Abstract
Cushing disease (CD) is a life-threatening disorder attributed to excess pituitary tumor-derived adrenocorticotrophic hormone (ACTH) and adrenal steroid secretion caused by pituitary tumors. Whereas CD was first described in 1932, the underlying genetic basis driving tumor growth and ACTH secretion remains unsolved. Here, we show that testicular orphan nuclear receptor 4 (TR4, nuclear receptor subfamily 2, group C, member 2) is overexpressed in human corticotroph tumors as well as in human and mouse corticotroph tumor cell lines. Forced overexpression of TR4 in both human and murine tumor cells increased proopiomelanocortin transcription, ACTH secretion, cellular proliferation, and tumor invasion rates in vitro. Conversely, knockdown of TR4 expression reversed all phenotypes. Mechanistically, we show that TR4 transcriptionally activates proopiomelanocortin through binding of a direct repeat 1 response element in the promoter, and that this is enhanced by MAPK-mediated TR4 phosphorylation. In vivo, TR4 overexpression promotes murine corticotroph tumor growth as well as enhances ACTH and corticosterone production, whereas TR4 knockdown decreases circulating ACTH and corticosterone levels in mice harboring ACTH-secreting tumors. Our findings directly link TR4 to the etiology of corticotroph tumors, hormone secretion, and cell growth as well as identify it as a potential target in the treatment of CD.
Pituitary tumors are common, with a reported overall prevalence of 15% in the general population (1). Although almost always benign, these tumors cause significant morbidity and mortality through mass effects and/or excess pituitary hormone secretion (2). Cushing disease (CD), due to a pituitary corticotroph tumor, results in excessive adrenocorticotrophic hormone (ACTH)-directed adrenal-derived steroid hypersecretion (3, 4). It results in various disabling symptoms including diabetes, hypertension, osteoporosis, obesity, and psychological disturbances and has a 3.8-fold increased mortality. Surgical removal of corticotroph adenomas is first-line therapy and although initial remission rates approximate 80% in expert centers, the disease recurs in up to 25% of cases where careful long-term follow-up is used (5). Additional therapies that directly target corticotroph tumor growth and/or ACTH production are still needed. Improved understanding of the mechanisms regulating expression of the ACTH precursor polypeptide proopiomelanocortin (POMC) may lead to unique therapies for Cushing disease.
Testicular orphan receptor 4 (TR4, nuclear receptor subfamily 2, group C, member 2) belongs to the nuclear receptor superfamily and encodes a 67-kDa protein (6). TR4 acts as a homodimer or heterodimer with TR2 and is a master transcriptional regulator in various processes including spermatogenesis, lipoprotein regulation, and CNS development. TR4 binds to AGGTCA DNA sequence motifs in direct repeat (DR) orientation with a variable number of spacer nucleotides to regulate target genes such as CD36, phosphoenolpyruvate carboxykinase, apolipoprotein E (ApoE), and beta-globin (7–14). TR4 expression has been demonstrated in rat and mouse pituitary gland and a potential TR4-binding site has been identified in the POMC promoter (15), leading us to examine potential actions of TR4 on POMC regulation.
Here, we demonstrate that in the normal human pituitary gland, TR4 is almost exclusively expressed in the cytosol of corticotroph cells, and that TR4 expression is markedly increased in corticotroph tumors from both humans and mice. We further demonstrate that TR4 is a potent regulator of POMC transcription, ACTH secretion and corticotroph tumor growth in vitro and in vivo thereby identifying TR4 as a potential unique therapeutic target in Cushing disease.
Results
ACTH-Secreting Corticotroph Tumors Exhibit Higher TR4 Expression.
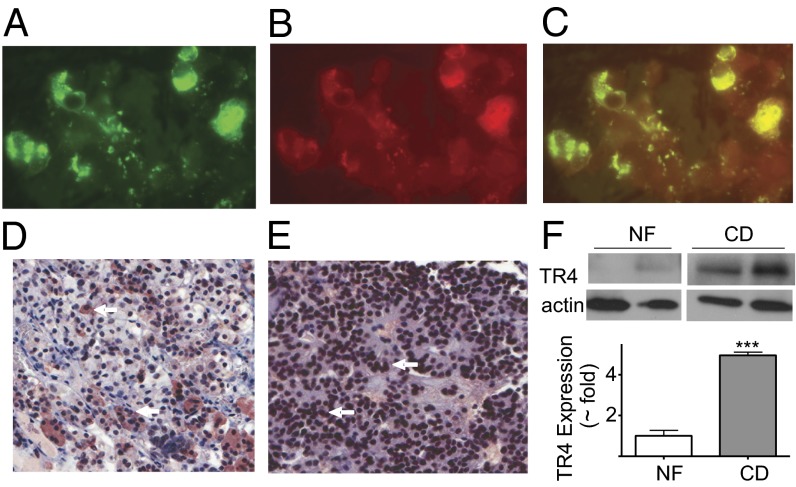
In normal human pituitary tissue, immunocytochemistry colocalized TR4 and ACTH in the cytoplasm of corticotroph cells (autopsy-derived tissue, n = 5, Fig. 1 A–D). Minimal TR4 immunoreactivity was observed in other pituitary cell subtypes and no TR4 expression was observed in the nucleus of normal pituitary cells.
Fig. 1.
Human ACTH-secreting pituitary tumor tissue showed higher TR4 expression compared with normal pituitary tissue. (A–C) Immunofluorescence images of normal corticotroph cells using fluorescently tagged antibodies to ACTH (green, A) and TR4 (red, B) colocalized TR4 and ACTH exclusively in the cytoplasm (C). Magnification (200×). Immunocytochemistry and hematoxylin & eosin staining showing (D) cytoplasmic TR4 expression (brown, arrowed) in a representative autopsy-derived normal pituitary tissue in contrast to strong nuclear TR4 expression in (E) human corticotroph tumor from patients with CD. (F) Western blot and quantification for TR4 expression in pituitary tumor of Cushing pituitary adenoma (CD) and nonfunctioning pituitary adenoma (NF).
In contrast, analysis of paraffin-embedded pituitary tumors demonstrated abundant TR4 expression in corticotroph tumors (++ to +++ expression in 10/12 tumors (84%), Table 1) which, unlike the normal pituitary corticotrophs, was clearly evident in the nucleus (Fig. 1E). The increased TR4 levels in tumors from CD patients were confirmed by Western blot analyses (Fig. 1F). In comparison with corticotroph tumors, lower TR4 expression was noted in the nucleus of somatotroph (3/5 tumors, 60%), lactotroph (2/5 tumors, 40%), and clinically nonfunctioning pituitary adenomas (3/5 tumors, 60%, Table S1).
Table 1.
TR4 immunostaining in corticotroph pituitary adenomas (n = 12) and autopsy-derived normal pituitary tissues (n = 5)
| Tumor type | Nuclear | Cytoplasmic |
| Corticotroph (n = 12) | ||
| 1 | ++, 25% | + |
| 2 | +++, 85 | + |
| 3 | +, 80% | + |
| 4 | -ve | -ve |
| 5 | -ve | -ve |
| 6 | +, 20% | + |
| 7 | ++, 70% | + |
| 8 | +++, 60% | + |
| 9 | +++, 70% | + |
| 10 | +++, 70% | + |
| 11 | +++, 70% | + |
| 12 | +++, 70% | + |
| Normal Pituitary (n = 5) | ||
| -ve | ++, 60%** |
Staining intensity: -ve, negative; +, weak; ++, moderate; +++, strong; %, immunopositive cells; **, predominantly corticotrophs.
TR4 Overexpression Induces POMC and ACTH in Murine Corticotroph Tumor Cells.
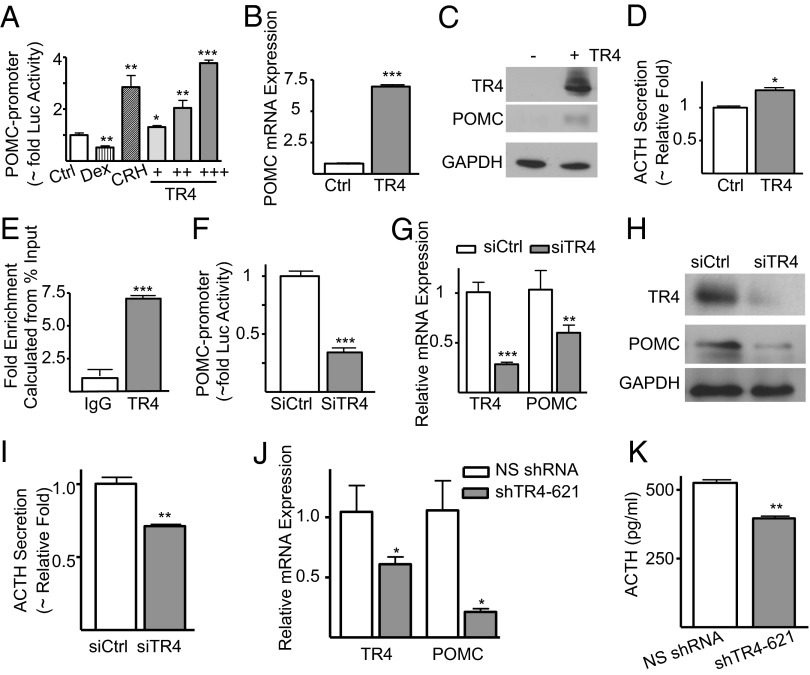
The marked increase in expression of TR4 in corticotroph tumors compared with normal pituitary corticotrophs, combined with its redistribution from the cytosol to the nucleus suggested an altered functional role for TR4 in tumor cells. As human pituitary tumors do not survive in long-term culture, we used murine corticotroph AtT20 cells to examine the role of TR4 in corticotroph tumor function in vitro. Like human CD, these cells express POMC, secrete ACTH, and produce a Cushing phenotype when inoculated s.c. in mice (16). Consistent with previous findings, dexamethasone (Dex, 100 nM for 8 h) treatment of murine pituitary corticotroph cell-line AtT20 cells transfected with a POMC-luciferase reporter construct led to a 50% decrease in POMC luciferase (POMC-Luc) activity, whereas treatment with corticotropin-releasing hormone (CRH, 100 nM for 8 h) increased POMC-Luc approximately threefold (Fig. 2A). In this in vitro system, overexpression of TR4 dose-dependently increased POMC transcription, leading to a maximal fourfold increase in POMC-Luc activity (Fig. 2A). Furthermore, AtT20 cells overexpressing TR4 showed a sevenfold increase in POMC mRNA expression (Fig. 2B), a fivefold increase in POMC protein levels (Fig. 2C), and a 30% increase in ACTH secretion (Fig. 2D) compared with vector-transfected controls.
Fig. 2.
TR4 regulates POMC expression and ACTH secretion in murine and human corticotroph tumor cells by directly binding to POMC. (A) AtT20 cells cotransfected with a rat POMC promoter-luciferase reporter plasmid (JA300) and control vector (pcDNAV5H6) or a TR4 expression plasmid (mTR4V5; +, ++, +++; 200, 400, 600 ng) were treated with dexamethasone (Dex) or corticotrophin releasing hormone (CRH). Data are shown as relative luciferase activity (∼fold). (B) Quantitative PCR (Q-PCR) of POMC mRNA, (C) Western blot of POMC protein expression, and (D) secreted ACTH levels in vector only (control) or TR4-overexpressing AtT20 cells. (E) Chromatin immunoprecipitation of AtT20 cells using TR4 or control (IgG) antibodies coupled to Q-PCR for the murine POMC promoter DR1 site (−854 to −637). (F) Relative POMC promoter activities in corticotroph AtT20 cells cotransfected with a rat POMC promoter-luciferase reporter plasmid (JA300) and either a control siRNA (siCtrl) or siRNA to TR4 (siTR4). (G) POMC mRNA, (H) POMC protein, (I) secreted ACTH levels in murine AtT20 cells, (J) TR4 and POMC mRNA expression, and (K) secreted ACTH levels in a primary culture of a freshly resected human corticotroph tumor following TR4 knockdown with a TR4 shRNA encoding lentivirus (siTR4-621) compared with nonsilencing shRNA encoding lentivirus (NS shRNA). Data are shown as mean ± SD; *P < 0.05, **P < 0.01, ***P < 0.001.
TR4 Directly Binds to the POMC Promoter.
The identification of a potential TR4-binding site in the POMC promoter sequence (−854 to −637 bp) raised the question that TR4 could directly regulate POMC expression. To address this question, we used chromatin immunoprecipitation (ChIP) coupled with real-time PCR. TR4 immunoprecipitates of AtT20 cells demonstrated a clear sevenfold enrichment of the proximal POMC promoter direct repeat 1 (DR1) element compared with control IgG-immunoprecipitates, indicating that TR4 directly binds the POMC promoter (Fig. 2E).
TR4 Knockdown Inhibits POMC and ACTH Expression in Vitro.
In contrast to our findings of increased POMC transcription and ACTH secretion following corticotroph tumor TR4 overexpression, TR4 knockdown using a small interfering RNA (siRNA) potently suppressed POMC luciferase reporter activity (70%) (Fig. 2F), reduced POMC mRNA and protein expression (Fig. 2 G and H), and decreased corticotroph tumor-derived ACTH secretion (all by 40%) (Fig. 2I) compared with control siRNA transfected cells (siCtrl).
In parallel studies, the effect of lentiviral-delivered TR4-targeting shRNA (shTR4-621) was evaluated in primary cultures of surgically resected human corticotroph tumors (n = 3). Notably, the induced ∼40% decrease in TR4 expression resulted in a marked decrease in POMC mRNA expression (∼80%) (Fig. 2J) and a ∼25% decrease in ACTH secretion (Fig. 2K). These results establish TR4 as a unique regulator of human and murine POMC transcription and ACTH secretion in vitro.
TR4 Regulates Corticotroph Tumor Proliferation and Invasion in Vitro.
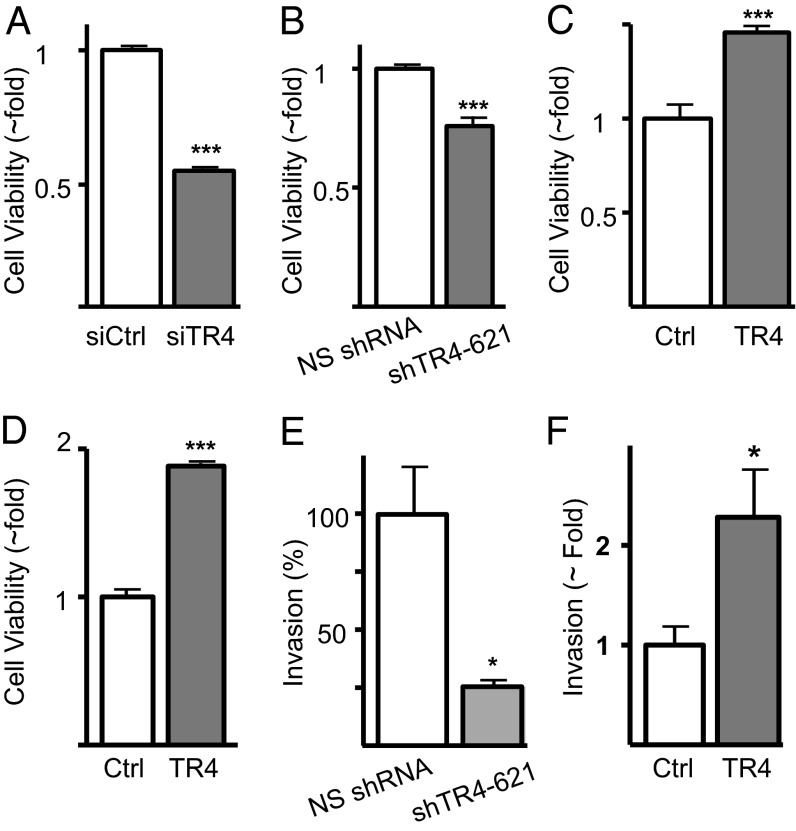
Whereas morbidity in CD is largely due to the effects of elevated ACTH and hypercortisolism, and human corticotroph adenomas are typically <1 cm and often <5 mm, corticotroph tumors are known to invade locally. To investigate the role of TR4 expression in corticotroph tumor invasion, we initially compared proliferation rates in murine corticotroph tumor cells after transient and stable TR4 knockdown. Transient and lentiviral-mediated stable TR4 knockdown resulted in ∼50% and ∼25% decreased corticotroph tumor proliferation, respectively, compared with siCtrl and nonsilencing shRNA transfectants (NS shRNA) (Fig. 3 A and B). Furthermore, transient and stable TR4 overexpression led to 1.5-fold and 1.7-fold increases in corticotroph tumor proliferation rates, respectively, compared with control transfectants (Fig. 3 C and D). Importantly, we also found that murine corticotroph tumor cell invasion rates correlated with TR4 expression, with a 75% reduction and threefold increase in invasion rates observed after TR4 knockdown and overexpression, respectively (Fig. 3 E and F).
Fig. 3.
Transient or stable TR4 knockdown inhibits, whereas TR4 overexpression increases corticotroph tumor cell proliferation and invasion. Relative proliferation rates in murine corticotroph tumor AtT20 cells following either (A) transient transfection of a TR4 targeting siRNA (siTR4) or control siRNA (siCtrl) or (B) stable infection with a TR4 shRNA (siTR4-621) encoded lentivirus or nonsilencing shRNA (NS shRNA) or (C) relative proliferation rates after either (C) transient or (D) stable TR4 overexpression in comparison with control vector (Ctrl) in murine corticotroph tumor AtT20 cells. (E) Murine corticotroph tumor invasion rates in corticotroph tumor cells after stable TR4 (E) knockdown or (F) overexpression in comparison with control transfectants. *P < 0.05, ***P < 0.001.
TR4 Knockdown Inhibits Corticotroph Tumor ACTH Secretion and Growth in Vivo.
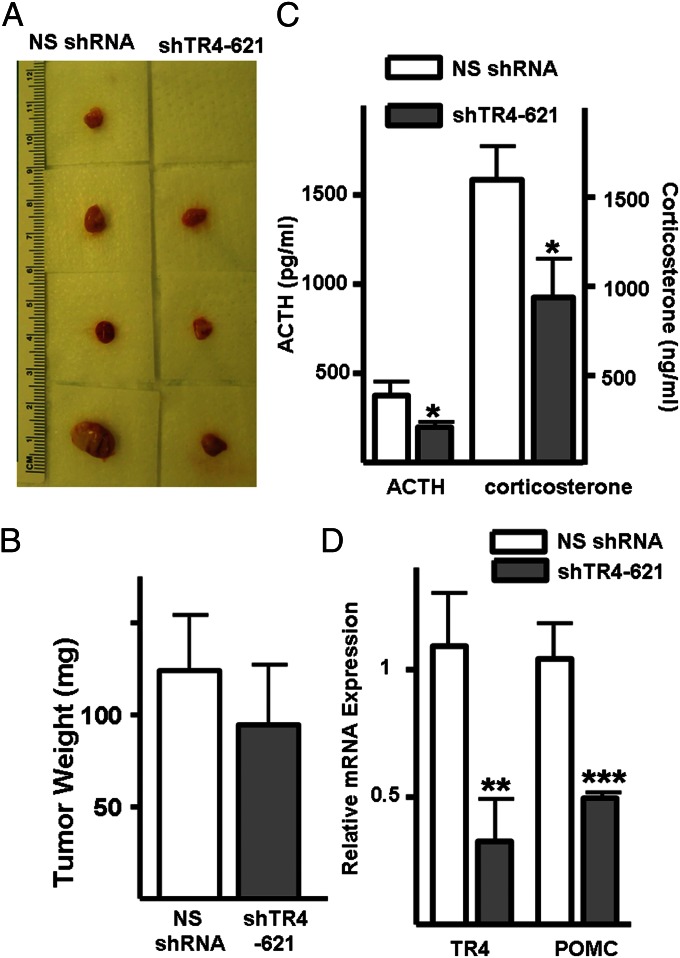
To examine the effects of TR4 expression on pituitary corticotroph tumor growth in vivo, AtT20 cells stably transfected with TR4 shRNA (shTR4-621) or nonsilencing shRNA (NS shRNA) were inoculated s.c. into 6-wk-old male nude mice. Mice inoculated with AtT20 cells with reduced TR4 expression (shTR4-621) developed tumors later than mice inoculated with control cells (Fig. 4A). At 3 wk postinoculation, tumors were detected in both groups and a similar rate of tumor growth was observed. Whereas lower tumor weights were observed in the mice inoculated with AtT20 cells with reduced TR4 expression (124.0 ± 29.3 vs. 94.5 ± 32.7 mg, n = 12, P > 0.05) this did not attain statistical significance (Fig. 4B). However, circulating ACTH and corticosterone levels were significantly reduced by ∼50% and 40%, respectively (ACTH: 376.4 ± 77.4 vs. 197.9 ± 30.4 pg/mL, P < 0.05 and corticosterone: 1585.9 ± 188.4 vs. 925.7 ± 217.5 pg/mL, P < 0.05) in mice harboring the TR4 shRNA-infected corticotroph tumor cells (shTR4-621) compared with control mice (Fig. 4C). Additionally, lower POMC and TR4 expression was detected in the tumors formed from TR4-knockdown cells compared with control tumors (Fig. 4D).
Fig. 4.
TR4 knockdown inhibits in vivo murine corticotroph tumor growth. Athymic nude mice were inoculated s.c. with either TR4 stable knockdown (shTR4-621) or nonsilencing shRNA-infected (NS shRNA) murine corticotroph tumor AtT20 cells (4 × 105 cells per mouse with 0.1 mL Matrigel). (A) Tumor depiction, (B) tumor weights, (C) circulating ACTH, and corticosterone levels derived from mice harboring stable TR4 knockdown cells compared with controls. (D) Real-time PCR confirmed reduced TR4 and POMC mRNA in stable TR4 knockdown tumor tissues compared with controls. Values are mean ± SD; *P < 0.05, **P < 0.01.
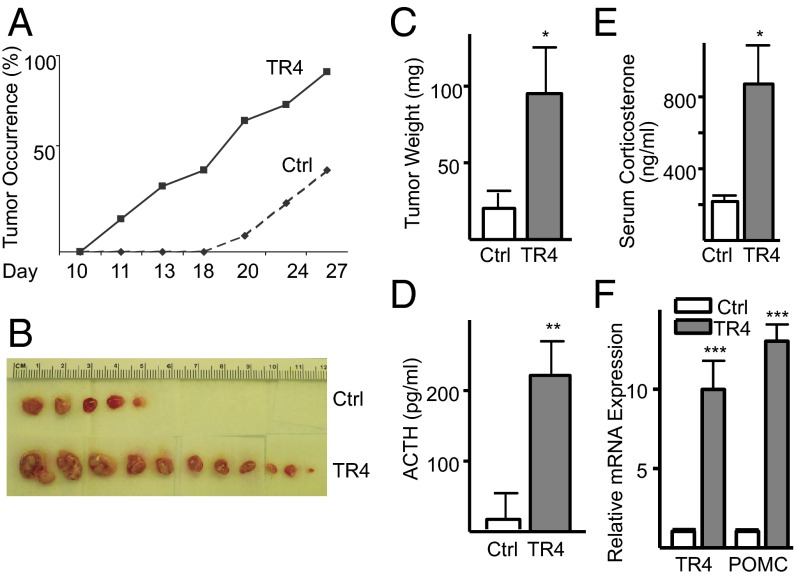
TR4 Overexpression Increases Corticotroph Tumor Growth and ACTH Secretion in Vivo.
To further explore the role of TR4 in tumor growth in vivo, murine corticotroph tumor cells stably overexpressing V5-tagged TR4 or control vector were s.c. inoculated into 6-wk-old male nude mice. Overexpression of TR4 induced a pronounced increase in the rate of tumor growth, with tumors detected 11 d after inoculation compared with 20 d for control cells (Fig. 5A). In addition, TR4 overexpression increased the penetrance of corticotroph tumors, with 11 of 12 mice in the TR4-overexpressing group harboring large tumors, whereas only 5 of 12 mice in the control group developed tumors after 27 d (Fig. 5B). Furthermore, mice harboring TR4-transfected corticotroph tumor cells exhibited larger tumor weights compared with control (99.4 ± 31.5 vs. 21.2 ± 10.5 mg, P < 0.05) (Fig. 5C). Consistent with the development of larger tumors at a higher frequency in mice injected with TR4 overexpressing cells, plasma ACTH and serum corticosterone levels were ∼5- and ∼4-fold higher, respectively, in mice harboring TR4-overexpressing corticotroph tumor cells compared with controls (ACTH: 59.7 ± 40.5 vs. 252.7 ± 43.8 pg/mL, P < 0.01 and corticosterone: 217.5 ± 32.9 vs. 870.9 ± 215.0 pg/mL, P < 0.05) (Fig. 5 D and E). These significant increases in secreted hormones were supported by marked increases in TR4 (∼10-fold) and POMC (∼12-fold) expression (Fig. 5F).
Fig. 5.
Athymic nude mice were inoculated subcutaneously with corticotroph tumor AtT20 cells (1 × 105 cells per mouse with 0.1 mL matrigel) either stably overexpressing TR4 plasmid mTR4V5H6 (TR4) or the pcDNAV5HisA control vector (Ctrl). (A) Tumor growth rates, (B) depiction, (C) tumor weights, and circulating (D) ACTH and (E) corticosterone levels derived from mice harboring stable TR4 overexpressing cells compared to controls. (F) Real-time PCR confirmed increased TR4 and POMC mRNA in stable TR4 transfectant tumor tissues compared to controls. Values are mean ± SD, *P < 0.05, **P < 0.01.
Phosphorylation-Mediated Regulation of TR4 on POMC Expression.
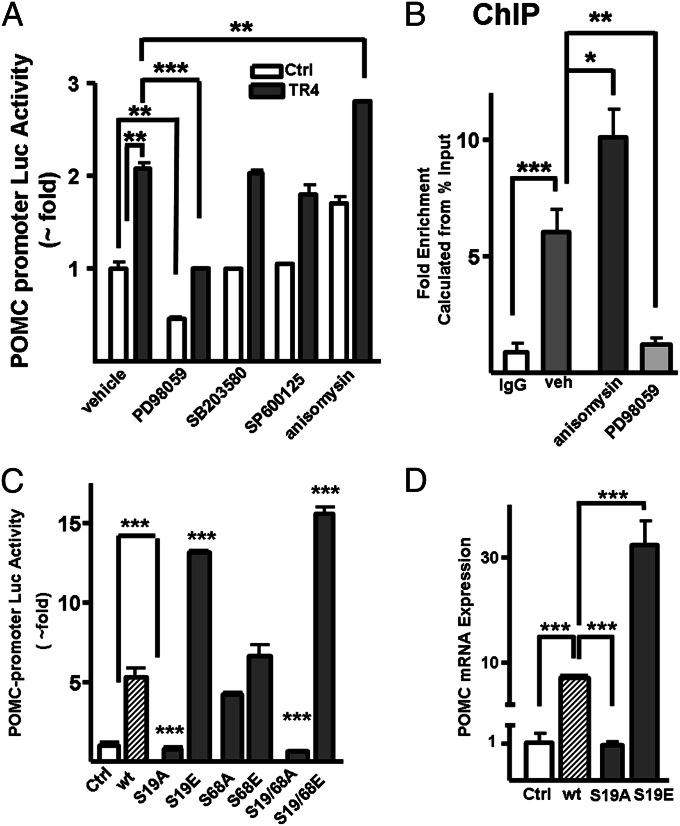
The transcriptional activity of TR4 has been reported to be regulated by MAPK-mediated phosphorylation (17). To examine the potential role of MAPK signaling in TR4-mediated actions on POMC transcription, we cotransfected TR4 and a POMC-luciferase reporter into corticotroph tumor AtT20 cells. TR4 overexpression alone led to a 2.1-fold increase in POMC-promoter activity (Fig. 6A). Addition of the MAPK activator, anisomycin caused a 1.9-fold increase in baseline POMC-promoter activity and a further 80% increase in POMC-luciferase in the TR4 transfected cells (Fig. 6A). Treatment of the control vector or TR4-transfected AtT20 cells with the protein 38 mitogen activated protein kinase (p38) and JNK MAPK inhibitors SB203580 (a specific inhibitor of p38α and p38β) and SP600125 (a Jun N-terminal kinase inhibitor), respectively, had no effect on constitutive or TR4-mediated POMC-luciferase activity (Fig. 6A). In contrast, treatment with the ERK inhibitor Parke–Davis (PD)98059 resulted in a >50% decrease in both the constitutive and TR4-induced POMC-promoter activity, implicating ERK-mediated TR4 phosphorylation as a regulatory step in POMC expression (Fig. 6A). To further explore this possibility, we examined whether ERK activation would alter TR4 binding to the POMC promoter. Immunoprecipitation with a TR4 antibody achieved a sixfold enrichment of the proximal POMC promoter DR1 element in vehicle-treated cells (Fig. 6B). Treatment with the MAPK activator anisomycin further increased TR4 binding to the POMC promoter 1.5-fold (Fig. 6B). Furthermore, treatment with the ERK1/2 inhibitor PD98059 decreased TR4 binding to the POMC promoter 5- and 9-fold, respectively, compared with vehicle- and anisomycin treatment alone (Fig. 6B) consistent with MAPK-mediated TR4-phosphorylation regulating TR4-POMC promoter binding.
Fig. 6.
TR4-mediated POMC regulation is altered by ERK-mediated phosphorylation. (A) AtT20 cells were cotransfected with a POMC promoter-luciferase reporter plasmid and either the TR4 expression plasmid mTR4V5 or a control vector, after which they were treated with vehicle (DMSO), a MAPK activator (anisomycin), an ERK inhibitor (PD98059), a p38 inhibitor (SB203580), or a JNK inhibitor (SP600125) for 18 h and harvested for luciferase assay. (B) ChIP assay using an antibody against TR4 or control mouse IgG (Ctrl) to precipitate the DNA transcriptome in AtT20 cells transiently transfected with TR4 or a control vector and then pretreated with vehicle (DMSO), 25 μM anisomysin or 10 μM PD98059 for 2 h. Q-PCR using primers specific to the murine POMC promoter DR1 site (−854 to −637) was performed in triplicate (C) POMC-promoter reporter luciferase activities in murine corticotroph tumor AtT20 cells cotransfected with a POMC promoter-luciferase reporter plasmid and either wild-type TR4 (WT) or encoding constitutively phosphorylated single (S19E) or double (S19/68E) or unphosphorylated single (S19A) or double (S19A/68A) mutant TR4 plasmids or control vector (Ctrl). (D) POMC mRNA expression in murine corticotroph tumor AtT20 cells transfected with either WT TR4, constitutively phosphorylated single (S19E), or unphosphorylated single (S19A) TR4 mutants. Values are mean ± SD; **P < 0.01, ***P < 0.001.
We next investigated the specific role(s) of the identified MAPK-mediated TR4 phosphorylation sites using mutations that mimic [serine to glutamic acid substitution at position 19 (S19E), S68E, and S19/68E] or prevent [serine to alanine substitution at position 19 (S19A), S68A, and S19/68A] TR4 phosphorylation. Notably, whereas the S19A TR4 mutant failed to induce POMC-promoter activity, the constitutively phosphorylated S19E TR4 mutant significantly increased POMC-promoter activity compared with wild-type TR4 (twofold), identifying S19 as a key regulatory site in TR4-mediated actions on POMC-promoter activity (Fig. 6C). In contrast, manipulation of the TR4 S68 site (S68A and S68E) did not significantly alter POMC-promoter activity compared with wild-type TR4 (Fig. 6C). Interestingly, the dual S19E/S68E TR4 mutant led to a further increase in POMC luciferase reporter activity compared with the S19E mutant alone. Consistent with the luciferase reporter assay, a dramatic decrease and a fourfold increase in POMC mRNA levels were found in cells transfected with the S19A and S19E TR4 mutant proteins, respectively (Fig. 6D). Together these studies implicate the MAPK-mediated phosphorylation of S19 in TR4 as an important regulatory mechanism in TR4-mediated POMC regulation.
Discussion
Whereas initial remission rates approach 90% for surgical tumor removal, long-term recurrence is seen in up to 25% of cases (2, 18). This necessitates further pituitary surgeries or radiation therapy but these ultimately cause hypopituitarism and incur great expense (19). Bilateral adrenalectomy can correct the hypercortisolemia but necessitates life-long glucocorticoid and mineralacorticoid replacement and can be complicated by rapid pituitary tumor growth (Nelson syndrome). At present, medical therapy is largely used either before surgery to assist biochemical control of hypercortisolism in patients with very elevated blood pressure and/or glucose levels or in the occasional patient who is unfit for or declines pituitary surgery. The glucocorticoid receptor antagonist, mefipristone has recently been approved to treat CD but response monitoring is challenging. Next generation somatostatin analogs have demonstrated long-term responses in ∼30% of treated patients but hyperglycemia may limit use in some patients (20, 21). Additionally, dopamine agonists such as cabergoline have shown efficacy either alone or in combination with ketoconazole in ∼40% of patients and other agents such as retinoic acid are in early stage development (22, 23).
Therefore, despite recent advances in medical therapy for CD, additional therapeutic approaches are needed (20–22, 24). Our finding of increased TR4 expression in corticotroph tumor cells, combined with its altered subcellular location, suggested that TR4 may be a unique therapeutic target for CD. In support of this notion, we demonstrated that TR4 regulated POMC expression and ACTH secretion in vitro, and showed that changes in TR4 expression levels induced corresponding changes in plasma ACTH and serum corticosterone levels in vivo. Furthermore, our studies demonstrated that TR4 expression positively impacts corticotroph tumor growth and invasion in vitro as well as corticotroph tumor growth in vivo.
These findings agree with previous studies that implicated TR4 in regulating cell proliferation and apoptosis, and mice lacking TR4 exhibited significant growth retardation and reduced embryonic and early perinatal survival (25–28).
Nuclear receptor phosphorylation has been shown to affect receptor stability, nuclear localization, transcriptional activity, and interaction with coregulators (27, 29). Previous studies established that TR4 activity can be modulated by the phosphorylation of two serine residues, S19 and S68. Our studies with mutant proteins (S19A and S19E) and pharmacological manipulation of MAPK activity clearly demonstrated regulation of TR4-mediated POMC expression by S19 phosphorylation, significantly extending earlier studies. Additionally, ChIP studies indicating increased binding of phosphorylated TR4 to the POMC promoter provides a mechanistic basis for the in vivo findings. Although we observed increased nuclear TR4 expression in human corticotroph tumors, no differences were detected in the nuclear localization of TR4 in corticotroph tumor AtT20 cells following MAPK agonist or antagonist treatment. This suggests that phosphorylation of TR4 is not required for its nuclear localization and implicates other mechanisms for the increased nuclear TR4 expression such as translocation by a yet unidentified ligand.
Prior studies have shown that phosphorylated or nonphosphorylated TR4 can act as a transcriptional repressor or enhancer and may vary depending on the tissue involved or the target in question (17, 29). For example, in a prior study using a luciferase reporter containing the DR1 response element derived from mouse hepatic control region 1 of the apoE gene, anisomysin suppressed, whereas PD98059 enhanced, TR4 actions on apoE (10). Likewise, the constitutively dephosphorylated TR4 mutants S19A and S68A resulted in higher apoE1 activation compared with wild-type TR4, whereas the constitutively phosphorylated TR4 mutants S19E and S68E resulted in decreased ApoE1 activity. These studies emphasize that TR4-directed transcriptional regulation may be controlled by phosphorylation in diverse ways depending on the promoter in question.
In summary, our findings demonstrate a previously unappreciated role for the orphan nuclear receptor TR4 in the regulation of corticotroph tumor POMC expression and ACTH secretion. Our results show that successful targeting of TR4 in corticotroph tumors could simultaneously result in reduced ACTH secretion and corticotroph tumor growth and may provide a unique therapeutic approach in Cushing disease treatment.
Materials and Methods
Plasmids, Transfection, and Reporter Assays.
Cells were transfected with a plasmid containing the -480bp rat POMC promoter luciferase reporter (gifted by Jacques Drouin, Institute de Recherches Cliniques de Montreal, Montreal, Canada), mouse V5-tagged TR4 (mTR4V5H6) or pcDNA3.1/V5-HisA vector from Invitrogen, TR4 siRNA or control siRNA (both from Santa Cruz) using lipofectamine (Invitrogen) for 18 h and treated with vehicle, CRH or Dex for an additional 6 h. Plasmid Renilla luciferase-simian virus 40 vector (Promega) was cotransfected in all experiments to correct for transfection efficiency variation. Luciferase activity was measured by Dual-Luciferase Assay kit (Promega) according to the manufacturer’s instructions. Data are presented as normalized fold changes compared with controls.
Immunocytochemistry.
Sections (10 μm) from paraffin-embedded tissues were immunostained using antibodies to human ACTH (1:1,000) (DAKO), TR4 (1:500) (Calbiochem) using the avidin-biotin-peroxidase method and avidin-biotin-FITC and -TRITC for double immunostaining. Negative controls used preabsorbed or nonimmune serum.
Chromatin Immunoprecipitation Assay.
ChIP assay to examine interactions between TR4 and POMC promoter DNA were performed in 3 × 106 AtT20 cells using Pierce agarose ChIP kit and anti-TR4 antibody or mouse IgG control. Coimmunoprecipitated DNA was analyzed by real-time PCR using paired POMC-promoter–specific primers (forward 5′-GTAGATTAGGCAGGCACCCCGACTG-3′ and reverse 5′-GAATGGTCTGGGTGGGGATTGTCTG-3′).
Stable Cell Transfections.
An AtT20 TR4 stable cell line was generated by transfecting AtT20 murine corticotroph tumor cell (ATCC) with a V5-tagged TR4 expressing plasmid (mTR4V5H6). Stable colonies were selected in 600 ng/mL geneticin. Empty vector pcDNA3.1/V5-HisA transfected control cells (AtT20-Ctrl) were simultaneously established. Stable TR4 knockdown cells were generated by infection of aliquots of AtT20 cells with GFP-IRES-puromycin-zeocin lentivirus (Thermo) encoding three TR4-targeting shRNAs (sh617, sh619, and sh621) and cells selected in 2.5 μg/mL puromycin. RT-PCR demonstrated ∼20% of TR4 expression with shTR4-621 compared with NS shRNA control. Control cells (NS shRNA) were generated using the same lentivirus encoding a nonsilencing shRNA.
Real-Time PCR.
Total RNA was extracted with Rneasy kit (Qiagen) and quantified by absorbance measurement at 260 and 280 nm. Total RNA was reverse transcribed into first-strand cDNA using iScript cDNA synthesis kit (Invitrogen) and quantitative RT-PCR was carried out using My iQ Real-time PCR Detection System (Bio-Rad Laboratories). Primer sequences (Invitrogen) were as follows: mouse actin forward 5′-GGCTGTATTCCCCTCCATCG-3′ and reverse 5′-CCAGTTGGTAACAATGCCATGT-3′; mouse TR4 forward 5′-GACTCTGCGGTAGCCTCAC-3′ and reverse 5′-AGGATGAACTGCTGTTTAGAGGA-3′; mouse POMC forward 5′-CATAGATGTGTGGAGCTGGTG-3′ and reverse 5′-CATCTCCGTTGCCAGGAAACAC-3′; human actin forward 5′-CACCATTGGCAATGAGCGGTTC-3′ and reverse 5′-AGGTCTTTGCGGATGTCCACGT-3′; human TR4 forward 5′-TCCCCACGCATCCAGATAATC-3′ and reverse 5′-GATGTGAAAACACTCAATGGGC-3′; and human POMC forward 5′-GCCAGTGTCAGGACCTCAC-3′ and reverse 5′-GGGAACATGGGAGTCTCGG-3′.
Western Blotting.
After treatments, cells were washed in cold PBS, protein extracted in 100 μL of radioimmuno precipitation assay (RIPA) buffer (Sigma-Aldrich) containing complete protease inhibitor mixture tablets (Sigma-Aldrich) and phosphatase inhibitor phosphor-STOP (Roche Molecular Biochemicals). Tissues were ground in liquid nitrogen before protein extraction in RIPA buffer. Protein concentrations were determined by DC protein assay reagent (Bio-Rad) and extracts resolved by SDS/PAGE on 8% gels. Membranes were blocked for 2 h at room temperature in TBS-Tween-20 containing 10% nonfat dried milk, washed, and then incubated with indicated primary antibodies. After washing, membranes were incubated with HRP-conjugated secondary antibodies and proteins visualized using SuperSignal Chemiluminescence Assay kit (Pierce).
Cell Proliferation and Invasion Assays.
AtT20 cell proliferation was performed in 96-well plates using the Cell-Titer-Glo luminescence cell viability assay (Promega). Invasion assays were performed using CytoSelect 24-well cell invasion assay kit. Briefly, wild-type, TR4 overexpressing or TR4 knockdown cells with paired vector controls were seeded into the upper chambers in serum-free media. The lower well compartments were filled with DMEM containing 10% FBS.
Hormone Assays.
RIAs for mouse ACTH and corticosterone were performed in duplicate using reagents purchased from CellBioTech and DRG International.
Primary Human Corticotroph Cultures.
Fresh surgically resected human ACTH-producing tumor tissues were washed, chopped, and digested with DMEM containing 0.5% BSA, 0.35% collagenase, and 0.1% hyaluronidase at 37 °C for 20 min. After centrifugation, cell pellets were resuspended in culture medium for 24 h and then infected with lentivirus encoding TR4 shRNA or nonsilencing shRNA. Forty-eight hours later, cells were incubated in serum-depleted DMEM with 0.3% BSA for 24 h and medium collected for ACTH RIA.
Animals.
Six- to eight-week-old male Nu/J (JAX) mice were injected s.c. with Ctrl or TR4 overexpressing stable transfectant cells (1 × 105 cells per mouse) or NS shRNA or shTR4-621 TR4 knockdown stable transfectant cells (4 × 105 cells per mouse). Mice were euthanized using CO2 inhalation, cardiac blood was collected, and tumors were excised and weighed. The use of mice was approved by the University of California Los Angeles (UCLA) Animal Research Committee and complied with all relevant federal guidelines and institutional policies.
Statistics.
Results are expressed as mean ± SEM. Differences were assessed by one-way ANOVA following Scheffé F test. P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institutes of Health (DK057978, HL105278, DK090962, HL088093, ES010337, and CA014195) as well as the Helmsley Charitable Trust, Samuel Waxman Cancer Research Foundation, UCLA Jonsson Comprehensive Cancer Center, and Ipsen/Biomeasure. J.W.J. is supported by grants from the Human Frontier Science Program (CDA00013/2011-C) and The Netherlands Organization for Scientific Research (VIDI Grant 016.126.338). R.M.E. is an Investigator of the Howard Hughes Medical Institute and March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306182110/-/DCSupplemental.
References
- 1.Ezzat S, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 2.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605–1617. doi: 10.1016/S0140-6736(06)68699-6. [DOI] [PubMed] [Google Scholar]
- 3.Grossman A. What is the cause of Cushing’s disease? Clin Endocrinol (Oxf) 1992;36(5):451–452. doi: 10.1111/j.1365-2265.1992.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 4.Krieger DT. Physiopathology of Cushing’s disease. Endocr Rev. 1983;4(1):22–43. doi: 10.1210/edrv-4-1-22. [DOI] [PubMed] [Google Scholar]
- 5.Tritos NA, Biller BM, Swearingen B. Medscape Management of Cushing disease. Nat Rev Endocrinol. 2011;7(5):279–289. doi: 10.1038/nrendo.2011.12. [DOI] [PubMed] [Google Scholar]
- 6.Liubinas SV, Porto LD, Kaye AH. Management of recurrent Cushing’s disease. J Clin Neurosci. 2011;18(1):7–12. doi: 10.1016/j.jocn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Praw SS, Heaney AP. Medical treatment of Cushing’s disease: Overview and recent findings. Int J Gen Med. 2009;2:209–217. doi: 10.2147/ijgm.s7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedroncelli AM. Medical treatment of Cushing’s disease: somatostatin analogues and pasireotide. Neuroendocrinology. 2010;92(Suppl 1):120–124. doi: 10.1159/000314352. [DOI] [PubMed] [Google Scholar]
- 9.Pivonello R, et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab. 2009;94(1):223–230. doi: 10.1210/jc.2008-1533. [DOI] [PubMed] [Google Scholar]
- 10.Pecori Giraldi F, et al. Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab. 2012;97(10):3577–3583. doi: 10.1210/jc.2012-2328. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, et al. Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc Natl Acad Sci USA. 1994;91(13):6040–6044. doi: 10.1073/pnas.91.13.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YF, Pan HJ, Burbach JP, Morkin E, Chang C. Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes. J Biol Chem. 1997;272(18):12215–12220. doi: 10.1074/jbc.272.18.12215. [DOI] [PubMed] [Google Scholar]
- 13.Young WJ, Lee YF, Smith SM, Chang C. A bidirectional regulation between the TR2/TR4 orphan receptors (TR2/TR4) and the ciliary neurotrophic factor (CNTF) signaling pathway. J Biol Chem. 1998;273(33):20877–20885. doi: 10.1074/jbc.273.33.20877. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Chinpaisal C, Wei LN. A novel nuclear receptor heterodimerization pathway mediated by orphan receptors TR2 and TR4. J Biol Chem. 1998;273(39):25209–25215. doi: 10.1074/jbc.273.39.25209. [DOI] [PubMed] [Google Scholar]
- 15.Xie S, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci USA. 2009;106(32):13353–13358. doi: 10.1073/pnas.0905724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu NC, et al. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes. 2007;56(12):2901–2909. doi: 10.2337/db07-0359. [DOI] [PubMed] [Google Scholar]
- 17.Kim E, et al. Disruption of TR4 orphan nuclear receptor reduces the expression of liver apolipoprotein E/C-I/C-II gene cluster. J Biol Chem. 2003;278(47):46919–46926. doi: 10.1074/jbc.M304088200. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe O, et al. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 2007;26(9):2295–2306. doi: 10.1038/sj.emboj.7601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Geen H, et al. Genome-wide binding of the orphan nuclear receptor TR4 suggests its general role in fundamental biological processes. BMC Genomics. 2010;11:689. doi: 10.1186/1471-2164-11-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LM, et al. Subfertility with defective folliculogenesis in female mice lacking testicular orphan nuclear receptor 4. Mol Endocrinol. 2008;22(4):858–867. doi: 10.1210/me.2007-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Wijk PA, van Neck JW, Rijnberk A, Croughs RJ, Mol JA. Proliferation of the murine corticotropic tumour cell line AtT20 is affected by hypophysiotrophic hormones, growth factors and glucocorticoids. Mol Cell Endocrinol. 1995;111(1):13–19. doi: 10.1016/0303-7207(95)03541-e. [DOI] [PubMed] [Google Scholar]
- 22.Huq MD, Gupta P, Tsai NP, Wei LN. Modulation of testicular receptor 4 activity by mitogen-activated protein kinase-mediated phosphorylation. Mol Cell Proteomics. 2006;5(11):2072–2082. doi: 10.1074/mcp.M600180-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Hassan-Smith ZK, et al. Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab. 2012;97(4):1194–1201. doi: 10.1210/jc.2011-2957. [DOI] [PubMed] [Google Scholar]
- 24.Bertagna X, Guignat L. [Recent progress in the treatment of Cushing’s disease] Ann Endocrinol (Paris) 2012;73(2):107–110. doi: 10.1016/j.ando.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Kim E, et al. TR4 orphan nuclear receptor functions as an apoptosis modulator via regulation of Bcl-2 gene expression. Biochem Biophys Res Commun. 2007;361(2):323–328. doi: 10.1016/j.bbrc.2007.06.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koritschoner NP, et al. The nuclear orphan receptor TR4 promotes proliferation of myeloid progenitor cells. Cell Growth Differ. 2001;12(11):563–572. [PubMed] [Google Scholar]
- 27.Lalevée S, Ferry C, Rochette-Egly C. Phosphorylation control of nuclear receptors. Methods Mol Biol. 2010;647:251–266. doi: 10.1007/978-1-60761-738-9_15. [DOI] [PubMed] [Google Scholar]
- 28.Collins LL, et al. Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci USA. 2004;101(42):15058–15063. doi: 10.1073/pnas.0405700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nucl Recept Signal. 2007;5:e005. doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.