Abstract
There is increasing interest in the presence of Staphylococcus aureus, specifically methicillin-resistant S. aureus (MRSA), on retail meat products. In this study, staphylococci were isolated from retail pork and retail beef in Georgia, and MRSA from the products was compared to human MRSA from the same geographic area using broth microdilution antimicrobial susceptibility testing, multilocus sequence typing (MLST), spa typing, SCCmec typing, and pulsed-field gel electrophoresis (PFGE). S. aureus was isolated from 45% (45/100) of pork products and 63% (63/100) of beef products; mecA was detected in S. aureus from both pork (3/100; 3%) and beef (4/100; 4%). Fifty percent (50/100) of human S. aureus also contained mecA. Multidrug resistance was detected among MRSA from all sources. All MRSA (n = 57) was SCCmec type IV, and nine different spa types were present among the isolates (t002, t008, t012, t024, t179, t337, t548, t681, and t1062). Four sequence types (ST5, ST8, ST9, and ST30) were detected using MLST; the majority of MRSA isolates belonged to ST8, followed by ST5. One retail beef MRSA isolate belonged to ST8, while the remaining three were ST5. In retail pork MRSA, ST5, ST9, and ST30 were observed. The majority of human MRSA isolates belonged to ST8. Thirty-seven MRSA isolates, one of which was a retail beef MRSA isolate, were pvl+. Using PFGE, MLST, and spa typing, three retail beef MRSA isolates were found to be identical in PFGE pattern, ST, and spa type to two human clonal MRSA isolates (USA100 and USA300). One additional retail beef MRSA isolate had a PFGE pattern similar to that of a human MRSA isolate, whereas none of the retail pork MRSA isolates had PFGE patterns similar to those of human MRSA isolates. These data suggest that the retail beef samples were contaminated by a human source, possibly during processing of the meat, and may present a source of MRSA for consumers and others who handle raw meat.
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that can be commonly found on the skin or in the nasal passages of most humans and animals (1). It has been implicated in a number of diseases in humans, ranging from minor, uncomplicated skin infections to more serious infections, such as bacteremia and pneumonia (1–3). Treatment of infections caused by S. aureus has been further complicated by antimicrobial resistance in the bacteria, particularly methicillin-resistant S. aureus (MRSA) (4). These infections were initially associated with hospitalization or surgery (health care-associated MRSA [HA-MRSA]); however, recently cases of MRSA have been identified in which no risk factors for MRSA infection were found (4). These cases are referred to as community-acquired MRSA (CA-MRSA) and represent a distinct group of bacteria as compared to HA-MRSA.
Because S. aureus can produce enterotoxins, the bacteria also pose a threat to humans who ingest food contaminated with the preformed toxins (5). Staphylococcal food poisoning, characterized by vomiting and diarrhea, is a leading cause of food-borne illness in the United States (6). In addition to causing staphylococcal food poisoning, food sources of S. aureus have recently expanded to include retail meat products from food-producing animals, including swine, poultry, and cattle (7). MRSA has also been isolated from retail meat and from meat-producing animals. Transmission of MRSA from food animals to humans was first realized for swine, since pig farmers and family members were found to be colonized with MRSA sequence type 398 (ST398) (8). Although methicillin-resistant ST398 was originally thought to be of animal origin and has been referred to as livestock-associated MRSA (LA-MRSA) (9), results from a recent study suggest that ST398 originated with humans as methicillin-susceptible S. aureus (MSSA) (10).
While close contact between food animals and humans is believed to increase the risk of transfer to humans (11), two humans MRSA cases described as ST398 have been documented whereby no animal contact was reported by the patients, their family members, or any of their other close contacts (12). This case study suggests that sources of ST398 other than animals may exist; one potential source may be MRSA-contaminated retail meat. The route of contamination of retail meat is thought to occur during slaughtering of MRSA-positive animals, in which the animal carcasses and the surrounding environment could serve as contamination points (13). In the United States, regional studies of retail meat for the presence of MRSA have found very few samples containing MRSA, and only one MRSA ST398 isolate was identified (14–19). Recently, a study of conventional and retail pork products found higher prevalence levels; almost 27% of the samples were contaminated with an ST398-associated MRSA type (20). A more recent study examined MRSA from swine herds through processing and to retail distribution (21). Results from that study showed that MRSA was detected at different stages of the pork production chain and persisted from farm to retail levels, serving as a good indication that contamination at retail levels may originate at the farm level.
The studies conducted to date have been limited to food animals and retail products. Although the studies have characterized MRSA isolates from those sources and compared their genotypic and phenotypic characteristics to what has been found in human infections, none of the studies have compared human MRSA and retail meat MRSA from the same geographical area to identify which MRSA types are circulating among humans and retail meat during a defined period of time. This study was conducted to determine if human MRSA isolates donated by a local hospital in Athens, GA, were genotypically or phenotypically related to retail meat MRSA isolated from beef and pork products from local grocery stores during the same time period. Antimicrobial susceptibility testing, presence of resistance genes, multilocus sequence typing (MLST), spa typing, pulsed-field gel electrophoresis (PFGE), and SCCmec typing were used to compare the MRSA isolates.
MATERIALS AND METHODS
Sample collection.
During a 12-week period in 2009, 100 (each) retail pork and beef products (prepackaged ground and whole pork and beef) were collected from 14 retail food stores in the Athens, GA, area representing both small (<1,000 stores) and major (>1,000 stores) chains of grocery stores. Approximately 10 items (each) of pork and beef were randomly collected weekly over the 12 weeks. Pork products included pork chops, ground pork, pork ears, pork feet, pork tails, pork cube steak, pork neck bones, pork stomach, pork spare ribs, pork liver, pork roast, pork hocks, and pork back bone. Beef products included steak (T-bone and New York strip), beef roast, ground beef, cubed beef steak, beef stew meat, beef tongue, and beef ribs. At least 2 lb (0.9 kg) of each meat item was purchased, and each product type was collected to ensure that cross-contamination from laboratory personnel to the product did not occur. Meat samples were refrigerated until processing on the same day of purchase. Human MRSA isolates were collected weekly during the same time period and were generously donated by a local hospital. Isolates were from anonymous human patients who were either admitted patients at the hospital or patients in the local emergency room. On condition of anonymity, none of the patient identifying information (gender, age, race, etc.), including the site of infection, was transferred to USDA personnel.
Isolation and identification.
Meat samples (2 lb) were placed in sterile bags, and 100 ml of phosphate-buffered saline (PBS) (1×) was added. Bags were vigorously shaken for 2 min to remove bacteria from the surface of the product, and then 1 ml of each rinsate was transferred to 9 ml BHI (brain heart infusion) (Becton, Dickinson, Sparks, MD) containing 6.5% NaCl and incubated for 24 to 48 h at 37°C. After incubation, a 1-ml aliquot from a positive BHI culture was transferred to mannitol salt broth (Becton, Dickinson) and incubated for 48 h at 37°C. A swab was used to transfer broth from positive cultures to mannitol salt agar plates for isolation of staphylococci. Plates were incubated for 24 to 48 h at 37°C. Three presumptive positive colonies were plated on blood agar and identified as presumptive S. aureus using the catalase test, coagulase test, and BD latex agglutination test (Becton, Dickinson). All resulting clones were identified to the genus and species levels using the Vitek 2 system (bioMérieux, Durham, NC) and the Vitek 2 Gram-positive identification cards according to the manufacturer's directions.
Antimicrobial susceptibility.
MICs (μg/ml) for S. aureus were determined by broth microdilution with the Sensititre semiautomated antimicrobial susceptibility system (Trek Diagnostic Systems, Inc., Cleveland, OH) and the Sensititre Gram-positive plate GPN3F according to the manufacturer's directions. Antimicrobials and breakpoints were as follows: ampicillin (AMP), ≥0.5 μg/ml; ceftriaxone (CEF), ≥64 μg/ml; ciprofloxacin (CIP), ≥4 μg/ml; clindamycin (CLI), ≥4 μg/ml; daptomycin, ≥4 μg/ml; erythromycin (ERY), ≥8 μg/ml; gatifloxacin (GAT), ≥2 μg/ml; gentamicin (GEN), ≥16, ≥500 μg/ml; levofloxacin (LEV), ≥4 μg/ml; linezolid, ≥8 μg/ml; oxacillin (OXA), ≥4 μg/ml; penicillin (PEN) G, ≥0.25 μg/ml; quinupristin-dalfopristin (Q/D), ≥4 μg/ml; rifampin (RIF), ≥4 μg/ml; streptomycin, ≥1,000 μg/ml; tetracycline (TET), ≥16 μg/ml; trimethoprim-sulfamethoxazole (TRI), ≥4 and 76 μg/ml; and vancomycin, ≥16 μg/ml). Resistance breakpoints for daptomycin (range, 0.25 to 8 μg/ml) and streptomycin (1,000 μg/ml) have not been established by CLSI. MIC values were manually recorded by using the Sensitouch system, and Clinical and Laboratory Standards Institute (CLSI) standards were used to determine resistance (22, 23). Results were analyzed using the software program WHONET 5.4 (www.who.int/drugresistance/whonetsoftware) to determine resistance profiles. S. aureus ATCC 29213 was used as a quality control strain.
Molecular characterization.
Presumptive S. aureus was confirmed as MRSA using a multiplex PCR to detect the presence of mecA (24). The multiplex also contained primers for detection of staphylococcal 16S rRNA genes, as an internal S. aureus control, and eight additional antimicrobial resistance genes frequently reported in S. aureus conferring resistance to macrolides [erm(A) and erm(C)], aminoglycosides (aacA-aphD), tetracycline [(tet(K)) and tet(M)], and streptogramins [vat(A), vat(B), and vat(C)]. Production of β-lactamases was carried out by PCR to detect the presence of blaZ (25). The SCCmec type (26), spa type (27, 28), and multilocus sequence type (MLST) (29) were determined as previously described. The presence of the Panton-Valentine leukocidin (PVL) gene was detected using PCR as described elsewhere (30). Macrorestriction patterns were determined using pulsed-field gel electrophoresis (PFGE) as previously described using 30 U of SmaI (Roche, Indianapolis, IN) (31). Cluster analysis was performed with the software program BioNumerics v6 (Applied Maths, Belgium) using the Dice coefficient and the unweighted pair group method (UPGMA). Optimization settings for dendrograms were 1% with a band tolerance of 0.869%. Positive and negative controls were included with all PCR runs performed. Positive controls were as follows: aacA-aphD-E. faecalis ATCC 49532, blaZ-S. aureus ST00266, erm(A)-S. aureus RN1389, erm(C)-S. aureus RN4220, mecA-S. aureus ATCC 33591, tet(K)-S. aureus RB 36-1 (this study), tet(M)-E. faecalis OG1-SSp, vat(A)-S. aureus CIP 107907, vat(B)-S. aureus CIP 108540, and vat(C)-S. aureus CIP 107908.
Statistical analysis.
Probability values of statistical significance among prevalences of S. aureus and MRSA from meat and human samples were determined using Fisher's exact test (SAS 9.2 software program; SAS Institute, Cary, NC). Statistical significance was defined as a probability value of ≤0.05.
RESULTS
Prevalence of staphylococci.
During 2009, 100 (each) retail pork products and retail beef products were collected for testing (Table 1). Of the retail meat samples, 13 different kinds of pork products and 7 different types of beef products were tested for the presence of staphylococci. Pork samples included cuts of pork containing portions of pig skin (pork ears, pork feet, pork hocks, and pork tails) and internal organs, such as pork stomach and pork liver; beef tongue was also tested for the presence of staphylococci. S. aureus was isolated from 108 retail meat samples (54%; 95% confidence interval, 52.2% to 55.8%). Significantly more S. aureus bacteria were isolated from retail beef than from retail pork (P = 0.0157). Overall, 45% (45/100) of the pork products and 63% of the beef products were positive for S. aureus; only pork stomach and pork liver were negative (Table 1). Other staphylococcal species were detected in the retail meat samples, including S. caprae, S. epidermidis, S. intermedius group, S. saprophyticus, S. sciuri, and S. xylosus from retail pork and S. saprophyticus, S. sciuri, S. xylosus, and S. warneri from retail beef. Every portion of retail meat tested was positive for either S. aureus or some other staphylococcal species (Table 1).
Table 1.
Prevalence of staphylococci from retail pork and beef products
| Item (na) | No. of samples | No. (%) of samples positive: |
Other Staphylococcus species (no. of positive samples) | |
|---|---|---|---|---|
| For S. aureus | For mecA | |||
| Pork (100) | ||||
| Pork chops | 31 | 11 (35.5) | 2 (6.5) | Staphylococcus caprae (1), Staphylococcus epidermidis (1), Staphylococcus intermedius group (1), Staphylococcus saprophyticus (1), Staphylococcus sciuri (2), Staphylococcus xylosus (2) |
| Ground pork | 14 | 7 (50) | 0 (0) | Staphylococcus saprophyticus (2), Staphylococcus sciuri (1) |
| Pork ears | 6 | 4 (66.7) | 0 (0) | Staphylococcus sciuri (2) |
| Pork feet | 13 | 8 (61.5) | 0 (0) | Staphylococcus sciuri (3), Staphylococcus saprophyticus (1) |
| Pork tails | 4 | 3 (75) | 0 (0) | Staphylococcus sciuri (1) |
| Pork cube steak | 6 | 2 (33.3) | 0 (0) | Staphylococcus epidermidis (1), Staphylococcus saprophyticus (1), Staphylococcus xylosus (1) |
| Pork neck bone | 5 | 2 (40) | 0 (0) | Staphylococcus sciuri (1) |
| Pork stomach | 3 | 0 (0) | 0 (0) | Staphylococcus sciuri (1), Staphylococcus saprophyticus (1) |
| Pork spare ribs | 14 | 5 (35.7) | 1 (7.1) | Staphylococcus intermedius group (1), Staphylococcus saprophyticus (1), Staphylococcus vitulinus (1), Staphylococcus xylosus (1) |
| Pork liver | 1 | 0 (0) | 0 (0) | Staphylococcus saprophyticus (1) |
| Pork roast | 1 | 1 (100) | 0 (0) | NDb |
| Pork hocks | 1 | 1 (100) | 0 (0) | ND |
| Pork back bone | 1 | 1 (100) | 0 (0) | ND |
| Beef (100) | ||||
| Steak | 29 | 18 (62) | 1 (3.4) | Staphylococcus saprophyticus (2), Staphylococcus sciuri (3) |
| Roast | 20 | 10 (50) | 1 (5) | Staphylococcus saprophyticus (2), Staphylococcus sciuri (1) |
| Ground beef | 26 | 18 (69.2) | 1 (3.8) | Staphylococcus saprophyticus (2), Staphylococcus sciuri (4), Staphylococcus xylosus (1) |
| Cubed beef | 12 | 9 (75) | 0 (0) | Staphylococcus sciuri (2) |
| Stew beef | 11 | 6 (54.5) | 1 (9.1) | Staphylococcus saprophyticus (2), Staphylococcus sciuri (1), Staphylococcus warneri (1) |
| Beef tongue | 1 | 1 (100) | 0 (0) | ND |
| Beef rib | 1 | 1 (100) | 0 (0) | ND |
| Human (100) | 100 | 100 (100) | 50 (50) | ND |
n, no. of samples.
ND, none detected.
S. aureus positive for mecA was identified in three retail pork samples (pork chops [n = 2] and pork spare ribs [n = 1]) and four retail beef samples (steak [n = 1], roast [n = 1], ground beef [n = 1], and stew beef [n = 1]) (Table 1). Two of the retail pork MRSA isolates originated with different samples (chops and ribs) from the same grocery store (store A), while the third positive sample (chops) was from a different grocery store (store B). Similarly, two of the positive beef samples (roast and stew beef) originated with one grocery store (store B), while the other two positive samples (steak and ground beef) originated with two different grocery stores (stores C and D); one grocery store (store B) was a common source of positive pork and beef samples (pork chops, steak, and stew meat). Human S. aureus isolates (n = 100), collected from a local hospital during the same time period, were confirmed as S. aureus by the hospital. Of those, 50% (50/100) of the human S. aureus isolates contained mecA. No significant difference was detected between MRSA prevalences among the pork and beef samples (P > 0.05); significantly more MRSA was obtained from human sources than from both of the retail meat sources (P < 0.0001).
Antimicrobial resistance.
Only the mecA-positive S. aureus isolates (n = 57) were selected for further study. With the exception of two retail pork isolates which were susceptible to oxacillin, mecA-positive S. aureus isolates were resistant to ampicillin, oxacillin, and penicillin (Table 2). All four MRSA isolates from retail beef were also resistant to ciprofloxacin, erythromycin, gatifloxacin, and levofloxacin. Seventy-five percent of the retail beef isolates were also resistant to ceftriaxone, while only one was resistant to clindamycin and tetracycline. MRSA isolates from retail pork were resistant to 10 of the 13 antimicrobials on the panel, including one to gentamicin; and like retail beef MRSA isolates, MRSA isolates from retail pork were also susceptible to rifampin and trimethoprim-sulfamethoxazole. This is in contrast to MRSA isolates from humans, since those isolates were the only ones resistant to rifampin and trimethoprim-sulfamethoxazole (Table 2).
Table 2.
Antimicrobial resistance profiles of MRSA from humans and retail meat
| Antimicrobiala | No. (%) of resistant isolates by source (nb) |
||
|---|---|---|---|
| Beef (4) | Pork (3) | Human (50) | |
| Ampicillin | 4 (100) | 3 (100) | 50 (100) |
| Ceftriaxone | 3 (75) | 1 (33.3) | 3 (6) |
| Ciprofloxacin | 4 (100) | 2 (66.7) | 22 (44) |
| Clindamycin | 1 (25) | 1 (33.3) | 9 (18) |
| Erythromycin | 4 (100) | 2 (66.7) | 47 (94) |
| Gatifloxacin | 4 (100) | 2 (66.7) | 37 (74) |
| Gentamicin | 0 (0) | 1 (33.3) | 0 (0) |
| Levofloxacin | 4 (100) | 1 (33.3) | 23 (46) |
| Oxacillin | 4 (100) | 1 (33.3) | 50 (100) |
| Penicillin | 4 (100) | 3 (100) | 50 (100) |
| Rifampin | 0 (0) | 0 (0) | 5 (10) |
| Tetracycline | 1 (25) | 0 (0) | 0 (0) |
| Trimethoprim-sulfamethoxazole | 0 (0) | 0 (0) | 1 (2) |
No isolates were resistant to daptomycin, linezolid, quinupristin-dalfopristin, streptomycin, or vancomycin.
n, no. of isolates tested.
Multidrug resistance (resistance to ≥3 antimicrobials) was also prevalent among the isolates (Table 3). Resistance to nine antimicrobials and four classes of antimicrobials was detected in one MRSA isolate from beef. The most common resistance pattern was ampicillin (AMP), erythromycin (ERY), gatifloxacin (GAT), oxacillin (OXA), penicillin (PEN) in 14 MRSA isolates from humans. Three MRSA isolates from retail beef had the same multidrug resistance pattern (AMP, ceftriaxone [CEF], ciprofloxacin [CIP], ERY, GAT, levofloxacin [LEV], OXA, PEN) (Table 3). Although no one pattern was shared by all three sources of MRSA, two human and one retail pork MRSA isolate were resistant to nine antimicrobials (three antimicrobial classes), exhibiting the pattern of AMP, CEF, CIP, CLI, ERY, GAT, LEV, OXA, PEN. Of the resistance genes tested, blaZ, mecA, erm(A), erm(C), and tet(K) were detected in some MRSA isolates (Fig. 1), while aacA-aphD, tet(M), vat(A), vat(B), and vat(C) were not detected in any of the isolates.
Table 3.
Multidrug resistance patterns among MRSA isolates from humans and retail meat
| Resistance patterna | No. of antimicrobials | No. of antimicrobial classes | No. of isolates with pattern by source (nb) |
|||
|---|---|---|---|---|---|---|
| Human (50) | Beef (4) | Pork (3) | Total | |||
| AMP, OXA, PEN | 3 | 1 | 3 | 0 | 0 | 3 |
| AMP, GEN, PEN | 3 | 2 | 0 | 0 | 1 | 1 |
| AMP, ERY, OXA, PEN | 4 | 2 | 7 | 0 | 0 | 7 |
| AMP, CLI, ERY, OXA, PEN | 5 | 2 | 2 | 0 | 0 | 2 |
| AMP, ERY, GAT, OXA, PEN | 5 | 3 | 14 | 0 | 0 | 14 |
| AMP, ERY, OXA, PEN, TRI | 5 | 3 | 1 | 0 | 0 | 1 |
| AMP, CIP, ERY, GAT, PEN | 5 | 3 | 0 | 0 | 1 | 1 |
| AMP, ERY, GAT, LEV, OXA, PEN | 6 | 3 | 1 | 0 | 0 | 1 |
| AMP, CIP, ERY, GAT, LEV, OXA, PEN | 7 | 3 | 9 | 0 | 0 | 9 |
| AMP, CEF, CIP, ERY, GAT, LEV, OXA, PEN | 8 | 3 | 1 | 3 | 0 | 4 |
| AMP, CIP, CLI, ERY, GAT, LEV, OXA, PEN | 8 | 3 | 5 | 0 | 0 | 5 |
| AMP, CIP, ERY, GAT, LEV, OXA, PEN, RIF | 8 | 4 | 5 | 0 | 0 | 5 |
| AMP, CEF, CIP, CLI, ERY, GAT, LEV, OXA, PEN | 9 | 3 | 2 | 0 | 1 | 3 |
| AMP, CIP, CLI, ERY, GAT, LEV, OXA, PEN, TET | 9 | 4 | 0 | 1 | 0 | 1 |
See the text for abbreviations.
n, no. of isolates tested.
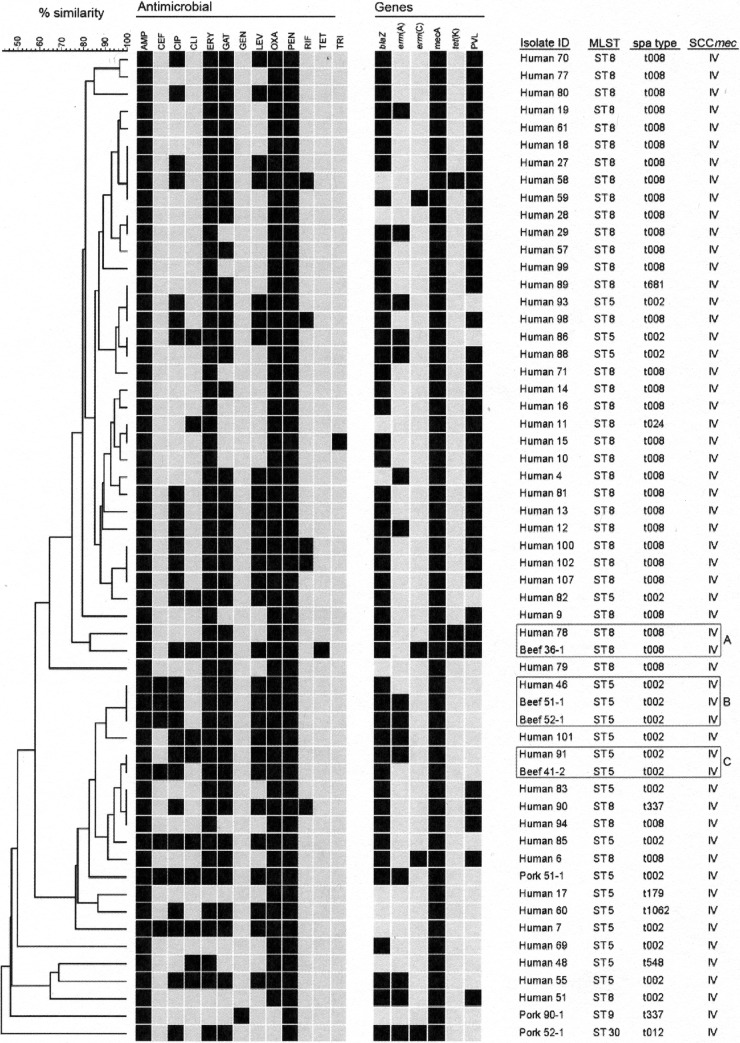
Fig 1.
PFGE analysis of MRSA isolates from retail pork, retail beef, and humans. DNA for PFGE was digested with SmaI. Levels of similarity were determined using the Dice coefficient and UPGMA. Clusters A, B, and C represent retail beef MRSA isolates which are similar or identical in pattern to human MRSA.
Molecular characteristics.
All MRSA isolates, including retail pork and beef isolates, were SCCmec type IV; subtyping of SCCmec type IV was not performed. Nine different spa types were detected among the isolates (t002, t008, t012, t024, t179, t337, t548, t681, and t1062) (Fig. 1). Of the 57 MRSA, 32 isolates (56.1%) were spa type t008, followed by 17 (29.8%) which were spa type t002. One MRSA isolate from retail beef was spa type t008, while the rest were from human MRSA. All three sources of MRSA (beef, human, and pork) had isolates of spa type t002; three of the retail beef MRSA isolates were of this spa type. One human and one pork MRSA isolate were spa type t337. The remaining retail pork spa type was t012 (Fig. 1). Four STs (ST5, ST8, ST9, and ST30) were detected using MLST. The majority of MRSA were ST8 (36/57; 63.2%), followed by ST5 (19/57; 33.3%). One retail beef MRSA isolate was ST8, while the remaining three were ST5; the retail pork MRSA isolates were of three different STs, ST5, ST9, and ST30. The majority of human MRSA isolates belonged to ST8 (35/57; 61.4%). Thirty-seven MRSA isolates one of which was a retail beef MRSA isolate (isolate 36-1), were positive for pvl (Fig. 1).
Genetic relatedness.
As determined by PFGE, the vast majority of MRSA isolates clustered at the top of the dendrogram were ST8, t008, and pvl+, while only four isolates were characterized as ST5/t002 (Fig. 1). In contrast, the majority of MRSA isolates (57%) clustered toward the bottom of the dendrogram were ST5/t002. Three isolates (human isolates 89, 93, and 98) were identical by PFGE but had different STs and spa types. One retail beef MRSA isolate (beef 36-1), originating with ground beef, was greater than 80% similar to a human MRSA isolate (human 78; cluster A). Both isolates were ST8/t008 and pvl+. The three other retail beef MRSA isolates were identical in pattern to two individual human MRSA isolates. Retail beef MRSA isolates 51-1 and 52-1 were identical in pattern to human MRSA isolate 78, and retail beef isolate 41-2 was identical to human isolate 91 (Fig. 1, clusters B and C). All of those isolates were ST5/t002, and isolates in cluster B also had identical antimicrobial resistance patterns. Retail beef MRSA isolates 51-1 and 52-1 originated with roast and stew beef from the same grocery store; isolate 41-2 originated with steak from a different grocery store. None of the PFGE patterns from the pork samples exhibited greater than 70% similarity to any of the patterns from human MRSA isolates.
DISCUSSION
Previous studies in the United States to detect the presence of MRSA in retail pork and beef have focused primarily on products popular with the consumer (i.e., ground pork and beef, pork chops) (14–19). In the southern United States, other cuts of pork and beef are regularly available in grocery stores in the area. In this study, in addition to testing the more common cuts of pork and beef, additional pork and beef products were also sampled to determine if they also are a source of staphylococci and/or MRSA. These samples included pork ears, pork feet, pork tails, and pork hocks, which included intact parts of the skin of the pig, as well as some internal organs, such as pork liver and beef tongue. A recent study included a number of cuts of pork different from those normally reported (pork riblet, pork ribs, pork sausage, pork steak, pork loin, and pork roast), which may or may not be the same as or similar to some of the products sampled in this study (20). However, although the cuts may be similar in name, comparisons between the individual items are not made in this study in order to avoid error between the samples.
Prevalence of S. aureus on retail pork products from other studies has ranged from 12% to 59.7% (14–20). Results from this study are similar to those in the other published reports, since S. aureus was found on 45% of pork products in this study. In contrast, while the prevalence of S. aureus in retail beef products from other studies has ranged from 20% to 37%, the prevalence of S. aureus from retail beef from this study was higher, at 63%. This could be due to the different beef products tested in this study compared to those in the other studies. Furthermore, the sample size was relatively low in this study, which may also account for the difference in percentages. It was surprising that the prevalence of S. aureus on steak (62%) was almost as high as the prevalence of S. aureus on ground beef (69.2%), suggesting that other cuts of meat, as well as the more popular items, should be tested for the presence of S. aureus and MRSA. These meat items may serve as reservoirs of resistant S. aureus, as with other retail meat samples.
In addition to the prevalence of S. aureus, the percentage of S. aureus isolates which were also MRSA was consistent with that in other studies. MRSA in retail pork has ranged from 0.14% to 6.6% and from 1.3% to 3.3% in retail beef; we detected 3% and 4% in retail pork and beef, respectively, from this study (14, 15, 17–20). While the sampling methods, samples, times of sampling, and geographical sampling locations were different among the studies, the consistency in the prevalence of S. aureus in pork and the MRSA prevalence in retail pork and beef among the studies lends some degree of assurance that all of these differences may not be significant enough to prevent comparison between the studies. This is subject to change over time as more studies are performed including testing of retail meat products from different regions of the United States, which may reflect cultural preferences.
Few studies have included human MRSA in comparison to MRSA from retail meat. One study characterized antimicrobial resistance in S. aureus from retail chicken carcasses and pet workers in Arkansas (16). Thirty percent of the human isolates were resistant to oxacillin, whereas 50% of the human isolates in this study were MRSA. Although the specific staphylococcal human infection site was unknown in the present study, the prevalence of MRSA was not surprising since the human isolates were obtained from the local hospital, indicating that some kind of infection was present or suspected, causing the patient to seek medical attention. Of interest was multidrug resistance in the human MRSA isolates. All human MRSA isolates were resistant to at least three antimicrobials and up to four different classes of antimicrobials. This is consistent with human MRSA from pet workers, where 94% of those isolates were resistant to two or more antimicrobials (16). Multidrug resistance in retail beef and pork MRSA was also prevalent, with isolates in both groups exhibiting resistance to three classes of antimicrobials. This has also been observed in other studies, where high levels of retail pork and retail beef isolates displayed resistance to three or more antimicrobials (≥2 antimicrobial classes) (15, 32). Most of the multidrug resistance can be attributed to the presence of a few resistance genes commonly found in staphylococci. In some isolates, resistance was detected without a corresponding resistance gene. This was most likely because only a few resistance genes were tested and the gene conferring resistance was not one of those tested. In two instances, tet(K) was detected, but tetracycline resistance was not, possibly due to an inactive or defective gene. Detection of mecA in two MRSA isolates from pork which were resistant to ampicillin and penicillin but susceptible to oxacillin could also be the result of a nonfunctional gene, other resistance genes (such as blaZ), or a heterororesistant population (33). Contrasting with results in other published reports, the lack of tetracycline resistance overall and especially in MRSA from meat products was remarkable. Results from this study were different from those in other studies which have detected high percentages of tetracycline resistance in S. aureus from retail pork and beef (15, 19, 32). High levels of tetracycline resistance in retail pork would be expected, since other studies have found very high levels of tetracycline resistance in swine (21, 34). Finally, one of the more important observations from antimicrobial susceptibility testing was that none of the human MRSA isolates or MRSA isolates from retail meat were resistant to clinically important antimicrobials, including daptomycin, linezolid, Q/D, or vancomycin (15).
Several molecular typing methods were used to characterize the isolates, including MLST, spa typing, SCCmec typing, and PFGE. PFGE is considered the “gold standard” for molecular typing of S. aureus and provides consistent results because the protocol is essentially standardized and has evolved as an efficient tool for epidemiological investigations (35). In the United States, the Centers for Disease Control and Prevention has established standardized protocols and a national database for typing MRSA (31). DNA sequencing technologies have advanced newer typing methods, such as MLST, which uses sequence analysis of housekeeping genes to discriminate between isolates (29). Both spa typing and SCCmec typing are specific for staphylococci and analyze polymorphisms in the protein A gene and variations in the mec element, respectively (36, 37). Each method has limitations, including typeability, reproducibility, discriminatory value, cost, time, and ease of use (35, 38–41). Combining these techniques enhances discriminatory power and provides means to trace epidemiologically related strains and to compare results from other studies (35, 41).
Although none of the retail meat MRSA isolates was identified as ST398, four STs (ST5, ST8, ST9, and ST30) were identified. ST5 and ST8 are both human-associated types predominant in HA-MRSA and CA-MRSA, respectively (42). These were found primarily in the human MRSA isolates, but one of the retail pork MRSA isolates and three retail beef MRSA isolates belonged to ST5, and one beef MRSA isolate was ST8. ST5 has been previously found in retail pork and beef in the United States, as has a poultry-adapted clone that was reported to have originated in humans (15, 17, 43). Like ST5, ST8 has also been found in retail meat, including chicken, turkey, pork, and beef (14, 15, 17, 18), and both have been found previously in live swine (34, 44, 45). Two of the retail pork isolates were susceptible to oxacillin although they contained mecA; one was identified as belonging to ST9, and the other was ST30, both associated with swine and characterized as MSSA (46, 47). ST9 has also been found in retail turkey samples in the United States (14). In addition to retail pork and beef, both STs have been associated with humans; ST9 has been previously isolated from healthy humans in Germany, while ST30 has been identified as CA-MRSA in European countries (46, 47).
Of the nine spa types identified, t002 and t008 were the most prevalent. These spa types are common among human MRSA isolates but have also been found previously in retail meat (14, 17, 18, 20). Three retail beef MRSA isolates and one retail pork MRSA isolate were spa type t002, and one retail beef MRSA isolate was spa type t008. The remaining two retail pork MRSA isolates were spa types t337 and t012, which have been found previously in retail pork and retail chicken, respectively (14). ST9/t337 is swine-associated MSSA, but t337 was also found in human MRSA (ST8/t337) which was resistant to oxacillin in this study. We are unaware if this spa type has been previously reported for human MRSA. In contrast to spa typing, only one SCCmec type, type IV, was detected in the isolates. SCCmec type IV is variable in size and diverse in composition; to date, at least seven subtypes have been described (48). In addition to being associated with CA-MRSA, SCCmec type IV has been found previously in MRSA in retail meat in the United States (14, 17, 18).
PFGE analysis of the isolates revealed nine groups of isolates with indistinguishable PFGE patterns. Two human MRSA isolates (human isolates 11 and 15) had identical PFGE patterns and STs but different spa types (t008 and t024). These two types are clones of USA300 but have different characteristics, including clindamycin resistance in t024 and pvl positivity in t008 (49). However, in this study, human MRSA isolate 11 (ST8/t024) was also pvl+, indicating that a rare clone may be circulating in the U.S. population. This clone was first reported during an outbreak of CA-MRSA in the Netherlands caused by ST8/t024 (pvl+) (50). Retail beef MRSA isolates in PFGE clusters B and C were 100% similar in pattern and also in ST and spa type to human MRSA. One additional retail beef MRSA (cluster A) had a PFGE pattern similar to that of a human MRSA isolate. Although the personnel at the grocery stores were not tested, these data suggest that the retail beef samples were contaminated from a human source sometime during processing of the meat. The possibility of human contamination of retail meat has also been proposed previously (17, 18). Furthermore, because the retail beef MRSA isolates were identical to two common human clones circulating in the U.S. population, this suggests that there may be a risk, however slight, that humans may become colonized with these clones if retail meat is not properly handled. Finally, although none of the retail pork MRSA was similar in PFGE pattern to human or retail beef MRSA, these isolates were also not similar to each other. Retail pork isolate 51-1 was the most similar to human MRSA but was also the only retail pork MRSA isolate identified as ST5/t002.
Conclusions.
In this study, we compared retail pork and beef MRSA to MRSA from human sources. Although this was a small study in a limited geographical area, this study serves as an indication that staphylococci and MRSA are present on retail meat, and some of those isolates are identical to those found in HA-MRSA and CA-MRSA infections. Because this study focused on antimicrobial-resistant isolates, primarily MRSA, a limiting factor of the present research is that only MRSA isolates from symptomatic humans were compared to the S. aureus bacteria isolated from meat products. Comparison of all S. aureus isolates regardless of antimicrobial resistance phenotype, may have revealed a more diverse collection of isolates rather than focusing on MRSA, which tends to be more clonal. Detection of the presence of known virulence factors in MSSA would be necessary, because although oxacillin resistance would be absent, virulence genes may be present and may also be a source of hazardous S. aureus for the human population. While the origin of the MRSA isolates from retail pork and beef remains unknown, there may be a slight risk of colonization of humans with MRSA from retail meat. Additional studies are needed in order to assess the risk of MRSA colonization to consumers and others who handle raw meat.
ACKNOWLEDGMENT
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 2. Negishi H, Matsuda T, Okuyama K, Sutoh S, Fujioka Y, Fujimoto S. 1998. Staphylococcus aureus causing chorioamnionitis and fetal death with intact membranes at term. A case report. J. Reprod. Med. 43:397–400 [PubMed] [Google Scholar]
- 3. Archer GL. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 4. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984 [DOI] [PubMed] [Google Scholar]
- 5. Hennekinne JA, De Buyser ML, Dragacci S. 2012. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol. Rev. 36:815–836 [DOI] [PubMed] [Google Scholar]
- 6. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kluytmans JA. 2010. Methicillin-resistant Staphylococcus aureus in food products: cause for concern or case for complacency? Clin. Microbiol. Infect. 16:11–15 [DOI] [PubMed] [Google Scholar]
- 8. Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. 2005. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 11:1965–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nemati M, Hermans K, Lipinska U, Denis O, Deplano A, Struelens M, Devriese LA, Pasmans F, Haesebrouck F. 2008. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: first detection of livestock-associated methicillin-resistant strain ST398. Antimicrob. Agents Chemother. 52:3817–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, Pearson T, Waters AE, Foster JT, Schupp J, Gillece J, Driebe E, Liu CM, Springer B, Zdovc I, Battisti A, Franco A, Zmudzki J, Schwarz S, Butaye P, Jouy E, Pomba C, Porrero MC, Ruimy R, Smith TC, Robinson DA, Weese JS, Arriola CS, Yu F, Laurent F, Keim P, Skov R, Aarestrup FM. 2012. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3(1):e00305–11 doi:10.1128/mBio.00305-11. (Erratum, 4(1):00520-12, 2013. doi:10.1128/mBio.00520-12.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Duijkeren E, Ikawaty R, Broekhuizen-Stins MJ, Jansen MD, Spalburg EC, de Neeling AJ, Allaart JG, van Nes A, Wagenaar JA, Fluit AC. 2008. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet. Microbiol. 126:383–389 [DOI] [PubMed] [Google Scholar]
- 12. Welinder-Olsson C, Floren-Johansson K, Larsson L, Oberg S, Karlsson L, Ahren C. 2008. Infection with Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus t034. Emerg. Infect. Dis. 14:1271–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Boer E, Zwartkruis-Nahuis JT, Wit B, Huijsdens XW, de Neeling AJ, Bosch T, van Oosterom RA, Vila A, Heuvelink AE. 2009. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int. J. Food Microbiol. 134:52–56 [DOI] [PubMed] [Google Scholar]
- 14. Hanson BM, Dressler AE, Harper AL, Scheibel RP, Wardyn SE, Roberts LK, Kroeger JS, Smith TC. 2011. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) on retail meat in Iowa. J. Infect. Public Health 4:169–174 [DOI] [PubMed] [Google Scholar]
- 15. Waters AE, Contente-Cuomo T, Buchhagen J, Liu CM, Watson L, Pearce K, Foster JT, Bowers J, Driebe EM, Engelthaler DM, Keim PS, Price LB. 2011. Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 52:1227–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanning I, Gilmore D, Pendleton S, Fleck S, Clement A, Park SH, Scott E, Ricke SC. 2012. Characterization of Staphylococcus aureus isolates from retail chicken carcasses and pet workers in Northwest Arkansas. J. Food Prot. 75:174–178 [DOI] [PubMed] [Google Scholar]
- 17. Pu S, Han F, Ge B. 2009. Isolation and characterization of methicillin-resistant Staphylococcus aureus strains from Louisiana retail meats. Appl. Environ. Microbiol. 75:265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhargava K, Wang X, Donabedian S, Zervos M, de Rocha L, Zhang Y. 2011. Methicillin-resistant Staphylococcus aureus in retail meat, Detroit, Michigan, USA. Emerg. Infect. Dis. 17:1135–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelman A, Soong YA, Dupuy N, Shafer D, Richbourg W, Johnson K, Brown T, Kestler E, Li Y, Zheng J, McDermott P, Meng J. 2011. Antimicrobial susceptibility of Staphylococcus aureus from retail ground meats. J. Food Prot. 74:1625–1629 [DOI] [PubMed] [Google Scholar]
- 20. O'Brien AM, Hanson BM, Farina SA, Wu JY, Simmering JE, Wardyn SE, Forshey BM, Kulick ME, Wallinga DB, Smith TC. 2012. MRSA in conventional and alternative retail pork products. PLoS One 7:e30092 doi:10.1371/journal.pone.0030092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molla B, Byrne M, Abley M, Mathews J, Jackson CR, Fedorka-Cray P, Sreevatsan S, Wang P, Gebreyes WA. 2012. Epidemiology and genotypic characteristics of methicillin-resistant Staphylococcus aureus (MRSA) of porcine origin. J. Clin. Microbiol. 50:3687–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed Approved standard M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 24. Strommenger B, Kettlitz C, Werner G, Witte W. 2003. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 41:4089–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malik S, Christensen H, Peng H, Barton MD. 2007. Presence and diversity of the beta-lactamase gene in cat and dog staphylococci. Vet. Microbiol. 123:162–168 [DOI] [PubMed] [Google Scholar]
- 26. Oliveira DC, de Lencastre H. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strommenger B, Braulke C, Heuck D, Schmidt C, Pasemann B, Nubel U, Witte W. 2008. spa typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J. Clin. Microbiol. 46:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strommenger B, Braulke C, Pasemann B, Schmidt C, Witte W. 2008. Multiplex PCR for rapid detection of Staphylococcus aureus isolates suspected to represent community-acquired strains. J. Clin. Microbiol. 46:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pu S, Wang F, Ge B. 2011. Characterization of toxin genes and antimicrobial susceptibility of Staphylococcus aureus isolates from Louisiana retail meats. Foodborne Pathog. Dis. 8:299–306 [DOI] [PubMed] [Google Scholar]
- 33. Sakoulas G, Gold HS, Venkataraman L, DeGirolami PC, Eliopoulos GM, Qian Q. 2001. Methicillin-resistant Staphylococcus aureus: comparison of susceptibility testing methods and analysis of mecA-positive susceptible strains. J. Clin. Microbiol. 39:3946–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dressler AE, Scheibel RP, Wardyn S, Harper AL, Hanson BM, Kroeger JS, Diekema DJ, Bender JB, Gray GC, Smith TC. 2012. Prevalence, antibiotic resistance and molecular characterisation of Staphylococcus aureus in pigs at agricultural fairs in the USA. Vet. Rec. 170:495 doi:10.1136/vr.100570 [DOI] [PubMed] [Google Scholar]
- 35. Strommenger B, Kettlitz C, Weniger T, Harmsen D, Friedrich AW, Witte W. 2006. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 44:2533–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frenay HM, Bunschoten AE, Schouls LM, van Leeuwen WJ, Vandenbroucke-Grauls CM, Verhoef J, Mooi FR. 1996. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur. J. Clin. Microbiol. Infect. Dis. 15:60–64 [DOI] [PubMed] [Google Scholar]
- 37. Frenay HM, Theelen JP, Schouls LM, Vandenbroucke-Grauls CM, Verhoef J, van Leeuwen WJ, Mooi FR. 1994. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J. Clin. Microbiol. 32:846–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cookson BD, Robinson DA, Monk AB, Murchan S, Deplano A, de Ryck R, Struelens MJ, Scheel C, Fussing V, Salmenlinna S, Vuopio-Varkila J, Cuny C, Witte W, Tassios PT, Legakis NJ, van Leeuwen W, van Belkum A, Vindel A, Garaizar J, Haeggman S, Olsson-Liljequist B, Ransjo U, Muller-Premru M, Hryniewicz W, Rossney A, O'Connell B, Short BD, Thomas J, O'Hanlon S, Enright MC. 2007. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J. Clin. Microbiol. 45:1830–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. David MZ, Taylor A, Lynfield R, Boxrud DJ, Short G, Zychowski D, Boyle-Vavra S, Daum RS. 26 December 2012. Comparing PFGE with MLST, spa typing, SCCmec typing, and PCR for PVL, arcA and opp3 in MRSA isolates at a U.S. medical center. J. Clin. Microbiol. doi:10.1128/JCM.02429-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faria NA, Carrico JA, Oliveira DC, Ramirez M, de Lencastre H. 2008. Analysis of typing methods for epidemiological surveillance of both methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J. Clin. Microbiol. 46:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vindel A, Cuevas O, Cercenado E, Marcos C, Bautista V, Castellares C, Trincado P, Boquete T, Perez-Vazquez M, Marin M, Bouza E. 2009. Methicillin-resistant Staphylococcus aureus in Spain: molecular epidemiology and utility of different typing methods. J. Clin. Microbiol. 47:1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalez BE, Rueda AM, Shelburne SA, III, Musher DM, Hamill RJ, Hulten KG. 2006. Community-associated strains of methicillin-resistant Staphylococcus aureus as the cause of healthcare-associated infection. Infect. Control Hosp. Epidemiol. 27:1051–1056 [DOI] [PubMed] [Google Scholar]
- 43. Lowder BV, Guinane CM, Ben Zakour NL, Weinert LA, Conway-Morris A, Cartwright RA, Simpson AJ, Rambaut A, Nubel U, Fitzgerald JR. 2009. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:19545–19550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Osadebe LU, Hanson B, Smith TC, Heimer R. 13 August 2012. Prevalence and characteristics of Staphylococcus aureus in Connecticut swine and swine farmers. Zoonoses Public Health. [Epub ahead of print.] doi:10.1111/j.1863-2378.2012.01527.x [DOI] [PubMed] [Google Scholar]
- 45. Khanna T, Friendship R, Dewey C, Weese JS. 2008. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 128:298–303 [DOI] [PubMed] [Google Scholar]
- 46. Kehrenberg C, Cuny C, Strommenger B, Schwarz S, Witte W. 2009. Methicillin-resistant and -susceptible Staphylococcus aureus strains of clonal lineages ST398 and ST9 from swine carry the multidrug resistance gene cfr. Antimicrob. Agents Chemother. 53:779–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meemken D, Blaha T, Tegeler R, Tenhagen BA, Guerra B, Hammerl JA, Hertwig S, Kasbohrer A, Appel B, Fetsch A. 2010. Livestock associated methicillin-resistant Staphylococcus aureus (LaMRSA) isolated from lesions of pigs at necropsy in northwest Germany between 2004 and 2007. Zoonoses Public Health 57:e143–e148 [DOI] [PubMed] [Google Scholar]
- 48. Milheirico C, Oliveira DC, de LH. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3374–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larsen AR, Goering R, Stegger M, Lindsay JA, Gould KA, Hinds J, Sorum M, Westh H, Boye K, Skov R. 2009. Two distinct clones of methicillin-resistant Staphylococcus aureus (MRSA) with the same USA300 pulsed-field gel electrophoresis profile: a potential pitfall for identification of USA300 community-associated MRSA. J. Clin. Microbiol. 47:3765–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huijsdens XW, Janssen M, Renders NH, Leenders A, van WP, Van S, van Driel VJK, Morroy G. 2008. Methicillin-resistant Staphylococcus aureus in a beauty salon, the Netherlands. Emerg. Infect. Dis. 14:1797–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]