Abstract
Spoligotyping has shown Mycobacterium tuberculosis strains to be composed of different lineages, and some of them are not just geographically restricted but also affect specific ethnic populations and are associated with outbreaks and drug resistance. We recently described a particular subtype within the Latin American-Mediterranean (LAM) family, called RDRio, widespread in Brazil. Moreover, recent data also indicate that RDRio is present in many countries on all continents and is associated with cavitary disease and multidrug resistance (MDR). To further explore the relationship between RDRio and MDR, we conducted a study in a tuberculosis (TB) reference center responsible for the care of MDR patients in Rio Grande do Sul, the southernmost Brazilian state. From a collection of 237 clinical isolates, RDRio alone was responsible for one-half of all MDR cases, including one large group composed of strains with identical IS6110-restriction fragment length polymorphism (RFLP) and having the LAM5 signature. We additionally had complete data records for 96 patients and could make comparisons between the presence and absence of RDRio. No difference in clinical, radiological or laboratory features was observed, but a significantly greater number of cases with MDR were described in patients infected with an RDRio strain (P = 0.0015). Altogether, RDRio was responsible for 38% of all TB cases. These data support and confirmed previous findings that RDRio is the main agent responsible for TB in Brazil and is associated with drug resistance. Considering that RDRio is a globally distributed genotype, such findings raise concern about the increase in MDR in certain human populations.
INTRODUCTION
Despite increasing efforts, tuberculosis (TB) continues to be a significant cause of morbidity and mortality around the globe, particularly in developing countries, where the increase in the number of multidrug-resistant (MDR) strains and the emergence of extensively drug-resistant (XDR) strains make the situation even more dramatic, posing a significant threat to the WHO goals of reducing the prevalence and deaths due to TB by 50% by 2015 and the elimination of TB as a public health problem by 2050 (http://www.who.int/tb/strategy/stop_tb_strategy/en/index.html). There are several factors responsible for this problem, most of them related to economic and social issues such as poverty, drug addiction, HIV-positive status, and difficult access to anti-TB drugs, among others. Although these factors are unquestionable, others directly related to the microorganism itself are also probably significant but less studied and understood.
The deciphering of the complete genome of Mycobacterium tuberculosis H37Rv in 1998 (1) led to a better understanding of its biology, facilitating the analysis and further expanding the use of molecular tools for interrogation of the genome, such as IS6110-restriction fragment length polymorphism (RFLP), spoligotyping, and mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing (2–4). Among these methods, spoligotyping proved to be a particularly useful tool. It is a standardized amplification method targeting the polymorphic Mtb complex-specific direct repeat (DR) locus and is visualized as a binary 43-digit pattern or reduced to an octal form of 15 digits (2). Compared to IS6110-RFLP and MIRU-VNTR typing, spoligotyping is unable to achieve the same level of discrimination; however, the assay is rapid, inexpensive, and robust, and the data can be easily exchanged between laboratories. Therefore, spoligotyping is often used as a first-line genotyping method and is the basis of the definition of the major genotype families of M. tuberculosis such as the Beijing, Haarlem, T, X, S, East African-Indian (EAI), Latin American-Mediterranean (LAM), and Central Asian (CAS) families, among others. Each of these patterns is included in the SpolDB4, an international database developed and maintained by the Pasteur Institute of Guadeloupe, which includes spoligotypes submitted by investigators from all over the world (5) and is available online (http://www.pasteur-guadeloupe.fr:8081/SITVITDemo). Some of the spoligotype families (also called spoligofamilies) are preferentially distributed in particular geographic regions, indicating that they are either spreading locally or better adapted to certain human populations (5–7). Importantly, some spoligofamilies have been associated with outbreaks and multidrug resistance, the Beijing (and Beijing-like) spoligofamily being the best-known example (8–11). Although predominant in far-east Asia, the Beijing spoligofamily is much less frequently found in the Americas, Europe, and Africa, where LAM, Haarlem, T, and S families predominate (5). The LAM family, a member of single nucleotide polymorphism (SNP) cluster VI (12), appears to be the most prevalent M. tuberculosis lineage globally, accounting for ∼15% of the global TB burden (5). Despite its success, little is yet known about the epidemiology, biological behavior, and clinical attributes of disease caused by strains of the LAM family. Similar to Beijing family strains, members of the LAM family have also been associated with drug resistance and outbreaks (13–16), including the recent description of a LAM strain as the leading cause of XDR-TB in South Africa (17).
An additional reason for studying the features of the spoligofamilies was the recent discovery of RDRio, a subfamily inside the larger LAM spoligofamily, which seems to be prevalent in several places in Brazil (18–23) but also in many other countries, particularly in Central and South America, Europe, and Africa (19). Analysis of TB isolates and registry data from New York, NY, between 2001 and 2005 showed that RDRio was responsible for 8% of all TB cases (24), a remarkably high rate considering the population diversity in that city. Moreover, RDRio was associated with cavitary disease in the Brazilian population (22) and a tendency (not statistically significant) to cause cavitary disease and increased transmission efficiency among U.S.-born blacks and Hispanics and persons of Latin American or Caribbean Heritage (24) as well as with drug resistance (24). To confirm and expand these findings, the present study was undertaken in Rio Grande do Sul (RS), the southernmost Brazilian state, for which no data on RDRio status and characteristics exist. In contrast to the other parts of the country, South Brazil received a high influx of Europeans (particularly Germans and Italians) in the beginning of the last century. This study was approved by the ethical committee of the School of Public Health of Rio Grande do Sul.
MATERIALS AND METHODS
Study setting.
According to the World Health Organization, Brazil is on the list of high-burden TB countries and among the 22 nations in which 80% of the world's new TB cases occur. In Brazil, 81,946 new TB cases were reported in 2010, and this was translated to incidence, prevalence, and mortality rates of, respectively, 43, 47, and 2.6 per 100,000 inhabitants (25). Isolates from this study were obtained from cases diagnosed at the TB clinic of the “Sanatório Partenon Hospital” (HSP), a regional TB reference center located in Porto Alegre City, the capital of Rio Grande do Sul State, Brazil. The center is the only institution in the state to care for MDR patients. Porto Alegre City has a population of 1.44 million inhabitants and a TB incidence of 93 per 100,000 habitants, the highest in RS, with 1,506 new cases in 2008 (Instituto Brasileiro de Geografia e Estatística [IBGE]; http://www.saude.rs.gov.br/dados/1293727576139Situa%E7%E3o_TB_RS_2.pdf). Overall, Rio Grande do Sul State has a TB incidence of 47 per 100,000 habitants.
Mycobacterial strains.
The present study comprises 237 M. tuberculosis isolates, each collected from different TB patients between 2004 and 2006. Testing of the isolates' drug susceptibility to isoniazid, rifampin, ethambutol, and streptomycin was performed at the Central Laboratory (LACEN) of Rio Grande do Sul State. MDR was defined as drug resistance to at least isoniazid and rifampin. The MDR isolates constitute the entire MDR collection available in the state at that time. The sensitive strains were a convenience sample chosen to match the MDR in terms of patient population and compliance with treatment. All strains were identified to the species level by morphological and biochemical analysis (26), and the tests for drug susceptibility were performed by the proportion method (27), using M. tuberculosis H37Rv ATCC 27294 as a susceptible control strain. Isolates with single-drug resistance were not included. The MDR isolates were additionally submitted to sequence analyses for polymorphisms in the katG and rpoB genes, and part of the results were published elsewhere (28).
Nucleic acid extraction.
Chromosomal DNA was extracted from cultures on Löwenstein-Jensen medium, using the cetyltrimethylammonium bromide (CTAB) method as previously described (29).
Spoligotyping.
Spoligotyping was performed using a commercial kit, according to the manufacturer's instructions (Isogen Biosciences B.V., Netherlands). The spoligotype patterns were compared with the updated SITVIT database, which provides information on the shared type distributions of M. tuberculosis spoligotypes worldwide.
Detection of RDRio.
A multiplex PCR was performed to differentiate RDRio from wild-type (WT) strains as previously described (19, 21, 22). Briefly, two sets of primer pairs were used to target either the IS1561 locus (positive only in WT strains and corresponding to a band size of 530 bp) or the region flanking the RDRio locus (positive only in RDRio strains and corresponding to a band size of 1,175 bp); the presence of both RDRio and WT strains in a single sample is indicated by the presence of both bands (22).
Clinical, radiological, and laboratory data.
Complete data records were available for 103 patients, but for 7 of them both RDRio and WT bands were identified in the same clinical isolate, indicating either mixed infection or laboratory contamination. These seven cases were excluded, leaving 96 patients for analysis, including 25 drug-susceptible and 71 drug-resistant cases. The MDR group was from secondary drug resistance cases, with multiple treatments. To better match this group, the drug-sensitive cases were also from patients with irregular treatment but who had not developed drug resistance. Clinical data included gender, race, presence of hemoptysis, weight loss, fever, alcoholism, and HIV status. Radiological data included the presence of lung cavitation, homogenous infiltrate, miliary pattern, pleural effusion, and mediastinal adenopathy. Bacteriological data included smear staining for acid-fast bacilli (AFB), culture, and sensitivity to anti-TB drugs.
Statistical analysis.
Continuous variables were compared by the t test and categorical variables by the X2 or Z-test (proportions), as appropriate. The comparison relied on logistic regression. Results for continuous variables were means ± standard deviations (SD). The two-sided 0.05 threshold was used for statistical significance. Analysis was performed using the statistical software XLSTAT (Addinsoft, France).
RESULTS
Among the 237 M. tuberculosis isolates, 122 (51%) were sensitive to all drugs and 115 (49%) were resistant to at least both isoniazid and rifampin, and among the latter set, 18 strains were also resistant to streptomycin and another 3 strains were resistant to ethambutol. A single strain was resistant to all four drugs, but at that time no further tests were performed to evaluate whether this strain was XDR. All isolates were spoligotyped and classified according to the spoligopatterns available in the SITVIT website. All spoligotyping patterns and genotypes (RDRio or WT) for each M. tuberculosis isolate are listed in Table 1.
Table 1.
Number of samples, MDR profile, RDRio genotype, and spoligotyping pattern for each M. tuberculosis patient strain from Porto Alegre Citya
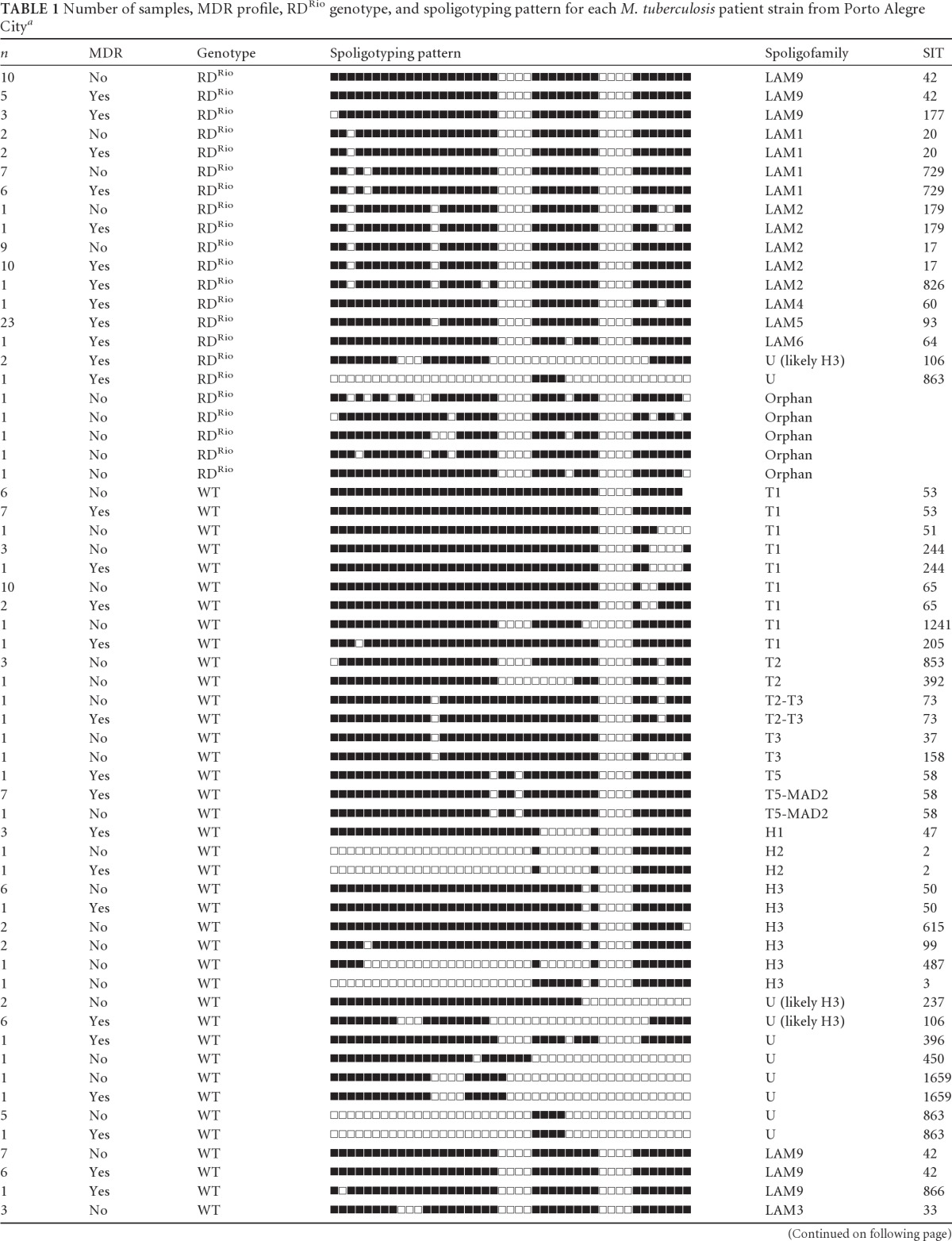
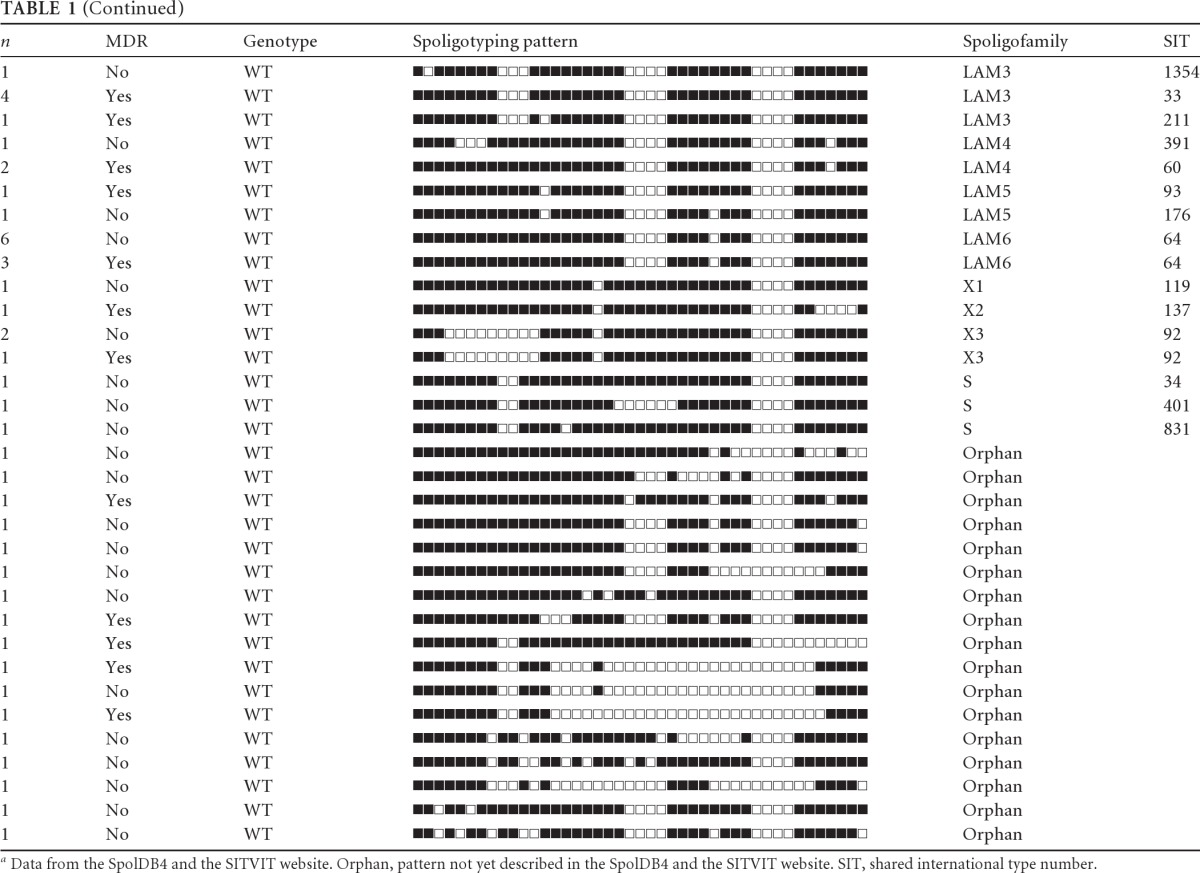
Data from the SpolDB4 and the SITVIT website. Orphan, pattern not yet described in the SpolDB4 and the SITVIT website. SIT, shared international type number.
In total, LAM strains account for 53% of the MTB collection (RDRio alone accounts for 38%), whereas T and Haarlem families account for 21% and 7.6%, respectively. The other families are represented in <5% of the strains. Among the 122 susceptible isolates, 53 were LAM (43%), 29 belonged to the T family (24%), 13 were Haarlem strains (11%), 3 were of the S family, and another 3 were of the X family (2% each). In addition, nine strains were classified as U or unknown (7%) and 14 isolates (11%) had no described spoligotype and were classified as orphans. In total, the susceptible strains bunched into 9 different clusters. RDRio was exclusively responsible for 64% (34/53) of the susceptible LAM strains, 28% (34/122) of all susceptible strains, and 48% (56/115) of the total resistance strains. This was statistically significant (P = 0.002). The 115 MDR isolates were classified as follows: 74 LAM strains (64%), 20 T family strains (17%), 5 Haarlem strains (4%), 2 S family strains (1.7%), 11 Unknown strains (9%), and 5 orphans (4%). RDRio alone was responsible for around one-half of all MDR strains (56/115), making up the largest family responsible for MDR strains in this study. In contrast, T and Haarlem families were responsible for 17% and 4%, respectively, of all MDR strains. IS6110-RFLP analysis was performed in all MDR LAM strains (data not shown). Noteworthy, a large cluster was found, composed of 22 isolates belonging to LAM5 (SIT 93), which presented three RFLP patterns: one with 20 isolates and two others belonging to a single pattern each. All were classified as RDRio. They had the same 315 AGC-ACC S-T mutation on the katG gene and the 531 TCG-TTG S-L mutation on the rpoB gene. This result identified a significant ongoing outbreak of RDRio strains.
Clinical, bacteriological, and radiological data and the presence of RDRio were analyzed for 96 patients with complete data records. The site of TB was pulmonary in 95% of the cases. A history of TB was described for 30% of the patients infected with susceptible M. tuberculosis strains and for 90% of those infected with MDR strains. In total, RDRio was found in 38% of all TB patients and in 34 of 71 MDR patients, constituting almost one half of all MDR strains (P = 0.0015) (Table 2). The other half was distributed among several spoligofamilies. The presence of RDRio was statistically associated with MDR even after logistic regression analysis. No other epidemiological, clinical, or radiological results were associated with TB caused by RDRio (Table 2).
Table 2.
Sociodemographic, clinical, radiological, and bacteriological data for pulmonary TB patients at the time of diagnosis
| Characteristic | RDRio (n = 37) | WT (n = 59) | P value |
|---|---|---|---|
| Gender (M/F) | 28/9 | 37/22 | 0.0683 |
| Race (white/not white) | 22/15 | 35/24 | 0.5804 |
| Weight loss | 4 | 10 | 0.2177 |
| Fever | 18 | 23 | 0.8736 |
| Hemoptysis | 17 | 23 | 0.8186 |
| Alcohol use | 18 | 27 | 0.7063 |
| Illicit drug use | 14 | 14 | 0.1465 |
| HIV positive | 13 | 16 | 0.5819 |
| Cavity or CXR | 17 | 25 | 0.3658 |
| Miliary infiltrate | 4 | 7 | 0.4367 |
| Pleural effusion | 4 | 2 | 0.0934 |
| Mediastinal adenopathy | 2 | 1 | 0.8185 |
| MDR | 34 | 37 | 0.0015 |
DISCUSSION
There is increasing evidence that specific M. tuberculosis strains possess unique genetic traits and virulence phenotypes. As examples, we mention strains within the W/Beijing spoligotype that produce a modified phenoglycolipid associated with an insertion in the pks15/1 gene and dampened induction of interleukin-6 (IL-6), IL-12, and tumor necrosis factor (TNF) (30, 31) and the CDC1551 strain that is associated with inducing a higher proinflammatory response (32). One of the mechanisms of Mycobacterium that could underlie the evasion from the host immune response is alteration of expression of the genes belonging to the PPE/PE_PGRS family, encoding surface proteins associated with mycobacterial virulence and host immune response (1, 33, 34). We previously speculated that in M. tuberculosis strains with the RDRio deletion, the loss of two PPE genes (PPE55 and PPE56) could minimize host immune recognition, leading to enhanced virulence and/or transmissibility (21). Indeed, both PPE55 and PPE56 have been shown to be expressed in vivo and upon entry into interferon-activated macrophages and are immunogenic in humans (35, 36). In addition, polymorphisms in PPE55 and/or PPE56 have been noted in several clinical strains of M. tuberculosis and M. tuberculosis complex (MTC) species (26, 37).
Independent of the possible mechanisms of associated virulence modification, it is clear that RDRio is presently causing TB in many parts of the world (19). The high transmissibility of RDRio strains is also suggested by studies showing an elevated secondary case rate in San Francisco, CA (38), and New York, NY (24), while a higher active-TB case rate in persons infected by RDRio strains was described in The Gambia (30). In Brazil, strains carrying RDRio have been isolated from one-third of the TB cases from two geographically distant metropolises (21, 22), a rate similar to that observed in the present study. Notably, in all of them RDRio was the main agent responsible for causing TB cases among all other spoligofamilies.
Though these data strongly suggest that RDRio may have some biological advantage, there is no proof that the clinical picture or outcome of patients infected by these strains is characteristic or even more severe than that caused by other lineages. One possible strategy used by RDRio is to induce more cavitation, as was previously demonstrated (22), which is a known risk factor for transmissibility. In the current study, however, we were unable to show an association with cavitation, and this is probably related to population selection, as the former study involved TB patients with suspected drug resistance or comorbidities. In contrast, the current study involved known MDR and drug-sensitive tuberculosis cases, where both groups included patients with multiples treatments and advanced cases, with high rates of cavitary disease, which is probably the reason for the absence of statistical difference in the presence of cavitary disease. It is interesting that preliminary data suggest that close contacts of patients infected by LAM strains (no data are available regarding the RDRio status) have more positive tuberculin skin test (TST) reactions than patients infected by other spoligofamilies (our unpublished data). As RDRio is the main component of the LAM family in Brazil, it is possible that this finding reflects a feature of the RDRio; but this needs to be further confirmed with new studies.
Our present results demonstrate that about one-half of all MDR cases were caused by RDRio strains, including a large cluster of LAM5 MDR strains as proved by IS6110-RFLP analysis. It should be noted that the number of patients infected by this cluster influenced the statistical result. To our knowledge, this is the first description of an outbreak of RDRio MDR strains. Additional data from other groups also indicate that RDRio could be associated with drug resistance. Gavín et al. described MDR strains of M. tuberculosis RDRio sublineage (LAM9 subfamily) in Spain from Equatorial Guinean patients (39), and Brown et al. showed that LAM1 (a marker for RDRio) was associated with resistance to both pyrazinamide and streptomycin in MTB cases from London, United Kingdom (33), while the study performed in New York, NY, demonstrated that RDRio was associated with resistance to isoniazid (24). Although this is a matter of speculation, it is interesting that a strain from LAM4, a subfamily that contains both WT and RDRio strains, is the major cause of XDR-TB in South Africa (17) and that both LAM9 (predominantly RDRio but also containing WT) and LAM1 (exclusively RDRio) subfamilies were major contributors to drug resistance in Russia (14, 16). Unfortunately, the status of RDRio in these strains is unknown, and the prevalence of RDRio in these places is yet to be defined. Our hypothesis is that RDRio strains are able to transmit more efficiently in certain ethnic populations such as U.S.-born blacks and Hispanics and persons who were born in Latin American and Caribbean countries as was previously demonstrated (24).
In summary, our data confirm that RDRio M. tuberculosis is also a major cause of TB in Southern Brazil. The mechanisms for such an impressive presence compared with other prevalent spoligofamilies are still undefined, and no particular clinical features have been associated with this genotype so far. Our hypothesis is that RDRio strains are able to transmit more efficiently in certain ethnic populations. In this context, the development of drug resistance in this successful lineage might bring additional obstacles to the control of tuberculosis, both in Brazil and in several other countries. Prospective cohort studies are needed to analyze the real contribution of the RDRio genotype to outbreaks, its role in the global TB burden, and the risk of significant development of MDR outbreaks caused by this lineage.
ACKNOWLEDGMENTS
We thank FAPERGS, CNPq, Hospital Santorio Partenon, CDCT/FEPPS, and the Laboratory of Molecular Biology Applied to Mycobacteria at IOC/FIOCRUZ for financial support and access to infrastructure.
Footnotes
Published ahead of print 16 January 2013
REFERENCES
- 1. Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentiles S, Hamlin N, Holroyd S, Hornsby T, Jegels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544 [DOI] [PubMed] [Google Scholar]
- 2. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suffys PN, Ivens de Araujo ME, Degrave WM. 1997. The changing face of the epidemiology of tuberculosis due to molecular strain typing—a review. Mem. Inst. Oswaldo Cruz 92:297–316 [DOI] [PubMed] [Google Scholar]
- 4. Supply P, Lesjean S, Savine E, Kremer K, van Soolingen D, Locht C. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, Allix C, Aristimuno L, Arora J, Baumanis V, Binder L, Cafrune P, Cataldi A, Cheong S, Diel R, Ellermeier C, Evans JT, Fauville-Dufaux M, Ferdinand S, Garcia de Viedma D, Garzelli C, Gazzola L, Gomes HM, Guttierez MC, Hawkey PM, van Helden PD, Kadival GV, Kreiswirth BN, Kremer K, Kubin M, Kulkarni SP, Liens B, Lillebaek T, Ho ML, Martin C, Martin C, Mokrousov I, Narvskaia O, Ngeow YF, Naumann L, Niemann S, Parwati I, Rahim Z, Rasolofo-Razanamparany V, Rasolonavalona T, Rossetti ML, Rusch-Gerdes S, Sajduda A, Samper S, Shemyakin IG, Singh UB, Somoskovi A, Skuce RA, van Soolingen D, Streicher EM, Suffys PN, Tortoli E, Tracevska T, Vincent V, Victor TC, Warren RM, Yap SF, Zaman K, Portaels F, Rastogi N, Sola C. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23 doi:10.1186/1471-2180-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filliol I, Motiwala AS, Cavatore M, Qi W, Hazbon MH, Bobadilla del Valle M, Fyfe J, Garcia-Garcia L, Rastogi N, Sola C, Zozio T, Guerrero MI, Leon CI, Crabtree J, Angiuoli S, Eisenach KD, Durmaz R, Joloba ML, Rendon A, Sifuentes-Osornio J, Ponce de Leon A, Cave MD, Fleischmann R, Whittam TS, Alland D. 2006. Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP Set. J. Bacteriol. 188:759–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gagneux S, DeRiemer K, Van T, Kato-Maeda M, Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu Y, Ma X, Graviss EA, Wang W, Jiang W, Xu B. 2011. A major subgroup of Beijing family Mycobacterium tuberculosis is associated with multidrug resistance and increased transmissibility. Epidemiol. Infect. 139:130–138 [DOI] [PubMed] [Google Scholar]
- 9. Kubica T, Agzamova R, Wright A, Aziz MA, Rakishev G, Bismilda V, Richter E, Rüsch-Gerdes S, Niemann S. 2005. The Beijing genotype is a major cause of drug-resistant tuberculosis in Kazakhstan. Int. J. Tuberc. Lung Dis. 9:646–653 [PubMed] [Google Scholar]
- 10. Niemann S, Diel R, Khechinashvili G, Gegia M, Mdivani N, Tang YW. 2010. Mycobacterium tuberculosis Beijing lineage favors the spread of multidrug-resistant tuberculosis in the Republic of Georgia. J. Clin. Microbiol. 48:3544–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purwar S, Chaudhari S, Katoch VM, Sampath A, Sharma P, Upadhyay P, Chauhan DS. 2011. Determination of drug susceptibility patterns and genotypes of Mycobacterium tuberculosis isolates from Kanpur district, North India. Infect. Genet. Evol. 11:469–475 [DOI] [PubMed] [Google Scholar]
- 12. Gutacker MM, Mathema B, Soini H, Shashkina E, Kreiswirth BN, Graviss EA, Musser JM. 2006. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J. Infect. Dis. 193:121–128 [DOI] [PubMed] [Google Scholar]
- 13. Candia N, Lopez B, Zozio T, Carrivale M, Diaz C, Russomando G, de Romero NJ, Jara JC, Barrera L, Rastogi N, Ritacco V. 2007. First insight into Mycobacterium tuberculosis genetic diversity in Paraguay. BMC Microbiol. 7:75 doi:10.1186/1471-2180-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ignatova A, Dubiley S, Stepanshina V, Shemyakin I. 2006. Predominance of multidrug-resistant LAM and Beijing family strains among Mycobacterium tuberculosis isolates recovered from prison inmates in Tula Region, Russia. J. Med. Microbiol. 55:1413–1418 [DOI] [PubMed] [Google Scholar]
- 15. Jagielski T, Augustynowicz-Kopec E, Zozio T, Rastogi N, Zwolska Z. 2010. Spoligotype-based comparative population structure analysis of multidrug-resistant and isoniazid-monoresistant Mycobacterium tuberculosis complex clinical isolates in Poland. J. Clin. Microbiol. 48:3899–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shemyakin IG, Stepanshina VN, Ivanov IY, Lipin MY, Anisimova VA, Onasenko AG, Korobova OV, Shinnick TM. 2004. Characterization of drug-resistant isolates of Mycobacterium tuberculosis derived from Russian inmates. Int. J. Tuberc. Lung Dis. 8:1194–1203 [PubMed] [Google Scholar]
- 17. Pillay M, Sturm AW. 2007. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin. Infect. Dis. 45:1409–1414 [DOI] [PubMed] [Google Scholar]
- 18. Cardoso Oelemann M, Gomes HM, Willery E, Possuelo L, Batista Lima KV, Allix-Béguec C, Locht C, Goguet de la Salmonière YO, Gutierrez MC, Suffys PN, Supply P. 2011. The forest behind the tree: phylogenetic exploration of a dominant Mycobacterium tuberculosis strain lineage from a high tuberculosis burden country. PLoS One 6:e18256 doi:10.1371/journal.pone.0018256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibson AL, Huard RC, Gey van Pittius NC, Lazzarini LCO, Driscoll J, Kurepina N, Zozio T, Sola C, Spindola SM, Kritski AL, Fitzgerald D, Kremer K, Mardassi H, Chitale P, Brinkworth J, de Viedma DG, Gicquel B, Pape JW, van Soolingen D, Kreiswirth BN, Warren RM, van Helden PD, Rastogi N, Suffys PN, Lapa e Silva J, Ho JL. 2008. Application of sensitive and specific molecular methods to uncover the global dissemination of the major RDRio sublineage of the Latin American-Mediterranean Mycobacterium tuberculosis spoligotype family. J. Clin. Microbiol. 46:1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomes HM, Elias AR, Oelemann MA, Pereira MA, Montes FF, Marsico AG, Kritski AL, Filho LD, Caldas PC, Possuelo LG, Cafrune P, Rossetti ML, Lucena N, Saad MH, Cavalcanti HR, Leite CQ, Brito RC, Lopes ML, Lima K, Souza M, Trindade RD, Zozio T, Sola C, Rastogi N, Suffys PN. 2012. Spoligotypes of Mycobacterium tuberculosis complex isolates from patients residents of 11 states of Brazil. Infect. Genet. Evol. 12:649–656 [DOI] [PubMed] [Google Scholar]
- 21. Lazzarini LC, Huard RC, Boechat NL, Gomes HM, Oelemann MC, Kurepina N, Shashkina E, Mello FC, Gibson AL, Virginio MJ, Marsico AG, Butler WR, Kreiswirth BN, Suffys PN, Lapa e Silva JR, Ho JL. 2007. Discovery of a novel Mycobacterium tuberculosis lineage that is a major cause of tuberculosis in Rio de Janeiro, Brazil. J. Clin. Microbiol. 45:3891–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lazzarini LC, Spindola SM, Bang H, Gibson AL, Weisenberg S, da Silva Carvalho W, Augusto CJ, Huard RC, Kritski AL, Ho JL. 2008. RDRio Mycobacterium tuberculosis infection is associated with a higher frequency of cavitary pulmonary disease. J. Clin. Microbiol. 46:2175–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Von Groll A, Martin A, Felix C, Prata PFS, Honscha G, Portaels F, Vandame P, da Silva PEA, Palomino JC. 2010. Fitness study of the RDRio lineage and Latin American-Mediterranean family of Mycobacterium tuberculosis in the city of Rio Grande, Brazil. FEMS Immunol. Med. Microbiol. 58:119–127 [DOI] [PubMed] [Google Scholar]
- 24. Weisenberg SA, Gibson AL, Huard RC, Kurepina N, Bang H, Lazzarini LC, Chiu Y, Li J, Ahuja S, Driscoll J, Kreiswirth BN, Ho JL. 2012. Distinct clinical and epidemiological features of tuberculosis in New York City caused by the RD(Rio) Mycobacterium tuberculosis sublineage. Infect. Genet. Evol. 12:664–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization 2011. WHO global report 2011. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf [Google Scholar]
- 26. Nolte FS, Metchock B. 1995. Mycobacterium, p 403–437 In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RI. (ed), Manual of clinical microbiology, 6th ed ASM Press, Washington, DC [Google Scholar]
- 27. Canetti G, Rist N, Grosset J. 1963. Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions: methodology, resistance criteria, results, and interpretation. Rev. Tuberc. Pneumol. 27:217–272 [PubMed] [Google Scholar]
- 28. Perizzolo PF, Dalla Costa ER, Ribeiro AW, Spies FS, Ribeiro MO, Dias CF, Unis G, Almeida da Silva P, Gomes HM, Suffys PN, Rossetti ML. 2012. Characteristics of multidrug-resistant Mycobacterium tuberculosis in southern Brazil. Tuberculosis 92:56–59 [DOI] [PubMed] [Google Scholar]
- 29. van Soolingen D, de Haas PE, Hermans PW, van Embden JD. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235:196–205 [DOI] [PubMed] [Google Scholar]
- 30. de Jong BC, Hill PC, Aiken H, Awine T, Antonio M, Adetifa IM, Jackson-Sillah DJ, Fox A, DeRiemer K, Gagneux S, Borgdorff MW, McAdam KPWJ, Corrah T, Small PM, Adegbola RA. 2008. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J. Infect. Dis. 198:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., III 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response p84. Nature 431:84–87 [DOI] [PubMed] [Google Scholar]
- 32. Valway SE, Sanchez MP, Shinnick TF, Orme I, Agerton T, Hoy D, Jones JS, Westmoreland H, Onorato IM. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633–639 [DOI] [PubMed] [Google Scholar]
- 33. Brown T, Nikolayevskyy V, Velji P, Drobniewski F. 2010. Associations between Mycobacterium tuberculosis strains and phenotypes. Emerg. Infect. Dis. 16:272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramakrishnan L, Federspiel NA, Falkow S. 2000. Granuloma specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436–1439 [DOI] [PubMed] [Google Scholar]
- 35. Talaat AM, Lyons R, Howard ST, Johnston SA. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. U. S. A. 101:4602–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voskuil MI, Schnappinger D, Rutherford R, Liu Y, Schoolnik GK. 2004. Regulation of the Mycobacterium tuberculosis PE/PPE genes. Tuberculosis 84:256–262 [DOI] [PubMed] [Google Scholar]
- 37. Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100:7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedman CR, Quinn GC, Kreiswirth BN, Perlman DC, Salomon N, Schluger N, Lutfey M, Berger J, Poltoratskaia N, Riley LW. 1997. Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J. Infect. Dis. 176:478–484 [DOI] [PubMed] [Google Scholar]
- 39. Gavín P, Iglesias MJ, Jiménez MS, Herrera-León L, Rodríguez-Valín E, Rastogi N, March J, González-Palacios R, Palenque E, Ayarza R, Hurra E, Campos-Herrero I, Vitoria MA, Lezcano MA, Revillo MJ, Martín C, Samper S. 2009. Multidrug-resistant Mycobacterium tuberculosis strain from Equatorial Guinea detected in Spain. Emerg. Infect. Dis. 15:1858–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brennan MJ, Delogu G. 2002. The PE multigene family: a “molecular mantra” for mycobacteria. Trends Microbiol. 10:246–249 [DOI] [PubMed] [Google Scholar]
- 41. Niobe-Eyangoh SN, Kuaban C, Sorlin P, Thonnon J, Vincent V, Gutierrez MC. 2004. Molecular characteristics of strains of the Cameroon family, the major group of Mycobacterium tuberculosis in a country with a high prevalence of tuberculosis. J. Clin. Microbiol. 42:5029–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]


