Abstract
CLSI method M27-A3 is not available for use with dimorphic fungi, such as those of the Paracoccidioides genus. In this study, we developed a microdilution method and added the alamarBlue reagent to test the responses of Paracoccidioides brasiliensis and Paracoccidioides lutzii against amphotericin B and itraconazole antifungals. The test proved to be sensitive, practical, and inexpensive and can be used to monitor the activity of low-growth microorganisms and their response to various drugs.
TEXT
Paracoccidioides brasiliensis and Paracoccidioides lutzii are dimorphic fungi and the etiologic agents of paracoccidioidomycosis, a disease with multiple clinical presentations, prolonged evolution, and high rates of mortality and morbidity (1–4). Until now, a lack of routine tests has made it difficult to determine whether patients who did not respond to treatment were infected with resistant strains (5–8). Amphotericin B (AMB) is still the first-choice drug despite its high toxicity, and itraconazole (ITZ) is effective for the mild and moderate forms of the disease (9, 10). A long course of therapy and sometimes even lifelong secondary prophylaxis are required, especially in AIDS patients, who have a high incidence of relapse (11). A more effective drug with a better safety profile than the currently available drugs would greatly improve the treatment of dimorphic fungal infections (10, 12).
CLSI (Clinical and Laboratory Standards Institute) document M27-A3 has proposed a method for determining the in vitro susceptibility of yeasts to different drugs. However, no standardized method is available for dimorphic fungi because of the difficulty in culturing them. For these reasons, more reliable assays are needed for use with the genus Paracoccidioides. The ability to determine the MICs of different compounds would inform clinicians in the choice of an antifungal agent and the management of therapy, as well as facilitating screens of new molecules for their antifungal activity.
However, the microdilution method used today presents difficulties in visually reading the MIC because there is a lack of significant fungal growth. Nevertheless, this test has been used in studies in the literature (12–15). In contrast, the microplate alamarBlue assay (MABA) has been evaluated by several authors in Aspergillus fumigatus (16), Mycobacterium spp. (17–19), and Trypanosoma cruzi (20). Resazurin, the active ingredient in the MABA (alamarBlue Biosource International, Invitrogen's BioSource division), is permeable, blue, and virtually nonfluorescent, but after entry into viable cells, resazurin is continuously reduced by the cells to resorufin, a highly fluorescent red compound.
The aim of our study was to compare the reference broth macrodilution microplate assay (MMA) and microdilution microplate assay (MMI) methods with the MABA to measure the presence of fungi of the Paracoccidioides genus and the activity of two drugs, AMB and ITZ.
The MMI and MMA were performed according to document M27-A3, and ITZ (Sigma) and AMB (Sigma) were prepared with RPMI 1640 medium (Gibco) supplemented with 2% glucose (RPMI 1640-2% Glc) (4). The final concentrations of ITZ and AMB varied from 1 to 0.002 mg/liter and 0.8 to 0.0015 mg/liter, respectively, after the addition of the inoculums. The antifungal activity was analyzed against the P. brasiliensis phylogenetic species S1 isolates 18 (São Paulo), D03, and 339 (São Paulo), S2 isolate 02 (Venezuela), and PS3 isolate Epm83 (Colombia) and P. lutzii strain 01 (ATCC MYA-826/Goiânia) and isolates EE (Mato Grosso) and 8334MMT (Goiânia). All the strains were isolated from human patients and were maintained in Fava-Netto medium at 37°C, and subcultures were performed every 4 days.
The cells were suspended in sterile saline and allowed to settle for several minutes to eliminate large aggregates, and then the supernatants were collected. The number of viable cells was estimated by staining with Trypan blue and counting with a hemocytometer, and the final concentration was 106 cells/ml. This suspension was diluted 1:50 in sterile saline and 1:20 in RPMI 1640-2% Glc for the MMI test. For the MMA test, the same dilutions were performed, but the first dilution was 1:100. The final inoculum used was 0.5 × 103 to 2.5 × 103 cells/ml after the addition of the antifungal. The plates were incubated at 35°C at 150 rpm for 48 h. After this period, the MABA was employed (according the manufacturer's instructions), and the plates were incubated for an additional 24 h, totaling 72 h for the MIC final reading. The lowest antifungal agent concentration that substantially inhibited the growth of the organism was visually determined at the point at which there was no change in the original blue color of the reagent.
Similar results were obtained when the MMA and MMI were compared for P. brasiliensis 18 (Fig. 1) and P. lutzii 01 (Table 1). The MICs ranged from 1 to 2 dilutions, which are acceptable values according to document M27-A3 (CLSI).
Fig 1.
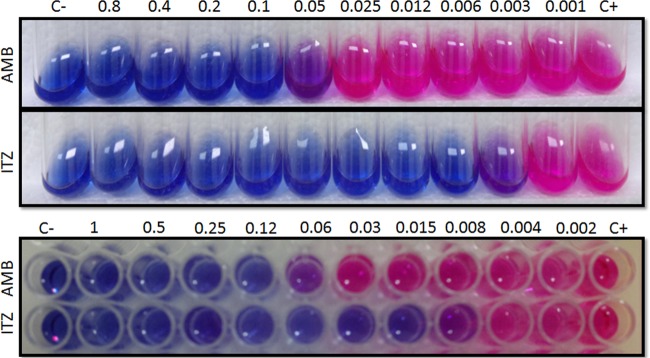
MMA and MMI tests developed with P. brasiliensis 18 against AMB and ITZ. The MIC values were determined after the addition of alamarBlue.
Table 1.
Comparison of MMA and MMI for isolates of two Paracoccidioides species
| Drug | MIC (mg/liter) in indicated assay for: |
|||
|---|---|---|---|---|
|
P. brasiliensis phylogenetic species isolate 18 |
P. lutzii strain 01 |
|||
| MMA | MMI | MMA | MMI | |
| AMB | 0.05 | 0.06 | 0.1 | 0.06 |
| ITZ | 0.003 | 0.008 | 0.001 | 0.004 |
The visual readings of MICs are easily performed, and the MIC values obtained by both methods are equivalent. Additionally, these values are similar to those described in the literature (12–15, 21–26).
There are several studies using MMA with the Paracoccidioides genus, and these generally follow the method suggested by Shadomy et al. (21–23, 27) or that suggested by the NCCLS standardized method in document M27-A (24, 25). The problem with this method is the high cost due to the need for larger amounts of culture medium and other reagents and materials. Also, there are in the literature studies (12–15) of MMI with the Paracoccidioides genus; however, they used larger final inoculums than those recommended in document M27-A3. This approach improved the ability to identify the MIC in the slow-growing fungus but is a significant modification in relation to the methods recommended in document M27-A3. Therefore, we followed the CLSI standardized method in document M27-A3, and the use of MABA made the MMI more sensitive and reliable, allowing the detection of fewer than 50 viable cells per well.
The use of MABA can replace the MFC (minimum fungicide concentration) test that is inconclusive in most cases because the long incubation of the plate results in dehydration of the solid medium and the consequent death of cells which might have been viable, leading to false-negative results. Similarly, the MIC could be considered equivalent to the MFC because it is a sensitive method to assay the viability of fungal cells.
Thus, the MMI/MABA has been shown to be a reliable and reproducible test for this genus. The test was extended to different isolates of the Paracoccidioides genus, confirming that this methodology can be applied for a wide range of isolates (Table 2). Comparing the MIC values determined for the antifungals against different isolates shows that MIC values for ITZ and AMB were within the expected ranges found in the literature (12–15). The MIC values varied independently of the Paracoccidioides species from 0.008 to 0.25 mg/liter for AMB and 0.008 to 0.03 mg/liter for ITZ.
Table 2.
MICs of AMB and ITZ in Paracoccidioides MABA test
| Drug | MIC (mg/liter) for indicated isolate or strain |
|||||||
|---|---|---|---|---|---|---|---|---|
|
P. lutzii |
P. brasiliensis phylogenetic species |
|||||||
| Strain 01-like isolate |
Strain 01 | S1 isolate |
S2 isolate 02 | PS3 isolate Epm83 | ||||
| EE | 8334MMT | 18 | D03 | 339 | ||||
| AMB | 0.25 | 0.25 | 0.06 | 0.12 | 0.008 | 0.06 | 0.12 | 0.06 |
| ITZ | 0.008 | 0.03 | 0.008 | 0.015 | 0.015 | 0.015 | 0.008 | 0.015 |
Thus, the Paracoccidioides MABA test is reliable, reproducible, quick, and an accurate tool for routine laboratory testing.
ACKNOWLEDGMENTS
This work was financially supported by the Brazilian organizations FAPESP, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and PADCFCF-UNESP. A. C. A. de Paula e Silva has a fellowship from CAPES.
We are very grateful to Eduardo Bagagli from the Biosciences Institute of Botucatu, UNESP—Universidade Estadual Paulista, for kindly providing the Paracoccidioides isolates used in this study.
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Bagagli E, Bosco SM, Theodoro RC, Franco M. 2006. Phylogenetic and evolutionary aspects of Paracoccidioides brasiliensis reveal a long coexistence with animal hosts that explain several biological features of the pathogen. Infect. Genet. Evol. 6:344–351 [DOI] [PubMed] [Google Scholar]
- 2. Bagagli E, Theodoro RC, Bosco SM, McEwen JG. 2008. Paracoccidioides brasiliensis: phylogenetic and ecological aspects. Mycopathologia 165:197–207 [DOI] [PubMed] [Google Scholar]
- 3. Matute DR, Sepulveda VE, Quesada LM, Goldman GH, Taylor JW, Restrepo A, McEwen JG. 2006. Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. J. Clin. Microbiol. 44:2153–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. 2009. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 52:273–283 [DOI] [PubMed] [Google Scholar]
- 5. Coutinho ZF, Silva D, Lazera M, Petri V, Oliveira RM, Sabroza PC, Wanke B. 2002. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad. Saude Publica 18:1441–1454 [DOI] [PubMed] [Google Scholar]
- 6. Lacaz CS. 1994. Paracoccidioides brasiliensis: morphology, evolutionary cycle, maintenance during saprophytic life, biology, virulence, taxonomy, p 13–25 In Franco M, Lacaz CS, Restrepo-Moreno A, Del Negro A. (ed), Paracoccidioidomycosis, CRC Press, Boca Raton, FL. [Google Scholar]
- 7. Ribeiro LC, Hahn RC, Favalessa OC, Tadano T, Fontes CJ. 2009. Systemic mycosis: factors associated with death among patients infected with the human immunodeficiency virus, Cuiabá, State of Mato Grosso, Brazil, 2005-2008. Rev. Soc. Bras. Med. Trop. 42:698–705 (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 8. San-Blas G, Mariño L, San-Blas F, Apitz-Castro R. 1993. Effect of ajoene on dimorphism of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 31:133–141 [DOI] [PubMed] [Google Scholar]
- 9. Franco M, Montenegro MR, Mendes RP, Marques SA, Dillon NL, Mota NG. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev. Soc. Bras. Med. Trop. 20:129–132 [DOI] [PubMed] [Google Scholar]
- 10. Shikanai-Yasuda MA, Fe Telles Filho Q, Mendes RP, Colombo AL, Moretti ML. 2006. Guidelines in paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 39:297–310 [DOI] [PubMed] [Google Scholar]
- 11. Morejón KM, Machado AA, Martinez R. 2009. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: a case-control study. Am. J. Trop. Med. Hyg. 80:359–366 [PubMed] [Google Scholar]
- 12. Nakai T, Uno J, Ikeda F, Tawara S, Nishimura K, Miyaji M. 2003. In vitro antifungal activity of micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob. Agents Chemother. 47:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Espinel-Ingroff A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam Micol. 20:121–136 [PubMed] [Google Scholar]
- 14. Johann S, Cisalpino PS, Watanabe GA, Cota BB, de Siqueira EP, Pizzolatti MG, Zani CL, de Resende MA. 2010. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against the pathogenic fungus Paracoccidioides brasiliensis. Pharm. Biol. 48:388–396 [DOI] [PubMed] [Google Scholar]
- 15. Johann S, Sá NP, Lima LA, Cisalpino PS, Cota BB, Alves TM, Siqueira EP, Zani CL. 2010. Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis. Ann. Clin. Microbiol. Antimicrob. 9:30 doi:10.1186/1476-0711-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monteiro MC, de la Cruz M, Cantizani J, Moreno C, Tormo JR, Mellado E, De Lucas JR, Asensio F, Valiante V, Brakhage AA, Latgé JP, Genilloud O, Vicente F. 2012. A new approach to drug discovery: high-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J. Biomol. Screen. 17:542–549 [DOI] [PubMed] [Google Scholar]
- 17. Collins LA, Franzeblau SG. 1997. Microplate Alamar Blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dixit P, Singh U, Sharma P, Jain A. 2012. Evaluation of nitrate reduction assay, resazurin microtiter assay and microscopic observation drug susceptibility assay for first line antitubercular drug susceptibility testing of clinical isolates of M. tuberculosis. J. Microbiol. Methods 88:122–126 [DOI] [PubMed] [Google Scholar]
- 19. Yajko DM, Madej JJ, Lancaster MV, Sanders CA, Cawthon VL, Gee B, Babst A, Hadley WK. 1995. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J. Clin. Microbiol. 33:2324–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rolón M, Vega C, Escario JA, Gómez-Barrio A. 2006. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 99:103–107 [DOI] [PubMed] [Google Scholar]
- 21. Hahn RC, Fontes CJ, Batista RD, Hamdan JS. 2002. In vitro comparison of activities of terbinafine and itraconazole against Paracoccidioides brasiliensis. J. Clin. Microbiol. 40:2828–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hahn RC, Hamdan JS. 2000. In vitro susceptibilities of Paracoccidioides brasiliensis yeast form to antifungal drugs. Mycoses 43:403–407 [PubMed] [Google Scholar]
- 23. Hahn RC, Morato Conceição YT, Santos NL, Ferreira JF, Hamdan JS. 2003. Disseminated paracoccidioidomycosis: correlation between clinical and in vitro resistance to ketoconazole and trimethoprim sulphamethoxazole. Mycoses 46:342–347 [DOI] [PubMed] [Google Scholar]
- 24. McGinnis MR, Pasarell L, Sutton DA, Fothergill AW, Cooper CR, Rinaldi MG. 1997. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob. Agents Chemother. 41:1832–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodero L, Canteros CE, Rivas C, Lee W, Davel G. 1999. In vitro sensitivity of Paracoccidioides brasiliensis to systemically used antifungal agents. Rev. Argent. Microbiol. 31:78–81 (In Spanish.) [PubMed] [Google Scholar]
- 26. Takahagi-Nakaira E, Sugizaki MF, Peraçoli MTS. 2009. Microdilution produce for antifungal susceptibility testing of Paracoccidioides brasiliensis to amphotericin B and itraconazole. J. Venom. Anim. Toxins Incl. Trop. Dis. 15:719 http://dx.doi.org/10.1590/S1678-91992009000400010 [Google Scholar]
- 27. Shadomy S, Espinel-Ingroff A, Cartwright RY. 1987. Estudios de laboratório com agents antifungicos: pruebas de susceptibilidade y bioensayos, p 1229–1238 In Lenette EH, Balows A, Hausler WJ, Shadomy HJ. (ed), Manual de microbiologia clínica, 4th ed Editorial Medica Panamericana, Buenos Aires, Argentina [Google Scholar]


