Abstract
Syndromic panels for infectious disease have been suggested to be of value in point-of-care diagnostics for developing countries and for biodefense. To test the performance of isothermal recombinase polymerase amplification (RPA) assays, we developed a panel of 10 RPAs for biothreat agents. The panel included RPAs for Francisella tularensis, Yersinia pestis, Bacillus anthracis, variola virus, and reverse transcriptase RPA (RT-RPA) assays for Rift Valley fever virus, Ebola virus, Sudan virus, and Marburg virus. Their analytical sensitivities ranged from 16 to 21 molecules detected (probit analysis) for the majority of RPA and RT-RPA assays. A magnetic bead-based total nucleic acid extraction method was combined with the RPAs and tested using inactivated whole organisms spiked into plasma. The RPA showed comparable sensitivities to real-time RCR assays in these extracts. The run times of the assays at 42°C ranged from 6 to 10 min, and they showed no cross-detection of any of the target genomes of the panel nor of the human genome. The RPAs therefore seem suitable for the implementation of syndromic panels onto microfluidic platforms.
INTRODUCTION
Syndromic panels for infectious and emerging infectious diseases have been suggested to be of value in point-of-care (POC) diagnostics for developing countries and for biodefense (1). Since the introduction of molecular diagnostics and in particular real-time PCR, ample proof of its sensitivity and specificity has been generated. Indeed, molecular diagnostics are deemed superior to bacterial culture techniques or serological diagnostics (2–4). It has even been suggested to entirely eliminate the old methods in order to streamline centralized laboratories for molecular diagnostics (5–7).
In recent years alternative isothermal amplification methods which can be categorized into (i) T7 promoter-driven amplifications (transcription-mediated amplification [TMA], nucleic acid sequence-based amplification [NASBA], and single primer isothermal amplification [SPIA]), (ii) strand displacement methods (strand displacement amplification [SDA], loop-mediated isothermal amplification [LAMP], and smart amplification [SmartAmp]), (iii) helicase-dependent amplification (HDA), (iv) recombinase polymerase amplification (RPA), and (v) rolling-circle amplification (RCA) methods (8–12) have been developed. Some were purposely designed for isothermal amplification starting from RNA (TMA, NASBA, and SPIA), whereas others initially targeted DNA (SDA, LAMP, HDA, RPA, and RCA) and were only later adapted for RNA targets. Nonspecific intercalating fluorophores or fluorescent primers have been used for real-time detection in LAMP, SDA, HDA, and RCA, and specific detection probe formats have been developed for NASBA, RCA, HDA, and RPA (13–17).
In isothermal and exponential RPA, the phage recombinase UvsX and its cofactor UvsY form a nucleoprotein complex with oligonucleotide primers to scan for homologous sequences in a DNA template. Recognition of a specific homologous sequence leads to the initiation of strand invasion of the complex and the opposing oligonucleotides are then extended by isothermal (42°C) strand displacement amplification via Sau polymerase (Staphylococcus aureus), yielding double-stranded DNA (dsDNA) amplificates very much like in PCR. Real-time amplificate detection can be performed by using TwistAmp exo probes. Exo probes carry internal fluorophore and quencher linked to thymine bases and separated by an abasic site mimic (tetrahydrofuran) localized approximately 15 nucleotides (nt) upstream from the 3′ end of the probe (45 to 55 nt). Once the probe hybridizes to its target sequence, the abasic site is recognized and cleaved by exonuclease III. The smaller downstream probe section carrying the quencher is released and fluorescence develops proportionally to the RPA-mediated amplification (15).
The second probe type for real-time fluorescent detection is the TwistAmp fpg probe, a 30-nt oligonucleotide, which carries a quencher at the 5′ end and the fluorophore at an internal position 4 to 5 nt downstream from the quencher via a C-O-C linker (or dR group). During hybridization of the probe the linker is cleaved by the DNA glycosylase FPG (Escherichia coli), thus causing separation of fluorophore and quencher and subsequently the proportional increase of fluorescence.
The purpose of the present study was to develop a panel of RPAs for a POC microfluidic platform. We describe the development of highly sensitive and specific fluorescent real-time RPA and RT-RPA assays for the detection of relevant category A bioterrorism agents, including Gram-positive and Gram-negative bacteria, and DNA and RNA viruses on a mobile ESEquant Tubescanner device. This mobile small-footprint device collects fluorescence signals over time, allowing for simultaneous real-time documentation of increasing fluorescence signals in an eight-tube strip (18, 19).
MATERIALS AND METHODS
Quantitative molecular standards.
For bacteria, quantitative pCRII backbone plasmid standards were generated for the pagA gene (Bacillus anthracis plasmid pX01) and the pla gene (Yersinia pestis) as described previously (20). A capC gene carrying plasmid (B. anthracis, plasmid pX02) was provided by the Robert-Koch-Institut (21). For DNA virus, the variola virus (VARV) HA gene was synthesized and ligated into pMA-RQ by Geneart, Regensburg, Germany, and the vaccinia virus (VACV) plasmid carrying the LE gene was provided by the Robert-Koch-Institut (22). For RNA viruses, quantitative Ebola virus (EBOV), Sudan virus (SUDV), and Marburg virus (MARV) NP-gene RNA standards were used as described previously (23, 24). A new quantitative sigma virus (SIGV) G gene based RNA standard was generated and transcribed as described previously (24).
Viral and bacterial material.
Genomic DNA of orthopoxviruses (VACV [Elstree 5], camelpox virus [CP19], monkeypox virus [MP4], and orthopoxvirus [OPV 90/3]) was provided by Hermann Meyer, Institute of Microbiology, German Armed Forces. Inactivated and gamma-irradiated bacteria and viruses were provided by the following institutes: B. anthracis spores (ATCC 14578); Yersinia pestis (03-1501) and Francisella tularensis (Ft 12) by the Center for Biosecurity 2, Robert Koch Institute, Berlin; VACV NYCBH strain (VR-1536) and Rift Valley fever virus (ZH548) by the Center for Biosecurity 1, Robert Koch Institute; MARV (Musoke strain) and EBOV (Zaire strain) by the Bernhard-Nocht Institute, Hamburg, Germany; and SIGV by the Institute of Virology, Göttingen, Germany. The organisms were cultured in the donating institutions at biosafety 3 or 4 levels.
Real-time PCR.
The quantitative standards for B. anthracis capC and pagA, Y. pestis pla, F. tularensis tul-4, VACV, RVFV, EBOV, SUDV, and MARV were tested using published real-time PCR protocols (22–24). A new real-time PCR amplicon was designed for the SIGV G gene and for the VARV HA gene. Real-time PCR assays for DNA and RNA targets were performed using the LightCycler Fast-Start DNA Master HybProbe kit and the LightCycler 480 RNA master hydrolysis probes, respectively, on a Light Cycler 2.0 (Roche, Mannheim, Germany) using the second derivative method for analysis. All real-time PCR assays showed the sensitivities reported in the original publications. The SIGV and the VARV assays showed analytical sensitivities of 10 molecules detected per reaction.
RPA conditions.
RPA was performed in a 50-μl volume using a TwistAmp Exo kit (TwistDX, Cambridge, United Kingdom) 420 nM RPA primers and 120 nM RPA probe, 14 mM magnesium acetate, and TwistAmp rehydration buffer. All reagents except for the template or sample DNA and the magnesium acetate were prepared in a mastermix, which was distributed into 0.2-ml reaction tubes, each containing a dried enzyme pellet. Magnesium acetate was pipetted into the tube lids. Subsequently, 1 μl of standard DNA or genomic DNA was added to the tubes. The lids were closed, the magnesium acetate was centrifuged into the tubes using a Minispin centrifuge, and the tubes were immediately placed into an ESEquant Tubescanner device (Qiagen Lake Constance, Stockach, Germany).
For RT-RPA 10 U of Transcriptor (Roche), 20 U of RNaseOUT, 2 mM dithiothreitol (DTT), and 22.4 mM magnesium acetate were added to the DNA-RPA mixture described above. The same amount of primers and TwistAmp fpg probe were used with the rehydration buffer and the enzyme pellets of the TwistAmp fpg kit. Subsequently, 1 μl of cDNA was added to the tubes. Fluorescence measurements (excitation, 470 nm; detection, 520 nm [FAM channel]) were performed at 42°C for 20 min. This reaction temperature was determined as optimal in terms of sensitivity from a temperature range of 39 to 42°C. The Tubescanner software permits evaluation of the increase of fluorescence above three standard deviations over the background determined in minute 1 (adaptable), i.e., threshold validation. Additionally, the slope of the curve as the mV/time can be used (slope adaptable), i.e., slope validation. For confirmation, the calculation of the second derivative of the turning point of the upward fluorescence development can be applied to individual fluorescence curves with a very low slope (18, 19).
Determination of sensitivity and specificity.
All quantitative DNA and RNA standards were tested by RPA in eight replicates, the threshold time (in minutes) was plotted against molecules detected, and a semilog regression was calculated. For exact determination, probit regression (25) was performed, and the sensitivity at 95% was calculated using Statistica software (StatSoft, Hamburg, Germany).
In order to assay the sensitivity of extraction and detection in samples containing representative whole organisms of each category in the panel, inactivated Bacillus anthracis spores (Gram positive), Y. pestis (Gram negative), VACV (DNA virus), and RVFV (RNA virus) were diluted in 10-fold steps in phosphate-buffered saline and spiked into plasma to achieve a final concentration of 101 to 104 genomic copies/ml. In addition, 2 μl of SIGV in a concentration of 105 genomic copies/ml was added to the prepared spiked plasma dilutions to monitor the performance of the extraction procedure.
Total nucleic acids from all bacterial and viral pathogens were prepared as spiked plasma samples using a single innuPREP MP basic kit A (Jena Analytik, Jena, Germany) and a magnetic bead separation rack combined with proteinase K treatment according to the manufacturer's instructions. The nucleic acids were eluted in 100 μl of nuclease-free distilled H2O, and 5 μl was subjected to PCR or RPA.
RESULTS
Amplicon design.
The design of the RPA primers differs from PCR primers, since the minimum length of 30 to 35 nt rather than the TM guides design. Since it is not clear which features of the 5′ end sequence of the primer actually supports the initiation of strand invasion, typically several primer pairs have to be tested. On average three, and at maximum eight, primer pairs were tested, and the final amplicon lengths in general ranged from 107 to 164 nt (Tables 1 and 2). Due to the high homology among the orthopoxvirus sequences. the most challenging design was that for VARV (Fig. 1). In the final design, the RPA probe overlaps the upstream primer sequences by 4 nt and covers a gap in the variola virus sequence, which is not present in the other orthopoxvirus sequences. Additionally, the downstream primer mismatched all other orthopoxvirus sequences at position 3 down from the 3′ end to specifically select for the VARV sequences according to the ARMS principle (26). An RPA for VACV was designed for the same region for use in the extraction experiments.
Table 1.
Details of RPA amplicon design
| Infectious agent | Target gene | Reference sequence, position(s) of RPA amplicon | RPA amplicon length (nt) | Sequences used in the design |
|---|---|---|---|---|
| Y. pestis | pla | AF053945, 7267–7420 | 153 | AF528089, AF053945, AL1009969, NC_003132, NC_004837, AE017046, NC_005816, CP001592, CP001596, NC_014027 |
| B. anthracis | pagA | CP001216, 144299–144424 | 125 | AF306778-83, AE011190, NC_003980, AE017336, CP001216, NC_012579, CP001599, NC_012656 |
| B. anthracis | capC | AF188935, 55735- 55885 | 150 | AF188935, AE01191, NC_003981, AE017335, NC_007323, CP001214, NC0125771, CP001597, NC_012655 |
| Variola virus | HA | X69168, 151606- 151732 | 126 | X69168, Y16780, DQ441416, DQ441418-48, DQ437500, DQ437581-91 |
| Vaccinia virus | HA | DQ121394, 165441- 165584 | 143 | M35027, U94848, AY243312, AY313847-48, AY603355, NC_006998, DQ121394 |
| Ebola virus | NP | AY142960, 1779–1943 | 164 | Jo44337, L11365, AF086833, AY142960, AF499101, AF272001, EU22440, AY354458, Y09358, AY054908, AY058895, EU051640-50 |
| Sudan virus | NP | AF173836, 1783- 1868 | 130 | AF173836, AY729654, NC_006432, EU338380 |
| Marburg virus | NP | FJ750959, 1121–1256 | 135 | Z12132, Z29337, NC_0016081, DQ217792, AY430365-66 |
| Marburg virus | NP | FJ750953, 1121–1260 | 107 | X68495, M72714, DQ447649-61, AY358025, FJ750953-59 |
| Sigma virus | G | X06171, 84–960 | 119 | X00171 |
Table 2.
Primers and probes
| Assay type and primer or exo probea | Sequence (5′–3′)b |
|---|---|
| DNA assays | |
| BA1 RPA FP | TACAGGGGATTTATCTATTCCTAGTTCTGAG |
| BA1 RPA RP | GTAGCAAATGTATATTCATCACTCTTCTTAAC |
| BA1 RPA P | GAAAATATTCCATCGGAAAACCAATATTTTCA-BTF-GCTATTTGGTCAGGAT-P |
| BA2 RPA FP | CTGGAACAATAACTCCAATACCACGGAATTCA |
| BA2 RPA RP | GGTGTTTCAAGATTCATGATTTTATATGGCCG |
| BA2 RPA P | TGGCATAACAGGATAACAATAATCAAATAAAAGT-BTF-AAACAAATACCTGTAATTAGC-P |
| YP RPA FP | CCAGTATCGCATTAATGATTTTGAGTTAAATGC |
| YP RPA RP | TCCAGCGTTAATTACGGTACCATAATAACGTGAG |
| YP RPA P | CGACTGGGTTCGGGCACATGATAATGATGAGCACTA-BTF-GAGAGATCTTACTT-P |
| VARV RPA FP | GAGAATCCACAACMGACAAGACKTCSGGAC |
| VARV RPA RP | TTGGCGGTTGATTTAGTAGTGACAATTTCA |
| VARV RPA P | TGTATGAGACAGTGTCTGTGACTGTATGA-BTF-TCTTTATTAGTAATTGGTCC-P |
| VACV RPA FP | ACATACACTAGTGATAGCATTAATACAGTAAG |
| VACV RPA RP | AGATGATGTACTTACTGTAGTGTATGAGACAGT |
| VACV RPA P | TCTTCTTATCAGTAATTGGTTCCGGAGTCTCG-BTF-TGTGGATTCTCCA-P |
| RNA assays | |
| EBO RPA FP | GACGACAATCCTGGCCATCAAGATGATGATCC |
| EBO RPA RP | CGTCCTCGTCTAGATCGAATAGGACCAAGTC |
| EBO RPA P | GATGATGGAAGCTACGGCGAATACCAGAG-BTF-CGGAAAACGGCATG-P |
| SUD RPA FP | CAACTATYCCAGGTGGTGTTGTTGACCCGT |
| SUD RPA RP | GGCTGTCRTCATCGTCGTCGTCCAAATTGAAGA |
| SUD RPA P | CTCCTGTGGTGCCTTCAGCCGAATCCTCG-BTF-CAGGATAATTATTACT-P |
| MAR1 RPA FP | CATGAACATCAGGAAATTCAAGCTATTGCMGARG |
| MAR1 RPA RP | CTAATTTTTCTCGTTTCTGGCTGAGGACGGC |
| MAR1 RPA P | TGTGTGTGATTTCAGTTTTYTGAAGGTGGAAY-BTF-TCTAATATCTTCC-P |
| MAR2 RPA FP | CGACATGAACACCAGGAAATTCAGGCCATCGCC |
| MAR2 RPA RP | CGAGCTAGTTTCTCTCGTTTCTGGCTGAGGAC |
| MAR2 RPA P | AATCTCAGTCTTCTGGAGATGGAACTGTTCTAA-BTF-TTTTCTCTCTTCGTC-P |
| SIGV RPA FP | TGACCATCCTAACTCTGTGACATTCCAAGT |
| SIGV RPA RP | GTTGACAGTGAGCTCTTGAATCTCTGGGTT |
| SIGV RPA P | ACTGATTTCCCTCCGTGTCCTCCCGGTACCAC-BTF-CCAAACTGCCGTTGTG-P |
| MARV FPG P | (dR-FAM)GCCGT-B-CTCAGCCAGAAACGAGARAAAYTAGC(C3-Spacer) |
| ZEBO FPG P | (dR-FAM)GGCG-B-ATACCAGAGTTACTCGGAAAACGGCATGAA-P |
| SIG FPG P | (dR-FAM)TGTC-B-TCCCGGTACCACTATCCAAACTGCCGTTGT-P |
FP or RP, forward or reverse primer; P, probe.
BTF, thymidine nucleotide carrying Blackhole Quencher 1, tetrahydrofuran spacer, and thymidine nucleotide carrying fluorescein; P, 3′phosphate to block elongation.
Fig 1.

Details of the RPA amplicon for VARV. All nucleotides in the alignment matching in the VARV sequence are presented as dots. Primer sequences are presented as full sequences. Gaps are presented as hyphens. VARV RPA FP is presented in sense, and VARV RPA P and RP are presented as reverse complement sequences. Gray fields: VARF RPA FP, degenerated IUB code positions; VARV RPA P, the TTT triplet used for the attachment of BTF (Table 2); VARF RPA RP, nucleotide at position 3 of the 3′ end mismatching all other orthopoxviruses. Sequences: cowpox virus, AY902252; camelpox virus, AF438165; monkeypox virus, AF380138; vaccinia virus, M35027; variola virus, X69198.
Assay development steps for RT-RPA.
The detailed development of DNA-RPA and RT-RPA was described for the assays for F. tularensis and RVFV, respectively, elsewhere (27, 28).
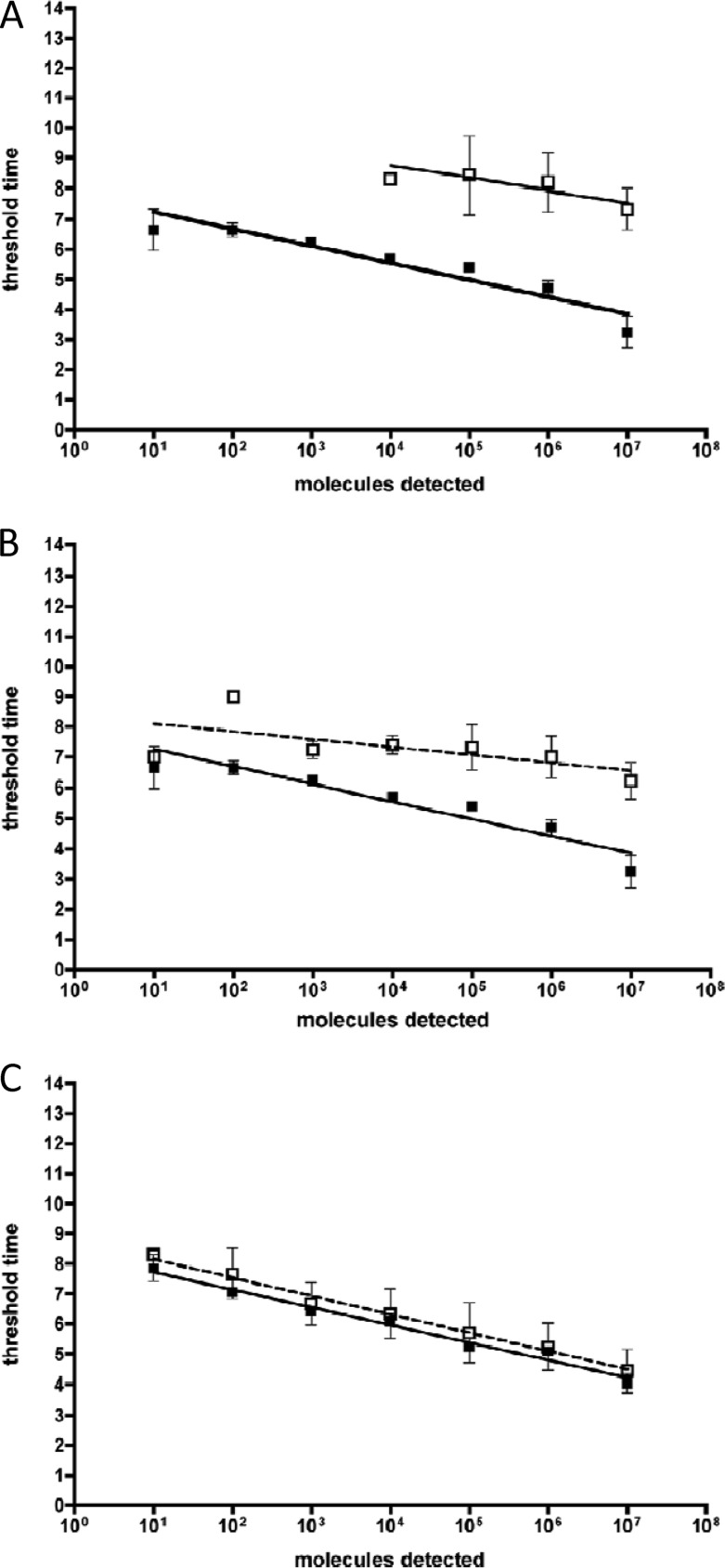
We also compare here the performance of the fluorescent TwistAmp exo probe and the TwistAmp fpg probe in RT-RPA. We designed exo and fpg probes for the same target regions for EBOV, MARV, and SIGV and tested them on the respective quantitative RNA standards. The sensitivities for the TwistAmp fpg probe assays were, respectively, 3-, 6-, and 3-log10 steps lower than the sensitivities of the TwistAmp exo probe assays. The results for EBOV are shown in Fig. 2A.
Fig 2.
Real time RT-RPA assay performance. (A) Comparison of exo and fpg probe performance in RT-RPA. Standard regression lines (SRLs) for EBOV one-step-RT-RPA were generated from eight data sets (exo probe, black squares) and three data sets (fpg probe, white squares). (B) Influence of background DNA on EBOV one-step-RT-RPA. Black squares, SRL as described above; white squares, SRL of the same assay with 70 ng of human genome DNA background. (C) Influence of background DNA on RPA. Black squares, SRL derived from eight data sets of B. anthracis RPA; white squares, SRL of the same assay with 70 ng of human genome DNA background.
RPA sensitivity.
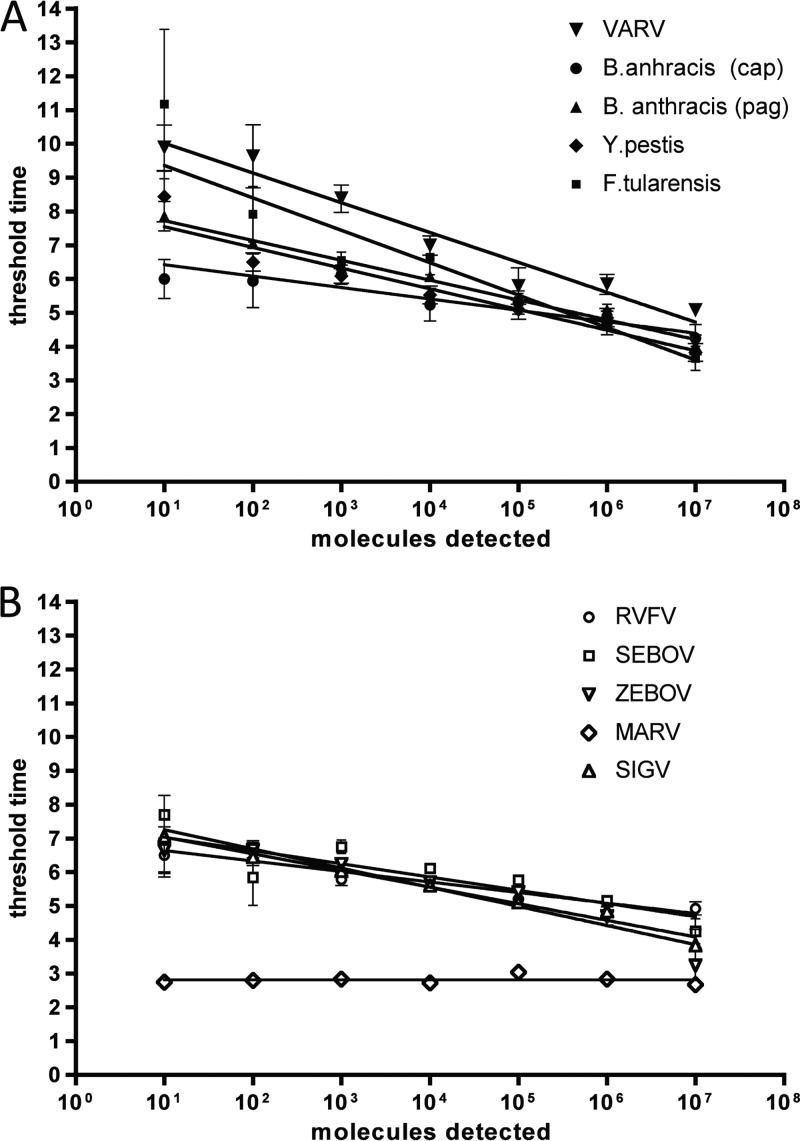
Quantitative molecular plasmid and RNA standards were generated and verified by real-time PCR (data not shown) and used to test the analytical sensitivities of all final RPAs (Fig. 3 and Table 3). The analytical sensitivity of the DNA-RPA assays was approximately 10 molecules detected (md) or as determined by probit analysis 16 to 19 md per reaction. Only the assay for the capC gene of B. anthracis plasmid pX02 showed a lower sensitivity of 100 to 1,000 md or as determined by probit analysis 778 md per reaction (Fig. 3A and Table 3). The standard deviation of the standard curve threshold time values ranged from 0.1 at 107 molecules to 2.6 at 10 md. The slopes of the semilog standard regression lines (SRLs) ranged from −0.33 to −0.96, indicating an efficiency of 1,072 to 11 if using the formula E = 101/slope used for real-time PCR assays, which at an idealized exponential efficiency have an efficiency of 1.
Fig 3.
SRLs of all developed assays, including assays for F. tularensis and Rift Valley fever virus (described elsewhere [35b]). The SRLs were derived from eight data sets each. (A) DNA-RPA assays; (B) one-step-RT-RPA assays.
Table 3.
Sensitivity of RPAs
| Infectious agent (target gene) | Real-time PCR sensitivity (three runs) (reference) | RPA sensitivity (eight runs) (reference) | RPA probit 95% sensitivity (eight runs) | RPA threshold time to sensitivity limit (min) | Sensitivity LAMP (reference) |
|---|---|---|---|---|---|
| DNA assays | |||||
| B. anthracis (pagA) | 101 to 102 (21) | 101 to 102 | 16 | 8 | 103 (27) |
| B. anthracis (capC) | 102 to 103 (21) | 102 to 103 | 778 | 7 | 103 (27) |
| F. tularensis (tul-4) | 102a (28) | 101 to 102 (35a) | 19 | 10 | NDc |
| Y. pestis (pla) | 2a (29) | 101 to 102 | 16 | 8 | ND |
| Variola virus | 101 to 102 (30) | 101 to 102 | 16 | 10 | 102b (31) |
| RNA assays | |||||
| Rift Valley fever virus (N) | 102 | 101 to 102 (35b) | 19 | 7 | 102 (32, 33) |
| Ebola virus (NP) | 102 | 101 to 102 | 21 | 7 | 101 (34) |
| Sudan virus (NP) | 101 | 101 to 102 | 17 | 8 | ND |
| Marburg virus (NP) | 101 | 101 to 102 | 21 | 8 | 102 (35) |
| Sigma virus (G) | 101 | 101 to 102 | 16 | 4 | ND |
Calculated from the fg value given in the source reference publication.
As determined from the monkeypox LAMP assay.
ND, not determined.
RT-RPA was performed, by adding Transcriptor RT enzyme (Roche) to the RPA mix. Optimal performance was observed at 22.4 mM magnesium acetate and 2 mM DTT, and the analytical sensitivities of the RPAs ranged from 10 to 100 md or as determined by probit analysis from 16 to 21 md per reaction (Fig. 3B and Table 3). The standard deviations of the standard curve threshold time values ranged from 0.2 at 107 md to 2.6 at 10 md. SRL slopes ranged from 0.9 × 10−6 to 0.56, indicating an efficiency (E = 101/slope) of 108 to 61.
Sensitivity of RPAs in whole-organism nucleic acid extracts.
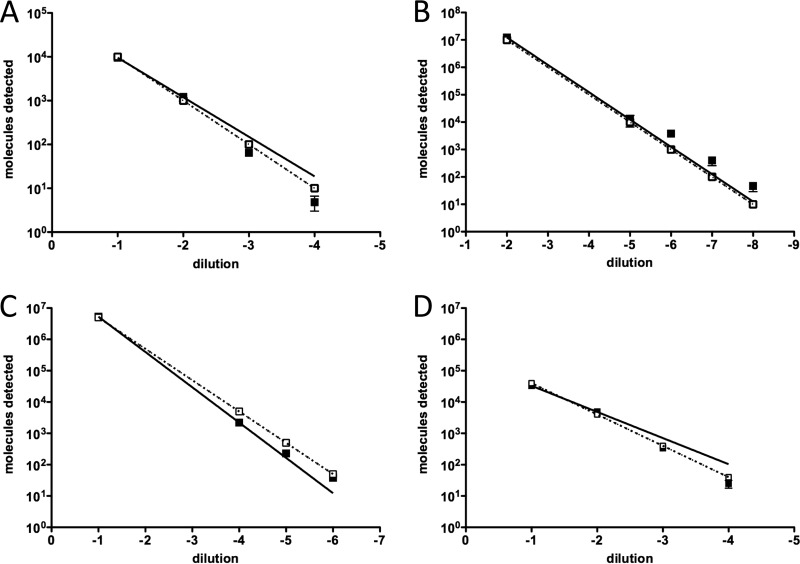
The extraction efficiency of an innuPREP MP basic kit was tested with plasma spiked with whole organisms of each organism category encountered in the biothreat panel using real-time PCR assays. The results of the performance of the innuPREP MP basic kit tested by real-time PCR analysis are illustrated in Fig. 4. Pathogens spiked in plasma at a range of 104 to 101 md per reaction were efficiently extracted by the innuPREP MP basic kit and were detected at high sensitivity by real-time PCR methods. In addition, the internal control was efficiently detected in extracts of all of the spiked samples as determined by real-time PCR (threshold cycle, 22.56 ± 0.51; n = 64). The results here demonstrate that this total nucleic acid extraction method is robust and highly reproducible. The same extracts were used for detection by the respective RPAs. The results of RPA performance are summarized in Table 4. The RT-RPA assays for F. tularensis and RVFV each specifically detected strains of respective strain panels as described elsewhere (35a, 35b).
Fig 4.
Extraction efficiency of the innuPREP MP basic kit. Plasma was spiked with whole organisms (range, 101 to 104 genomic copies/ml), and nucleic acids were extracted. Eluates were tested by respective quantitative real-time PCR assays in triplicate. The amount of measured molecules detected (md) per reaction is plotted against the 10-fold serial dilution of pathogens in plasma. The dotted line represents the calculated 100% efficiency of extraction. (A) B. anthracis (Gram-positive) extracts tested with pag-PCR; (B) Y. pestis (Gram negative) tested with pla-PCR; (C) VACV tested with LE-PCR; (D) MARV tested with NP-PCR.
Table 4.
Comparison of assay sensitivity in nuclear extracts
| Infectious agent | Real-time sensitivity in extracts (md) |
|
|---|---|---|
| PCR | RPA | |
| B. anthracisa (Gram positive) | 10 | 10 |
| Y. pestis (Gram negative) | 10 | 10 |
| Vaccinia virus (DNA virus) | 10 | 10 |
| RVFV (RNA virus) | 100 | 100 |
| SIGV (RNA virus IPC) | 100 | 1,000 |
pagA was used for detection.
Specificity.
The specificity of all RPAs was determined by cross-testing human genome DNA and the nucleic acids of all of the other biothreat agents in the panel, i.e., genomic DNA of Y. pestis (strain 03-1501), F. tularensis subsp. holarctica (strain LVS), B. anthracis (strain 3007), VACV (VR-1536), the VARV plasmid, and the genomic RNAs of EBOV, SUDV, MARV, and SIGV. Only specific detection was observed. Additionally, the RPA for VARV did not detect the genomic DNA of tested orthopoxviruses. These were all detected by the VACV RPA, which did not detect the VARV plasmid. The RPAs for the detection of bacteria were tested against a panel of bacterial genomes as described elsewhere (35a; see also Table S1 in the supplemental material) and showed exclusively specific detection.
To assay the influence of human genomic DNA on the RPAs, we determined the concentrations of human DNA in the eluates of negative sera extracted with the RNeasy kit (Qiagen). We then added the determined average amount of 70 ng of human genomic DNA/μl to RPA and RT-RPA reactions. The added background DNA delayed threshold time points negligibly for RPA and up to 3 min for one-step RT-RPA. It had no effect on the sensitivity (Fig. 2B and C).
DISCUSSION
In order to develop a panel of isothermal detection assays for category A bioterrorism agents, we assessed recombinase polymerase amplification (RPA) for the following reasons: (i) it is an exponential amplification, with specific amplificate confirmation using a fluorescent probe; (ii) it contains GP32, a single-strand binding protein and a good enhancer for the amplification of RNA molecules with complicated secondary structures (36); (iii) it needs only three conserved regions for oligonucleotide design; and (iv) available dried pellet reagents facilitate field use or point of care applications.
With regard to analytical sensitivity and specificity, the RPAs developed showed a performance equal to PCR (Table 3) and showed no cross-detection among their respective targets. Compared to PCR, however, the RPA reaction time was much shorter and, surprisingly, one-step-RT-RPA assays were quicker (limit of detection [LOD] reached at 4 to 8 min) than RPAs (LOD reached at 7 to 10 min).
The SRLs of the RT-RPA assays showed even lower slope values, indicating very fast reaction kinetics. We assume that this might be due to an additive effect of the fluorogenic detection of (i) RNA templates, (ii) the initially generated cDNA (ssDNA; generically detected in T7 promoter-driven isothermal assays such as TMA or NASBA), and (iii) the RPA products (dsDNA). Alternatively, the initiation of RPA may be facilitated by single-stranded cDNA.
The published Km values for exonuclease III (Km = 6.3 × 10−9 M [nicks/min]) (37) and FPG (Km = 7 × 10−9 M [excisions/min]) (38) range in the same order of magnitude, implying comparable activity levels. Nevertheless, the assays using cleavage of fpg probes showed a significantly reduced sensitivity compared to the exo probe assays (Fig. 2A), suggesting that in RPA the FPG enzyme kinetics are not as favorable to real-time detection as those of exonuclease III.
The results of the whole-organism extraction experiments indicate that the magnetic bead-based total nucleic acid extraction kit used showed efficient extraction of DNA and RNA for all tested organism categories. Moreover, it was demonstrated that real-time PCR and RPA show comparable detection sensitivities in these extracts (Table 4).
LAMP assays may also be considered a good option for isothermal detection and miniaturization (9). In general, LAMP assays need four to six primers, leading to longer amplicons and possibly more difficult design in the case of highly variable RNA viruses, whereas the RPA design with three oligonucleotides offers almost the same flexibility as real-time PCR. However, the longer TwistAmp exo probes can be a design obstacle, which can be partly circumvented by allowing probe and primer to overlap. The use of LNA nucleotides might help to reduce probe length, as has been shown for TaqMan probes (39, 40).
In comparison, published LAMP assays for B. anthracis, monkeypox virus, RVFV, MARV, and EBOV (Table 3) have longer run times (18 to 60 min) at 60 to 63°C than the RPAs but show about the same sensitivity (Table 3). However, not all LAMP assays have been adapted for real-time fluorescence since some of them use the turbidity index for readout.
The current advantage of RPA is that the reaction mixtures containing enzymes, nucleotides, and buffer are provided in dried pellets, which is very amenable to POC or field use. This is now also possible for RT-RPA (35b). The only ingredients that need to be added are primers, probe, and sample.
With a small footprint of 17.4 by 18.8 cm and a weight of 1 kg (including the laptop), the ESEquant Tubescanner system is significantly lighter and smaller than all other available state of the art mobile PCR cyclers such as the SmartCycler, Rapid, and Razor (5 to 35 kg) cyclers or the Loopamp real-time Turbidimeter 2.0 for LAMP assays (5 kg). At 4,000 euros, the ESEquant Tubescanner is also considerably less expensive than any of the mobile PCR devices. In combination with the ESEquant Tubescanner, RPA is therefore a very attractive nucleic acid detection method that could easily be installed in hospitals or laboratories that cannot afford real-time PCR cyclers.
The only constraints of isothermal amplification methods are enzyme activity rates since there is no dependency on rapid temperature ramping as in PCR. This feature makes them more amenable to engineer microfluidic lab-on-chip devices than PCR. A recent review on miniaturization efforts for NASBA, LAMP, HDA, SDA, RCA, and RPA pointed out that low-temperature isothermal methods such as SDA, NASBA, RCA, and RPA show an advantage for miniaturization since they need much less energy input and are therefore better candidates for battery driven handheld devices than high-temperature isothermal reactions (LAMP, SmartAmp, and HDA) (9).
The implementation of RPA on centrifugational LabDisks was recently described (41). This type of cartridge could fulfill the requirements for simple benchtop devices if sample preparation were included. It would come closest to a lab on a cartridge, in contrast to the majority of current miniaturized molecular assay systems, which have aptly been described as “chip in lab” rather than “lab on chip” platforms (9).
In summary we have developed a panel of very rapid and highly sensitive isothermal real-time RPAs for the detection of category A bioterrorism agents covering Gram-negative and Gram-positive bacteria, DNA viruses, and RNA viruses. We also showed that a commercially available magnetic bead-based total nucleic extraction kit, which could be used in resource-poor settings, can be efficiently combined with RPA. We now seek to integrate all assays onto a microfluidic POC device and test this syndromic panel of RPAs on clinical samples.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Federal Ministry of Education and Research (BMBF)-funded PROBE (Potential Release-Oriented Biothreat Emergency Diagnostics) project 13N10114 under the research program for civil security of the German Federal Government as part of the high-tech strategy for Germany and by the Shanghai Municipal Education Commission (the Eastern Scholar Project) and Shanghai Municipal Science and Technology Commission (project 10540503000)
Footnotes
Published ahead of print 23 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02704-12.
REFERENCES
- 1. Peeling RW, Mabey D. 2010. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 16:1062–1069 [DOI] [PubMed] [Google Scholar]
- 2. Cuchacovich R. 2006. Clinical implications of the polymerase chain reaction: an update. Dis. Clin. N. Am. 20:735–758 [DOI] [PubMed] [Google Scholar]
- 3. Rothman R, Ramachandran P, Yang S, Hardick A, Won H, Kecojevic A, Quianzon C, Hsieh YH, Gaydos C. 2010. Use of quantitative broad-based polymerase chain reaction for detection and identification of common bacterial pathogens in cerebrospinal fluid. Acad. Emerg. Med. 17:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang S, Rothman RE. 2004. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 4:337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carman B. 2001. Molecular techniques should now replace cell culture in diagnostic virology laboratories. Rev. Med. Virol. 11:347–349 [DOI] [PubMed] [Google Scholar]
- 6. Gunson RN, Bennett S, Maclean A, Carman WF. 2008. Using multiplex real-time PCR in order to streamline a routine diagnostic service. J. Clin. Virol. 43:372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunson RN, Collins TC, Carman WF. 2006. Practical experience of high throughput real-time PCR in the routine diagnostic virology setting. J. Clin. Virol. 35:355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andras SC, Power JB, Cocking EC, Davey MR. 2001. Strategies for signal amplification in nucleic acid detection. Mol. Biotechnol. 19:29–44 [DOI] [PubMed] [Google Scholar]
- 9. Asiello PJ, Baeumner AJ. 2011. Miniaturized isothermal nucleic acid amplification: a review. Lab. Chip 11:1420–1430 [DOI] [PubMed] [Google Scholar]
- 10. Gill P, Ghaemi A. 2008. Nucleic acid isothermal amplification technologies: a review. Nucleosides Nucleotides Nucleic Acids 27:224–243 [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Easley CJ. 2011. Isothermal DNA amplification in bioanalysis: strategies and applications. Bioanalysis 3:227–239 [DOI] [PubMed] [Google Scholar]
- 12. Stougaard M, Juul S, Andersen FF, Knudsen BR. 2011. Strategies for highly sensitive biomarker detection by rolling circle amplification of signals from nucleic acid composed sensors. Integr. Biol. (Camb.) 3:982–992 [DOI] [PubMed] [Google Scholar]
- 13. Leone G, van Schijndel H, van Gemen B, Kramer FR, Schoen CD. 1998. Molecular beacon probes combined with amplification by NASBA enable homogeneous, real-time detection of RNA. Nucleic Acids Res. 26:2150–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsson M, Gullberg M, Dahl F, Szuhai K, Raap AK. 2002. Real-time monitoring of rolling-circle amplification using a modified molecular beacon design. Nucleic Acids Res. 30:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piepenburg O, Williams CH, Stemple DL, Armes NA. 2006. DNA detection using recombination proteins. PLoS Biol. 4:e204 doi:10.1371/journal.pbio.0040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tong Y, Tang W, Kim HJ, Pan X, Ranalli T, Kong H. 2008. Development of isothermal TaqMan assays for detection of biothreat organisms. Biotechniques 45:543–557 [DOI] [PubMed] [Google Scholar]
- 17. Vincent M, Xu Y, Kong H. 2004. Helicase-dependent isothermal DNA amplification. EMBO Rep. 5:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haberstroh K, Reiff E-C. 2007. Fluoreszenzmessung wird alltagstauglich, p 29–33 In Sensortechnik aktuell 2007. Oldenbourg Industrieverlag GmbH, Oldenbourg, Germany [Google Scholar]
- 19. Qiagen 2011. ESEQuant tube scanner product information. Qiagen, Venlo, Netherlands: http://www.qiagen.com/Products/ESEQuantTubeScanner.aspx?r=603 [Google Scholar]
- 20. Weidmann M, Meyer-Konig U, Hufert FT. 2003. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J. Clin. Microbiol. 41:1565–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellerbrok H, Nattermann H, Ozel M, Beutin L, Appel B, Pauli G. 2002. Rapid and sensitive identification of pathogenic and apathogenic Bacillus anthracis by real-time PCR. FEMS Microbiol. Lett. 214:51–59 [DOI] [PubMed] [Google Scholar]
- 22. Kramski M, Drozd A, Lichtfuss GF, Dabrowski PW, Ellerbrok H. 2011. Rapid detection of anti-vaccinia virus neutralizing antibodies. Virol. J. 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weidmann M, Muhlberger E, Hufert FT. 2004. Rapid detection protocol for filoviruses. J. Clin. Virol. 30:94–99 [DOI] [PubMed] [Google Scholar]
- 24. Weidmann M, Sanchez-Seco MP, Sall AA, Ly PO, Thiongane Y, Lo MM, Schley H, Hufert FT. 2008. Rapid detection of important human pathogenic phleboviruses. J. Clin. Virol. 41:138–142 [DOI] [PubMed] [Google Scholar]
- 25. Smieja M, Mahony JB, Goldsmith CH, Chong S, Petrich A, Chernesky M. 2001. Replicate PCR testing and probit analysis for detection and quantitation of Chlamydia pneumoniae in clinical specimens. J. Clin. Microbiol. 39:1796–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF. 1989. Analysis of any point mutation in DNA: the amplification refractory mutation system (ARMS). Nucleic Acids Res. 17:2503–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurosaki Y, Sakuma T, Fukuma A, Fujinami Y, Kawamoto K, Kamo N, Makino SI, Yasuda J. 2009. A simple and sensitive method for detection of Bacillus anthracis by loop-mediated isothermal amplification. J. Appl. Microbiol. 107:1947–1956 [DOI] [PubMed] [Google Scholar]
- 28. Emanuel PA, Bell R, Dang JL, McClanahan R, David JC, Burgess RJ, Thompson J, Collins L, Hadfield T. 2003. Detection of Francisella tularensis within infected mouse tissues by using a hand-held PCR thermocycler. J. Clin. Microbiol. 41:689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matero P, Hemmila H, Tomaso H, Piiparinen H, Rantakokko-Jalava K, Nuotio L, Nikkari S. 2011. Rapid field detection assays for Bacillus anthracis, Brucella spp., Francisella tularensis, and Yersinia pestis. Clin. Microbiol. Infect. 17:34–43 [DOI] [PubMed] [Google Scholar]
- 30. Putkuri N, Piiparinen H, Vaheri A, Vapalahti O. 2009. Detection of human orthopoxvirus infections and differentiation of smallpox virus with real-time PCR. J. Med. Virol. 81:146–152 [DOI] [PubMed] [Google Scholar]
- 31. Iizuka I, Saijo M, Shiota T, Ami Y, Suzaki Y, Nagata N, Hasegawa H, Sakai K, Fukushi S, Mizutani T, Ogata M, Nakauchi M, Kurane I, Mizuguchi M, Morikawa S. 2009. Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J. Med. Virol. 81:1102–1108 [DOI] [PubMed] [Google Scholar]
- 32. Le Roux CA, Kubo T, Grobbelaar AA, van Vuren PJ, Weyer J, Nel LH, Swanepoel R, Morita K, Paweska JT. 2009. Development and evaluation of a real-time reverse transcription-loop-mediated isothermal amplification assay for rapid detection of Rift Valley fever virus in clinical specimens. J. Clin. Microbiol. 47:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peyrefitte CN, Boubis L, Coudrier D, Bouloy M, Grandadam M, Tolou HJ, Plumet S. 2008. Real-time reverse-transcription loop-mediated isothermal amplification for rapid detection of Rift Valley fever virus. J. Clin. Microbiol. 46:3653–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kurosaki Y, Takada A, Ebihara H, Grolla A, Kamo N, Feldmann H, Kawaoka Y, Yasuda J. 2007. Rapid and simple detection of Ebola virus by reverse transcription-loop-mediated isothermal amplification. J. Virol. Methods 141:78–83 [DOI] [PubMed] [Google Scholar]
- 35. Kurosaki Y, Grolla A, Fukuma A, Feldmann H, Yasuda J. 2010. Development and evaluation of a simple assay for Marburg virus detection using a reverse transcription-loop-mediated isothermal amplification method. J. Clin. Microbiol. 48:2330–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a. Euler M, Wang Y, Otto P, Tomaso H, Escudero R, Anda P, Hufert FT, Weidmann M. 2012. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J. Clin. Microbiol. 50:2234–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35b. Euler M, Wang Y, Nentwich O, Piepenburg O, Hufert FT, Weidmann M. 2012. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J. Clin. Virol. 54:308–312 [DOI] [PubMed] [Google Scholar]
- 36. Weidmann M, Rudaz V, Nunes MR, Vasconcelos PF, Hufert FT. 2003. Rapid detection of human pathogenic orthobunyaviruses. J. Clin. Microbiol. 41:3299–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kow YW, Wallace SS. 1985. Exonuclease III recognizes urea residues in oxidized DNA. Proc. Natl. Acad. Sci. U. S. A. 82:8354–8358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boiteux S, O'Connor TR, Lederer F, Gouyette A, Laval J. 1990. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J. Biol. Chem. 265:3916–3922 [PubMed] [Google Scholar]
- 39. Fessehaie A, Block CC, Shepherd LM, Misra MK. 2007. Evaluation of LNA, MGB and non-modified DNA probes to improve the detection limit of TaqMan real-time PCR assay for Pantoea stewartii subsp stewartii. Phytopathology 97:S35–S35 [Google Scholar]
- 40. Weidmann M, Faye O, Faye O, Kranaster R, Marx A, Nunes MR, Vasconcelos PF, Hufert FT, Sall AA. 2010. Improved LNA probe-based assay for the detection of African and South American yellow fever virus strains. J. Clin. Virol. 48:187–192 [DOI] [PubMed] [Google Scholar]
- 41. Lutz S, Weber P, Focke M, Faltin B, Hoffmann J, Muller C, Mark D, Roth G, Munday P, Armes N, Piepenburg O, Zengerle R, von Stetten F. 2010. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab. Chip 10:887–893 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.