Abstract
The limitations of conventional methods of identification of Mycobacterium tuberculosis have led to the development of several nucleic acid amplification techniques which have the advantage of being rapid, sensitive, and specific. However, their expense or the need for technical expertise makes it difficult to use them in regions in which tuberculosis is endemic. A novel PCR restriction analysis (PRA) of the hsp65 gene was therefore developed for rapid screening of clinical isolates to identify Mycobacterium spp. The restriction enzymes NruI and BamHI were selected to obtain a limited number of restriction patterns to further differentiate between Mycobacterium tuberculosis complex (MTBC) and nontuberculous mycobacteria (NTM). Three hundred ten isolates from clinical specimens and 24 reference strains were tested. The assay correctly identified 295 of the 310 culture isolates as MTBC, while the remaining 15 isolates were identified as NTM. Of the isolates tested, 135 MTBC strains and all 15 NTM were also confirmed by PRA using Sau96I and CfoI. Thirty-eight randomly selected MTBC strains and all 15 NTM were further confirmed by sequencing. The NruI/BamHI PRA was simple, as it did not require any elaborate analyses. It was cost-effective, rapid, highly sensitive, and specific and did not require technical expertise. The assay can, therefore, be used as a simple screening test not only to detect Mycobacterium spp. but also to differentiate MTBC from NTM in peripheral laboratories with minimal availability of funds.
INTRODUCTION
Tuberculosis is a major cause of death due to a single infectious agent. Early diagnosis and treatment of tuberculosis would not only improve patient outcome but also help control this disease by reducing transmission. Unfortunately, conventional methods of bacteriological diagnosis of tuberculosis are time-consuming and labor-intensive. Moreover, although Mycobacterium tuberculosis is the most important Mycobacterium species from a public health perspective, nontuberculous mycobacteria (NTM) are increasingly being reported in various parts of the world. Though NTM are ubiquitous organisms, normally found in the environment (1), several species are pathogenic to humans. Hence, rapid identification of Mycobacterium spp. is imperative for appropriate diagnosis and treatment, especially in immunocompromised individuals. However, laboratories in peripheral areas, especially in developing countries, are still not able to differentiate between M. tuberculosis and NTM. Routinely, identification of mycobacteria is achieved by their growth characteristics in culture (viz. colony morphology, rate of growth, and pigmentation) and biochemical tests, which are often inconclusive (2, 3).
The quest for rapid identification techniques has led to the development of several genotypic techniques. Direct gene sequencing (4, 5), though highly specific in discriminating between mycobacteria, is usually used in reference laboratories (6). Though a number of probe-based assays have been introduced (7, 8, 9), the high costs of commercially available assays, such as AccuProbe (Gen-Probe Inc., San Diego, CA) and Inno-LiPA Mycobacteria v2 (Innogenetics N.V., Ghent, Belgium), have restricted their large-scale use in most clinical laboratories, especially in high-burden countries in which tuberculosis is endemic (6).
PCR restriction analysis (PRA), a simple, easy-to-read, reproducible, and rapid molecular technique, has been used in recent years for identification of Mycobacterium species. PRA techniques have been developed for several mycobacterial genes, such as hsp65 (10, 11), the 16S-23S rRNA gene spacer (12, 13), and rpoB (6, 14). However, most of these techniques require the use of an algorithm to identify the Mycobacterium species. Also, the formation of short restrictions or small differences between bands requires the use of NuSieve or MetaPhor agarose, both of which are expensive (10, 15, 16), or polyacrylamide gel, which is difficult to handle, thus making the assay technically demanding. We aimed to develop a simple PRA technique to rapidly identify M. tuberculosis complex (MTBC) and to further differentiate it from NTM. The assay does not require technical expertise and can be used as a screening assay in diagnostic laboratories in areas of tuberculosis endemicity.
MATERIALS AND METHODS
Clinical isolates.
A total of 310 clinical isolates were obtained from patients suspected of pulmonary tuberculosis who were admitted to the Rajan Babu Institute of Respiratory Medicine and Tuberculosis (RBIPMT), Delhi, and Vallabhbhai Patel Chest Institute (VPCI) during the time period of 2007 to 2010. Of the 310 isolates, 250 were obtained from RBIPMT, which serves as a referral center for patients of tuberculosis in North India, while 60 were obtained from VPCI, which serves as a referral hospital in North India for chest diseases. The patients were adults ≥18 years old who were not coinfected with HIV. The study was approved by the institutional ethical committee, and written and informed consent was taken from the patients. The clinical isolates were subjected to biochemical identification using niacin, nitrate reduction, and semiquantitative catalase tests by the standard procedure (2).
Reference strains.
In addition to the 310 clinical isolates, 24 reference strains were also included in the study. The reference strains used were Mycobacterium tuberculosis (H37Rv), Mycobacterium bovis (ATCC 19210T), Mycobacterium microti (ATCC 25584), Mycobacterium avium (MTCC, IMTECH, Chandigarh, India), Mycobacterium intracellulare (ATCC 13950), Mycobacterium gordonae (ATCC 14470), Mycobacterium fortuitum (ATCC 6841), Mycobacterium kansasii (ATCC 21982), Mycobacterium phlei (ATCC 11758), Mycobacterium smegmatis (ATCC 19420), Mycobacterium terrae (ATCC 15755), Mycobacterium vaccae (ATCC 15483), Mycobacterium malmoense (ATCC 29571), Mycobacterium xenopi (ATCC 19250), Mycobacterium simiae (ATCC 25275T), Nocardia brasiliensis (ATCC 19296), Nocardia asteroides (ATCC 19247), Escherichia coli (ATCC 35218), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (ATCC 33348), Enterococcus faecalis (ATCC 19433), Staphylococcus aureus (ATCC 25923), Haemophilus influenzae (ATCC 35891), and Streptococcus pneumoniae (ATCC BAA-255).
DNA extraction from cultures and spiked sputum sample.
Chromosomal DNA was extracted from 310 clinical mycobacterial isolates, the reference strain H37Rv, and 14 reference nontuberculous mycobacterial strains by the cetyltrimethylammonium bromide (CTAB) method as described previously (17). DNA was extracted from cultures of bacteria other than Mycobacterium spp. (n = 9) by boiling. Briefly, a loopful of mycobacterial growth was transferred to a microcentrifuge tube containing 100 μl of 1% Triton X-100 and 50 μl of sterile double-distilled water. The suspension was vortexed and boiled at 100°C for 30 min. The suspension was centrifuged at 8,000 rpm for 10 min, and the clear supernatant containing mycobacterial DNA was taken for PCR.
A smear-negative sputum sample was spiked with serial dilutions of H37Rv to test the lower limit of detection with the assay. DNA was extracted from the spiked sputum samples as described earlier (18).
Development of the assay.
Sequences of 34 mycobacterial species were obtained from GenBank (www.ncbi.nlm.nih.gov/GenBank) (Table 1), and target (HSP N3, 5′-AAGAAGTGGGGTGCCCCC-3′) and antisense (HSP N4, 5′-CTTGGTCTCGACCTCCTTG-3′) primers specific for hsp65 of the mycobacterial species were designed. Restriction enzymes compatible with the 300-bp region of the hsp65 gene of MTBC were screened by the BioEdit software so that the restricted bands were large enough to be visualized on agarose gel. A search for restriction enzymes was made to obtain only two bands through digestion with the enzymes to differentiate between MTBC and NTM. The restriction enzymes NruI and BamHI were selected for further experiments since both enzymes restricted the 300-bp region of the hsp65 gene of MTBC whereas the NTM were restricted by either NruI or BamHI but not both.
Table 1.
Strains of Mycobacterium spp. used as sources of hsp65 sequences for in silico analyses of the restriction enzymes NruI and BamHI
| Mycobacterium organism | Strain |
|---|---|
| M. tuberculosis | BX842573 |
| M. bovis | BX248333 |
| M. bovis BCG | CP002095 |
| M. canettii | NC_015848 |
| M. gastri | U17931 |
| M. scrofulaceum | U17955 |
| M. asiaticum | U17921 |
| M. malmoense | U17948 |
| M. genavense | U17932 |
| M. gordonae | U17933 |
| M. shimoidei | U17956 |
| M. xenopi | U17959 |
| M. neoaurum | U17950 |
| M. nonchromogenicum | U17951 |
| M. ulcerans | U34034 |
| M. habana | AF129011 |
| M. fortuitum | AF140677 |
| M. chelonae | AF071142 |
| M. fallax | U17930 |
| M. agri | U17920 |
| M. rhodesiae | U17954 |
| M. vaccae | U17958 |
| M. chitae | U17929 |
| M. senegalense | AF071137 |
| M. peregrinum | AF071136 |
| M. mucogenicum | AF071135 |
| M. confluentis | AF071132 |
| M. brumae | AF071129 |
| M. pulveris | U17953 |
| M. abscessus | AF071139 |
| M. terrae | AF257468 |
| M. simiae | AF247570 |
| M. avium | U17922, AF281650, AF126032, U17922, U85632, AF234261, U17922, AF126031, AF126033, AF126030 |
| M. intracellulare | U85637, U55828, U85638, U85638, U55830, U85636, U85635, U17944, AF126035, U55829, AF126034, U85633, U17943 |
| M. smegmatis | AJ307653, AF547876, AY299161 |
| M. kansasii | U17947 |
| M. marinum | NC_010612, CP000854 |
(i) PCR amplification.
Amplification of 310 clinical isolates of Mycobacterium spp., 24 reference strains, and a sputum sample spiked with various concentrations of H37Rv was performed in an Eppendorf Mastercycler with the primer set HSP N3 and HSP N4, amplifying a 300-bp region of the hsp65 gene. A total of 5 μl of the extracted DNA or 7 μl of the supernatant collected from processing the spiked sputum sample was used as the template for PCR. The 30-μl PCR mix consisted of 10 pM each primer, 200 μM each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 0.8 U Taq polymerase (Biotools, B&M Labs, Madrid, Spain). The thermal profile consisted of an initial denaturation for 10 min at 94°C, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and primer extension at 72°C for 1 min. Final extension was performed at 72°C for 10 min. The PCR product was analyzed by electrophoresis on a 1.5% agarose gel, with a 50-bp DNA ladder (Fermentas Life Sciences, Lithuania) used as a marker.
(ii) Positive and negative controls used in the PCR assay.
Each PCR run contained a positive control (H37Rv DNA) and a negative control (double-distilled water). For PCR directly from spiked sputum samples, the samples were also tested for the presence of the human β-actin gene as described earlier (19). This served as an internal control in each PCR.
(iii) Restriction analysis with NruI and BamHI.
Restriction digestion of the 300-bp product, obtained from PCR of all the clinical isolates, the reference strains, and the spiked sputum sample, was carried out with 1 U each of NruI and BamHI (Fermentas Life Sciences, Lithuania) in separate tubes. Restriction digestion was carried out with 10 μl of the amplicon at 37°C for 2 h. The products were electrophoresed on 2.5% agarose gel with a 50-bp DNA marker (Fermentas Life Sciences, Lithuania).
PRA using Sau96I and CfoI.
To test the efficacy of the assay and confirm the test developed by us, 150 clinical mycobacterial isolates were also tested by PRA using the enzymes Sau96I and CfoI as previously described (10). Sau96I/CfoI PRA was also able to further identify the isolated NTM to the species level.
Sequencing.
PCR products obtained by amplification of the hsp65 gene with the primers HSP N3 and HSP N4 of a subset of clinical isolates (n = 53) were subjected to sequencing by an ABI automated sequencer (Ocimum Biosolutions, Bangalore, India). Sequences were identified by similarity using BLASTN, available at NCBI (www.blast.ncbi.nlm.nih.gov/blast.cgi). Species identification was confirmed if a 97% match was achieved with any sequence deposited in the database according to the criteria proposed by McNabb et al. (20).
Sensitivity of the assay when used directly on clinical samples.
With the intent to identify M. tuberculosis directly in clinical specimens, we wanted to estimate the lower limit of detection of the assay using the primers HSP N3 and HSP N4. The sensitivity of the assay was tested on a smear-negative sputum sample obtained from a patient not suffering from tuberculosis. The sample was spiked with serial dilutions of H37Rv (21) to obtain a final concentration of 100, 101, 102, 103, 104, 105, or 106 organisms/μl. DNA was extracted from the spiked sputum samples as described above. The suspensions were subjected to PCR with the primer set HSP N3 and HSP N4. PRA was performed on the amplicons obtained with the restriction enzymes NruI and BamHI as described previously.
Quality control.
Quality laboratory practices were strictly followed at every step. Sterile distilled water was used for reagent preparation. Positive and negative controls were included with every new batch of medium and reagents.
Statistical analysis.
Kappa values were determined to find out the agreement between NruI/BamHI and Sau96I/CfoI assays by using GraphPad software (GraphPad, La Jolla, CA).
RESULTS
Identification of mycobacteria by biochemical reactions.
All the clinical isolates were subjected to biochemical identification by niacin, nitrate reduction, and semiquantitative catalase tests. Of the 310 clinical isolates, 236 were identified as M. tuberculosis whereas 8 isolates were identified as NTM. However, 66 isolates were not able to be identified definitively on the basis of biochemical reactions.
Identification of mycobacteria by hsp65 PRA using the restriction enzymes NruI and BamHI.
The DNA obtained from 310 cultures of Mycobacterium spp. and 15 mycobacterial reference strains, including H37Rv, was used to amplify a 300-bp region of the hsp65 gene using the primers HSP N3 and HSP N4. All mycobacterial isolates were amplified by the primers. PCR with the primers HSP N3 and HSP N4 was also carried out with all the nonmycobacterial reference strains (viz. Nocardia brasiliensis, Nocardia asteroides, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae) obtained from ATCC and MTCC (IMTECH, Chandigarh). However, none of the nonmycobacterial species were amplified.
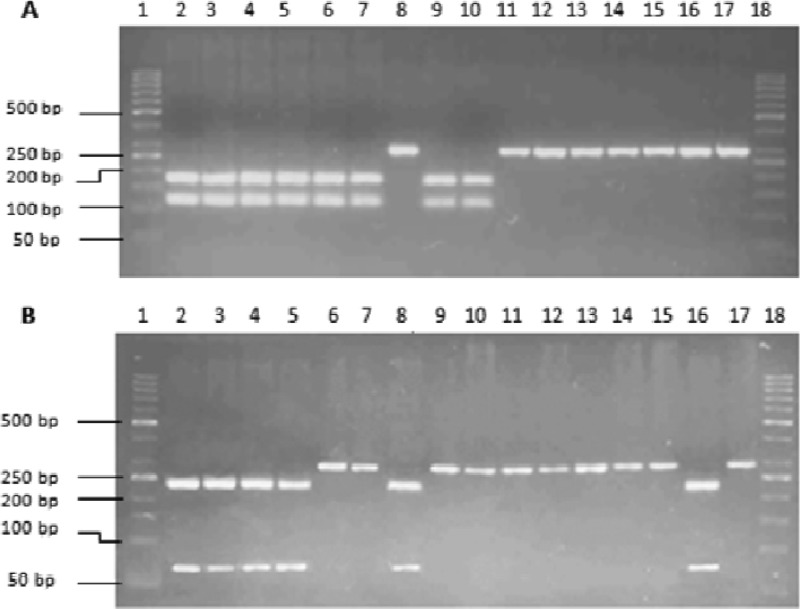
Restriction enzymes compatible with the 300-bp region of hsp65 were screened by the BioEdit software. The enzyme NruI was found to restrict the amplified sequences of M. tuberculosis complex at TCG/CGA, giving two bands of 121 bp and 179 bp (Fig. 1A), while BamHI restricted the same sequences at G/GATCC, giving two bands of 66 bp and 234 bp (Fig. 1B).
Fig 1.
(A) Digestion of PCR amplicons of reference strains of Mycobacterium spp. with NruI. Lane 1, 50-bp DNA marker. Lanes 2 to 17, restriction digests of H37Rv, M. bovis, M. bovis BCG, M. microti, M. smegmatis, M. simiae, M. xenopi, M. vaccae, M. malmoense, M. kansasii, M. fortuitum, M. avium, M. intracellulare, M. gordonae, M. phlei, and M. terrae, respectively. Lane 18, 50-bp marker. (B) Digestion of PCR amplicons of reference strains of Mycobacterium spp. with BamHI. Lane 1, 50-bp DNA marker. Lanes 2 to 17, restriction digests of H37Rv, M. bovis, M. bovis BCG, M. microti, M. smegmatis, M. simiae, M. xenopi, M. vaccae, M. malmoense, M. kansasii, M. fortuitum, M. avium, M. intracellulare, M. gordonae, M. phlei, and M. terrae, respectively. Lane 18, 50-bp marker.
We labeled the restricted pattern with NruI “A” and the unrestricted pattern “a.” The restricted pattern of BamHI was labeled “B,” and the unrestricted pattern was labeled “b” (Table 2). M. tuberculosis, M. bovis, and M. microti were the only mycobacterial species among all the species tested which were restricted by both NruI and BamHI (restriction pattern “AB”) (Table 2). Of all the clinical culture isolates tested, 295 were identified as M. tuberculosis complex by NruI/BamHI PRA. The 295 MTBC strains thus identified included 59 of the 66 isolates that had not been identified definitively on the basis of biochemical reactions. The remaining 7/66 isolates that were not able to be identified definitively by conventional methods were determined to be NTM by NruI/BamHI PRA.
Table 2.
Restriction pattern of reference strains of Mycobacterium spp. using the restriction enzymes NruI and BamHI
| Mycobacterium sp. | Restriction patterna |
|
|---|---|---|
| NruI | BamHI | |
| H37Rv | A | B |
| M. bovis | A | B |
| M. microti | A | B |
| M. smegmatis | A | b |
| M. simiae | A | b |
| M. xenopi | a | B |
| M. vaccae | A | b |
| M. malmoense | A | b |
| M. kansasii | a | b |
| M. fortuitum | a | b |
| M. avium | a | b |
| M. intracellulare | a | b |
| M. gordonae | a | b |
| M. phlei | a | B |
| M. terrae | a | b |
A, restricted pattern of NruI with bands of 121 bp and 179 bp; a, unrestricted pattern of NruI; B, restricted pattern of BamHI with bands of 66 bp and 234 bp; b, unrestricted pattern of BamHI.
Testing the efficacy of NruI/BamHI PRA.
To test the ability of PRA using the restriction enzymes NruI and BamHI to correctly differentiate between MTBC and NTM, 150 clinical isolates were also subjected to PRA using the enzymes Sau96I and CfoI. These included the 66 isolates that had not been identified on the basis of biochemical reactions alone. All the isolates identified as NTM (n = 15) by NruI/BamHI PRA were subjected to Sau96I/CfoI PRA. Of the 150 mycobacterial isolates, 135 were identified as MTBC by both NruI/BamHI PRA and Sau96I/CfoI PRA. Of the 15 isolates identified as NTM using the enzymes NruI and BamHI, all were confirmed as NTM using Sau96I and CfoI (Table 3).
Table 3.
Comparison between PRA for the NTM isolated using NruI/BamHI and Sau96I/CfoIa
| Strain | Result by: |
||
|---|---|---|---|
| NruI/BamHI method (present assay) | Sau96I/CfoI method (10) | Sequencingb | |
| RB 1 | NTM | M. fortuitum | M. fortuitum |
| RB 2 | NTM | M. intracellulare | M. intracellulare |
| RB 3 | NTM | M. intracellulare | 93% M. avium/93% M. intracellulare |
| RB 4 | NTM | M. intracellulare | 92% M. avium/92% M. intracellulare |
| RB 5 | NTM | M. avium | M. avium |
| RB 6 | NTM | M. intracellulare | M. intracellulare |
| RB 7 | NTM | M. avium | 96% M. avium/96% M. intracellulare |
| RB 8 | NTM | M. intracellulare | M. intracellulare |
| RB 9 | NTM | M. fortuitum | M. fortuitum |
| RB 10 | NTM | M. fortuitum | M. fortuitum |
| VP 11 | NTM | M. abscessus | M. abscessus |
| VP 12 | NTM | M. intracellulare | M. intracellulare |
| VP 13 | NTM | M. intracellulare | M. intracellulare |
| VP 14 | NTM | M. abscessus | M. abscessus |
| VP 15 | NTM | M. abscessus | M. abscessus |
The restriction pattern using NruI was a for all strains, and the restriction pattern using BamHI was b for all strains.
Percent identification was ≥97% unless otherwise specified.
Sequencing.
Of the 53 isolates sequenced, 38 isolates, selected randomly, had been restricted by both NruI and BamHI and were identified as M. tuberculosis by sequencing, thus confirming our results. These 38 isolates also included 12 of the 66 isolates which had not been identified biochemically but had been identified as M. tuberculosis by PRA. Of the remaining 15 clinical isolates sequenced, all had been determined to be NTM by PRA using NruI/BamHI as well as Sau96I/CfoI, although 7 of these isolates had not been identified by conventional methods. The isolates were identified as M. avium/M. intracellulare (n = 9), M. fortuitum (n = 3), or Mycobacterium abscessus (n = 3) by sequencing (Table 3). The NruI/BamHI assay was thus 100% concordant with sequencing (Kappa value = 1).
Sensitivity of the assay.
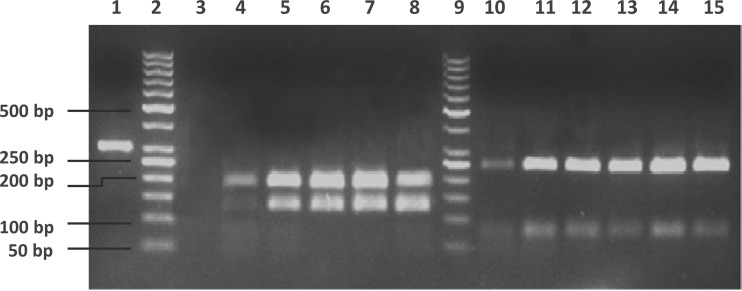
To detect the sensitivity of PRA using the primers HSP N3 and HSP N4 and the enzymes NruI and BamHI directly on sputum samples, DNA was extracted from serial dilutions of H37Rv used to spike a smear-negative sputum sample to obtain a final concentration of 100, 101, 102, 103, 104, 105, or 106 organisms/μl and used as the template for hsp65 PCR. PRA was performed on the amplicons obtained with the restriction enzymes NruI and BamHI. The lower limit of identification with BamHI was 10 organisms/μl; however, a reliable PCR restriction analysis with both NruI and BamHI was only possible down to a concentration of 102 organisms/μl (Fig. 2).
Fig 2.
NruI and BamHI digests of amplicons from the PCR conducted on serial dilutions of H37Rv in a spiked sputum sample with the HSP N3 and N4 primers. No PCR amplicon was obtained with the spiked specimen with 100 organisms/μl. Lane 1, undigested H37Rv amplicon. Lanes 2 and 9, 50-bp marker. Lanes 3 to 8, NruI digests of PCR amplicons from a smear-negative sputum sample spiked with 101, 102, 103, 104, 105, or 106 organisms/μl, respectively. Lanes 10 to 15, BamHI digests of PCR amplicons from a smear-negative sputum sample spiked with 101, 102, 103, 104, 105, or 106 organisms/μl, respectively.
DISCUSSION
Accurate detection of Mycobacterium tuberculosis in clinical specimens may provide significant advantages for control of tuberculosis. Moreover, though the number of NTM isolated in clinical specimens has increased over the years, laboratories in peripheral areas, especially in developing countries, are still not able to differentiate between M. tuberculosis and NTM. Clinical microbiologists in these laboratories often presumptively identify cultures of M. tuberculosis on the basis of colony characteristics on culture, which may show variations in morphology. Even laboratories sufficiently equipped to perform biochemical assays are not able to make a definitive diagnosis due to interassay variations and inherent limitations of phenotypic methods in an accurate identification of NTM (22). This was also illustrated in the present study, in which biochemical tests were inconclusive for 21% of isolates.
Commercially available molecular techniques, widely used in developed countries for definite identification, are expensive and not used in resource-poor countries and peripheral laboratories. PRA is simple to perform, easy to read, and reproducible and is used in several laboratories to identify NTM. PRA has been developed to target mycobacterial genes, such as hsp65, the 16S-23S rRNA gene spacer region, and rpoB (10, 12, 14). Most PRA techniques used, however, are impeded by difficulties such as minor differences in band sizes between species, the occurrence of new patterns which have not been reported earlier (10), and the need to use elaborate algorithms to identify species. We therefore used an hsp65 PRA which would not require an algorithm and would produce band sizes large enough to be differentiated on agarose. Of the 310 culture isolates tested by NruI/BamHI PRA, 295 were identified as MTBC.
The 15 NTM were further analyzed by Sau96I/CfoI (Table 3) to verify the results obtained with NruI/BamHI PRA and to identify them to the species level. The most common NTM identified was M. avium/M. intracellulare (60%). Sau96I/CfoI PRA was able to further identify them as M. avium (13.3%) and M. intracellulare (46.6%). The NTM M. fortuitum and M. abscessus were isolated from 3 (20%) samples each.
The protocol using Sau96I and CfoI had the advantage of being able to provide species identification for the most commonly encountered mycobacteria (10). However, the assay was technically demanding and needed to be visualized on polyacrylamide gel to be able to identify small bands clearly. An imaging system was required to visualize the bands, as they then had to be analyzed by an extensive algorithm to identify various NTM (10). The protocol using NruI and BamHI that we developed needed only an agarose gel, was more rapid, and did not require an algorithm to differentiate between NTM and M. tuberculosis complex. If the amplicon was restricted by both NruI and BamHI, the isolate was identified as MTBC. Since no other mycobacterial species were restricted by both these enzymes, the identification of MTBC was easy. The NTM detected were then taken up for further analysis by techniques which can differentiate between the various species or by sequencing. This reduced the workload and time for detection, as MTBC species were able to be identified within 5 h of obtaining a positive culture.
The assay was 100% sensitive and specific when performed on culture isolates, as it was able to correctly identify 295 of the 310 isolates as M. tuberculosis complex and the remaining 15 isolates as NTM. The results of 150 clinical isolates were confirmed by the hsp65 PRA developed by Wong et al. (10), and the results of 53 isolates were confirmed by sequencing, which served as the gold standard. Moreover, none of the reference nonmycobacterial strains were amplified by HSP N3 and HSP N4.
Our final aim is to be able to use NruI/BamHI PRA directly on clinical specimens. Hence, we tested the sensitivity of the assay on a smear-negative sputum sample spiked with serial dilutions of H37Rv. The sensitivity of the assay in detecting mycobacteria directly was found to be 102 organisms/μl. In the future, we wish to apply PRA to the direct investigation of smear-positive and smear-negative clinical samples.
To conclude, the protocol we designed was sensitive and specific. In addition, it was cost-effective and simple and was able to be used as a rapid screening assay for MTBC. The drawback of the assay was that it was not able to further identify the NTM. However, the assay was not intended to be used to differentiate between the different mycobacterial species. It was devised to overcome the basic problem of differentiating between M. tuberculosis and NTM definitively using a simple and rapid approach so that it can be used in peripheral laboratories by laboratory personnel with minimum training.
ACKNOWLEDGMENTS
This work was supported by a grant from the Indian Council of Medical Research.
There are no conflicts of interest to declare.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Shojaei H, Heidarieh P, Hashemi A, Feizabadi MM, Daei Naser A. 2011. Species identification of neglected non-tuberculous mycobacteria in a developing country. Jpn. J. Infect. Dis. 64:265–271 [PubMed] [Google Scholar]
- 2. Kent PT, Kubica GP. 1985. A guide for the level III laboratory. Centers for Disease Control, Atlanta, GA [Google Scholar]
- 3. Springer B, Stockman L, Teschner K, Roberts GD, Bottger EC. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J. Clin. Microbiol. 34:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, Gaillard JL, Pierre-Audigier C. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adékambi T, Drancourt M. 2004. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 54:2095–2105 [DOI] [PubMed] [Google Scholar]
- 6. Ong CS, Ngeow YF, Yap SF, Tay ST. 2010. Evaluation of PCR-restriction analysis targeting hsp65 and rpoB genes for the typing of mycobacterial isolates in Malaysia. J. Med. Microbiol. 59:1311–1316 [DOI] [PubMed] [Google Scholar]
- 7. Reisner BS, Gatson AM, Woods GL. 1994. Use of Gen-Probe AccuProbes to identify Mycobacterium avium complex, Mycobacterium tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J. Clin. Microbiol. 32:2995–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kox LF, Jansen HM, Kuijper S, Kolk AH. 1997. Multiplex PCR assay for immediate identification of the infecting species in patients with mycobacterial disease. J. Clin. Microbiol. 35:1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tortoli E, Mariottini A, Mazzarelli G. 2003. Evaluation of INNO-LiPA MYCOBACTERIA v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J. Clin. Microbiol. 41:4418–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong DA, Yip PC, Cheung DT, Kam KM. 2001. Simple and rational approach to the identification of Mycobacterium tuberculosis, Mycobacterium avium complex species, and other commonly isolated mycobacteria. J. Clin. Microbiol. 39:3768–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dziadek J, Sajduda A, Boruń TM. 2001. Specificity of insertion sequence-based PCR assays for Mycobacterium tuberculosis complex. Int. J. Tuberc. Lung Dis. 5:569–574 [PubMed] [Google Scholar]
- 12. Roth A, Reischl U, Streubel A, Naumann L, Kroppenstedt RM, Habicht M, Fischer M, Mauch H. 2000. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 38:1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deepa P, Therese KL, Madhavan HN. 2005. Application of PCR-based restriction fragment length polymorphism for the identification of mycobacterial isolates. Indian J. Med. Res. 121:694–700 [PubMed] [Google Scholar]
- 14. Kim BJ, Lee KH, Park BN, Kim SJ, Bai GH, Kook YH. 2001. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 39:2102–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. 2000. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J. Clin. Microbiol. 38:2966–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varma-Basil M, Pathak R, Singh K, Dwivedi SKD, Garima K, Kumar S, Sharma D, Dhiman B, Bose M. 2010. Direct early identification of Mycobacterium tuberculosis by PCR-restriction fragment length polymorphism analysis from clinical samples. Jpn. J. Infect. Dis. 63:55–57 [PubMed] [Google Scholar]
- 19. Bonecini-Almeida MG, Ho JL, Boéchat N, Huard RC, Chitale S, Doo H, Geng J, Rego L, Lazzarini LC, Kritski AL, Johnson WD, Jr, McCaffrey TA, Silva JR. 2004. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor β (TGF-β) and analysis of TGF-β receptors I and II in active tuberculosis. Infect. Immun. 72:2628–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNabb A, Eisler D, Adie K, Amos M, Rodrigues M, Stephens G, Black WA, Isaac-Renton J. 2004. Assessment of partial sequencing of the 65-kilodalton heat shock protein gene (hsp65) for routine identification of Mycobacterium species isolated from clinical sources. J. Clin. Microbiol. 42:3000–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marin MD, de Viedma G, Ruiz-Serrano MJ, Bouza E. 2004. Rapid direct detection of multiple rifampin and isoniazid resistance mutations in Mycobacterium tuberculosis in respiratory samples by real-time PCR. Antimicrob. Agents Chemother. 48:4293–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shenai S, Rodrigues C, Mehta A. 2010. Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. Int. J. Tuberc. Lung Dis. 14:1001–1008 [PubMed] [Google Scholar]