mar Biol Ecol. 1991;147(1):37–46. [Google Scholar]
- 65.Weerman EJ, Herman PMJ, van de Koppel J. Macrobenthos abundance and distribution on a spatially patterned intertidal flat. Mar Ecol Prog Ser. 2011;440:95–103. [Google Scholar]
- 66.Admiraal W. The ecology of estuarine sediment-inhabiting diatoms. In: Round FE, Chapman DJ, editors. Progress in Phycological Research. Biopress, Bristol, UK; 1984. pp. 269–314. [Google Scholar]
- 67.Grace JB. Comparing groups using structural equations. In: Pugesek BH, Tomer A, von Eye A, editors. Structural Equation Modeling: Applications in Ecological and Evolutionary Biology. Cambridge, UK: Cambridge Univ. Press; 2003. pp. 281–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.