Abstract
Plasmodium knowlesi can cause severe and fatal human malaria in Southeast Asia. Rapid diagnosis of all Plasmodium species is essential for initiation of effective treatment. Rapid diagnostic tests (RDTs) are sensitive for detection of uncomplicated and severe falciparum malaria but have not been systematically evaluated in knowlesi malaria. At a tertiary referral hospital in Sabah, Malaysia, we prospectively evaluated the sensitivity of two combination RDTs for the diagnosis of uncomplicated and severe malaria from all three potentially fatal Plasmodium species, using a pan-Plasmodium lactate dehydrogenase (pLDH)-P. falciparum histidine-rich protein 2 (PfHRP2) RDT (First Response) and a pan-Plasmodium aldolase-PfHRP2 RDT (ParaHIT). Among 293 hospitalized adults with PCR-confirmed Plasmodium monoinfection, the sensitivity of the pLDH component of the pLDH-PfHRP2 RDT was 74% (95/129; 95% confidence interval [CI], 65 to 80%), 91% (110/121; 95% CI, 84 to 95%), and 95% (41/43; 95% CI, 85 to 99%) for PCR-confirmed P. knowlesi, P. falciparum, and P. vivax infections, respectively, and 88% (30/34; 95% CI, 73 to 95%), 90% (38/42; 95% CI, 78 to 96%), and 100% (12/12; 95% CI, 76 to 100%) among patients tested before antimalarial treatment was begun. Sensitivity in severe malaria was 95% (36/38; 95% CI, 83 to 99), 100% (13/13; 95% CI, 77 to 100), and 100% (7/7; 95% CI, 65 to 100%), respectively. The aldolase component of the aldolase-PfHRP2 RDT performed poorly in all Plasmodium species. The pLDH-based RDT was highly sensitive for the diagnosis of severe malaria from all species; however, neither the pLDH- nor aldolase-based RDT demonstrated sufficiently high overall sensitivity for P. knowlesi. More sensitive RDTs are needed in regions of P. knowlesi endemicity.
INTRODUCTION
The simian parasite Plasmodium knowlesi is a common cause of human malaria in all age groups in Malaysian Borneo (1–5). In Sabah, Malaysia, the incidence of knowlesi malaria has increased with the control of Plasmodium falciparum and Plasmodium vivax (6) and accounts for up to 80% of malaria admissions to district hospitals (1, 3–5). The geographic range of P. knowlesi corresponds to the overlapping distribution of the simian hosts and the forest-dwelling Anopheles leucosphyrus group of mosquitoes and extends across all of Southeast Asia from southern China, Taiwan, Philippines, and Indonesia to Bangladesh and eastern India. Human P. knowlesi infection has been reported in many of these countries and in travelers returning to regions where the parasite is not endemic (7, 8). The 24-h replication cycle of P. knowlesi may be associated with rapidly increasing parasite counts with consequent complications and fatality rates comparable to those of P. falciparum (9, 10). However, risk of death appears to be low with early initiation of intravenous artesunate and oral artemisinin combination therapy for severe and nonsevere disease, respectively (9, 11). Prompt diagnosis of P. knowlesi infection is therefore essential to allow early commencement of effective treatment.
In Malaysia and much of Southeast Asia, P. knowlesi is coendemic with the two major human Plasmodium species, P. falciparum and P. vivax. The diagnosis of malaria relies primarily on microscopic examination of stained blood smears. However, microscopy is associated with several limitations, including the need for well-trained staff and appropriately maintained microscopes. Maintaining high skill levels among microscopists can be particularly difficult in areas of low malaria transmission. Rapid diagnostic tests (RDTs) provide an alternative method of detection which can be performed by staff with minimal training and are now widely used in many areas of malaria endemicity. The antigens detected by RDTs include P. falciparum-specific histidine-rich protein 2 (PfHRP2), genus-specific aldolase (pan-aldolase), and pan-Plasmodium lactate dehydrogenase (pan-pLDH) (12), as well as P. vivax-specific (13, 14) and P. falciparum-specific (15) pLDH. Limited data from case reports demonstrate that RDTs detecting pan-aldolase and pan-pLDH may be used to detect P. knowlesi (16–20); however, the sensitivity of any RDT for the diagnosis of P. knowlesi has not been evaluated.
Data on the sensitivity of RDTs in the diagnosis of severe malaria from any Plasmodium species are also limited. Only four studies have evaluated the use of PfHRP2-based RDTs in severe falciparum malaria (21–24), with one of these also reporting on the use of a pLDH-based RDT (21) and another reporting on the use of an aldolase-based RDT (24). Importantly, there are no data on the sensitivity of the best available RDTs in the diagnosis of severe malaria from the other two major causes of severe malaria in Asia, P. knowlesi and P. vivax. The latter species, previously thought to be benign, is now recognized as a cause of severe and fatal malaria in areas of endemicity, particularly in the presence of comorbidities (25, 26). Determining the sensitivity of RDTs in severe malaria from any Plasmodium species is important as a false-negative result may lead to delayed or inappropriate treatment with consequent death.
We prospectively evaluated the sensitivity of a pan-pLDH-PfHRP2 RDT and a pan-aldolase-PfHRP2 RDT for the diagnosis of severe and nonsevere knowlesi, falciparum, and vivax malaria at a tertiary referral hospital. We chose the best-performing pan-pLDH and pan-aldolase RDTs, respectively, from the WHO RDT product testing rounds 1 and 2 that were commercially available at the time of the study (27).
MATERIALS AND METHODS
Study site and referral system.
This study was conducted at Queen Elizabeth Hospital (QEH), an adult tertiary referral hospital in Kota Kinabalu, Sabah, Malaysia. The hospital services the West Coast and Kudat Divisions of Sabah, with six district hospitals and a population of 1.14 million. From mid-2010, in response to ongoing malaria deaths in Sabah (9, 10), new guidelines were implemented, including tertiary hospital referral for all malaria patients with a thick blood film reported as 4+ (indicating >10 parasites/high-power microscopy field) or with any evidence of severe malaria. Treatment was commenced pretransfer, and a pretreatment blood film was sent with the patient. Local health clinics within the Kota Kinabalu area were required to refer all malaria patients to QEH for admission, with treatment commencing on arrival.
Subjects.
All patients admitted to QEH with a microscopic diagnosis of malaria were assessed for eligibility from September 2010 to October 2011 as part of a prospective study of the epidemiology, clinical spectrum, and pathophysiology of knowlesi malaria, reported separately (11). Nonpregnant patients of ≥12 years old were enrolled if they were within 18 h of commencing malaria treatment (with 18 h chosen for both pathophysiological and logistical purposes), had no major comorbidities, and had not previously been enrolled in the study. Patients who had mixed species infection (n = 16) or were PCR negative were retrospectively excluded. Patients were classified as having severe malaria using modified 2010 WHO severe malaria criteria (11, 28, 29). Written informed consent was provided by patients or their relatives. Approvals were obtained from the Ethics Committees of the Malaysian Ministry of Health and Menzies School of Health Research.
RDT selection.
WHO RDT product testing performance results from rounds 1 and 2 were used to identify the RDTs with the highest reported sensitivities for both P. falciparum and P. vivax (27). We then selected the pan-pLDH and pan-aldolase RDT with the highest reported sensitivities (27) that could be sourced commercially at the time of study: the First Response Malaria Antigen pLDH/HRP2 Combo Card Test (First Response; Premier Medical Corporation Ltd., Mumbai, India), which detects pan-pLDH and PfHRP2, and the ParaHIT Total Dipstick (ParaHIT; SPAN Diagnostics, Ltd., Surat, India), which detects pan-aldolase and PfHRP2.
The panel detection scores (PDSs; a composite index of test positivity as well as of intertest and interlot consistency in performance [30]) for the First Response RDT were 84% and 100% for detection of P. falciparum at low (200 parasites/μl) and high (2,000 to 5,000 parasites/μl) densities, respectively, and 75% and 100% for the detection of P. vivax at low and high densities, respectively. For the ParaHIT RDT, the PDSs at low and high densities were 64% and 99%, respectively, for detection of P. falciparum and 10% and 98%, respectively, for the detection of P. vivax.
Study procedures.
Standardized data forms were used to record demographic and clinical information. Venous blood was collected in a labeled tube containing citrate, theophylline, adenosine, and dipyridamole (CTAD), and thick and thin blood smears were prepared. RDTs were performed on enrolment according to the manufacturers' instructions, and results were recorded by one of two research laboratory technicians unaware of the microscopy result. Results were recorded as follows: negative, no clearly visible band; 1, faint band; 2, a band darker than 1 but lighter than that of the control; 3, same as the control band; 4, a band darker than the control. Results were cross-checked regularly by the study clinician to ensure consistent reporting between laboratory technicians. The ParaHIT RDT was unavailable for a 4-week period during April to May 2011, with its use then discontinued in September 2011.
Blood smear examination was performed by microscopists at referring district hospitals or at QEH, with slides later cross-checked by an experienced research microscopist. Parasite density was quantified by the research microscopist using pretreatment slides and reported as parasites per 200 leukocytes or per 1,000 erythrocytes and converted to the number of parasites/μl using the patient's leukocyte count or hematocrit, respectively. Where pretreatment slides were unavailable (6%), referring hospital microscopy was used, and the grades 1+ to 4+ were converted into numbers of parasites/μl using the relevant median parasite density. Parasite DNA was extracted and PCR was performed as reported previously (11), using previously described methods for P. falciparum, P. vivax, Plasmodium ovale, and Plasmodium malariae (31), and P. knowlesi (32) detection.
Statistical analysis.
Data were analyzed using STATA, version 10.1 (StataCorp LP, College Station, TX). Intergroup differences were compared using a Kruskal-Wallis test for nonnormally distributed continuous variables, and a χ2 test was used for categorical variables. The sensitivity of RDTs for each species was defined as the total number of positive results divided by the total number of samples positive for that species by PCR; 95% confidence intervals (CIs) were estimated using Wilson's method. Spearman's correlation coefficient was used to assess the association between parasite count and intensity of the RDT band.
RESULTS
Baseline demographics and clinical features.
From September 2010 to October 2011, 293 patients had pan-pLDH-PfHRP2 with or without aldolase-PfHRP2 RDTs performed, including 129 patients with P. knowlesi, 121 with P. falciparum, and 43 with P. vivax infections. Baseline demographics, reported previously (11), are shown in Table 1. Patients with knowlesi malaria were significantly older than those with falciparum or vivax malaria, with a median age of 46 years versus 27 and 24 years, respectively (P < 0.001). The majority (73%) of patients were male, and this did not differ between infecting species. Most patients (66%) with knowlesi malaria were referred from district hospitals, and 74% had commenced antimalarial treatment prior to enrolment in the study. The median time from commencing treatment to enrolment for patients with knowlesi malaria was 5.1 h (range, 0 to 18 h). Severe malaria occurred in 38/129 (29%) patients with P. knowlesi, 13/121 (11%) with P. falciparum, and 7/43 (16%) with P. vivax infections (Table 1).
Table 1.
Baseline demographic and clinical features
| Parameter | Value by infecting organisma |
P value | ||
|---|---|---|---|---|
| P. knowlesi (n = 129) | P. falciparum (n = 121) | P. vivax (n = 43) | ||
| Age (yr) | ||||
| Median (IQR) | 46 (29–58) | 27 (17–40) | 24 (18–42) | <0.001 |
| Range | 14–83 | 13–78 | 13–79 | |
| No. of males (%) | 96 (74) | 87 (71) | 33 (77) | 0.769 |
| Source of referral (no. of patients [%]) | <0.001 | |||
| QEH or local health clinic | 44 (34) | 75 (61) | 22 (51) | |
| District Hospital | 86 (66) | 47 (39) | 21 (49) | |
| No. of patients (%) enrolled pretreatment | 34 (26) | 42 (34) | 12 (28) | 0.231 |
| Median time on malaria treatment (h [IQR]) | 5.13 (0–11.47) | 4.04 (0–11.69) | 4.81 (0–9.68) | 0.726 |
| No. of patients (%) with severe malaria | 38 (29%) | 13 (11%) | 7 (46%) | <0.001 |
| Median parasite density count/μl (IQR [range]) | ||||
| All patients | 8,537 (1,980–35,495 [32–584,015]) | 11,610 (4,387–36,977 [16–959,383]) | 4,938 (2,848–12,927 [72–84,403]) | 0.009 |
| Nonsevere malaria | 4,841 (1,581–14,994 [65–62,360]) | 10,937 (4,002–32,326 [16–218,921]) | 4,753 (2,369–10,316 [72–46,849]) | <0.001 |
| Severe malaria | 80,359 (25,874–168,279 [32–584,015]) | 72,270 (27,905–273,909 [625–959,383]) | 10,243 (4,387–40,895 [460–84,403]) | 0.070 |
| Severity criteria among patients with severe malaria | ||||
| Median no. of criteria (range) | 2 (1–6) | 3 (1–4) | 1 (1–2) | 0.039 |
| No. of patients (%) with: | ||||
| Hyperparasitemia | 18 (47) | 2 (15) | 0 | |
| Respiratory distress | 14 (37) | 4 (31) | 1 (14) | |
| Hypotension | 13 (34) | 6 (49) | 3 (43) | |
| Jaundice | 20 (53) | 9 (69) | 2 (29) | |
| Acute kidney injury | 9 (24) | 3 (23) | 0 | |
| Metabolic acidosis | 4 (11) | 4 (31) | 0 | |
| Severe anemia | 2 (5) | 2 (15) | 0 | |
| Abnormal bleeding | 2 (5) | 1 (8) | 1 (14) | |
| Multiple convulsions | 0 | 0 | 1 (14) | |
| Coma | 0 | 0 | 0 | |
Table includes data previously reported (11).
Boldface indicates significant values.
Pan-pLDH-PfHRP2 (First Response) RDT.
Results of the pLDH component of the First Response RDT are shown in Fig. 1 and 2 and in Table S1 in the supplemental material. Among all patients with knowlesi malaria, 95/129 had positive results, giving a sensitivity of 74% (95% CI, 65 to 80%). The sensitivity of the test was higher when only pretreatment samples were analyzed, with 30/34 positive results (sensitivity, 88% [95% CI, 73 to 95%]) (see Table S1). The four patients with pretreatment samples and negative pLDH results had parasite counts of 75, 282, 320, and 11,078 parasites/μl. The lowest parasite count among patients with pretreatment samples and a positive pLDH result was 907 parasites/μl.
Fig 1.
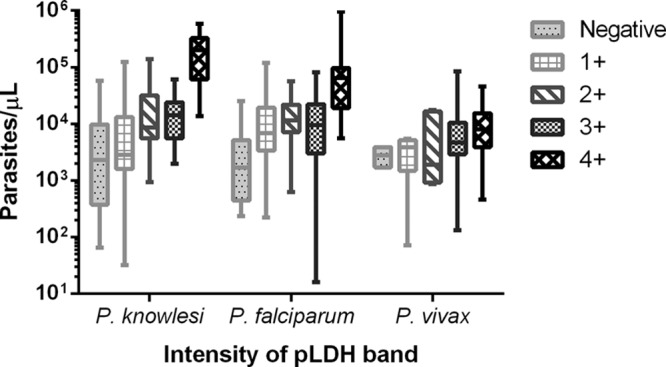
Parasite count by pLDH-band intensity for all samples. Horizontal lines indicate medians; boxes indicate interquartile ranges; vertical lines indicate ranges. Among patients with P. knowlesi infection, the pLDH band was negative in 34 (26%), 1+ in 34 (26%), 2+ in 17 (13%), 3+ in 17 (13%), 4+ in 24 (18%), and recorded only as positive in 3 (2%). Among patients with P. falciparum infection, the pLDH band was negative in 11 (9%), 1+ in 35 (29%), 2+ in 17 (14%), 3+ in 24 (20%), 4+ in 30 (25%), and recorded as positive in 4 (3%). Among patients with P. vivax infection, the pLDH band was negative in 2 (5%), 1+ in 4 (9%), 2+ in 5 (12%), 3+ in 7 (16%), 4+ in 20 (47%), and recorded as positive in 5 (12%).
Fig 2.
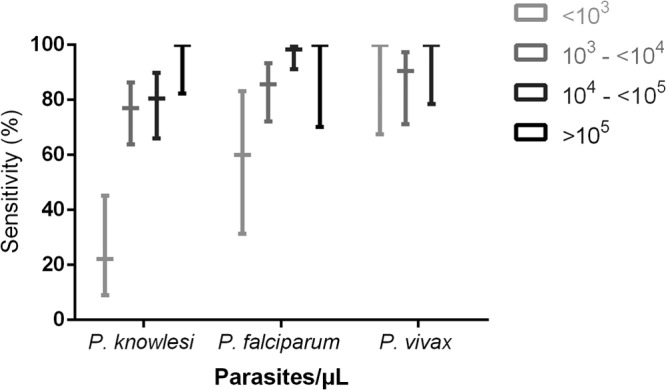
Sensitivity of the pLDH-component of the First Response RDT by parasite count. Wide horizontal bars indicate sensitivity. Vertical lines represent 95% confidence intervals. Among patients with P. knowlesi infection, 18 (14%) had <103 parasites/μl, 52 (40%) had 103 to <104 parasites/μl, 41 (32%) had 104 to <105 parasites/μl, and 18 (14%) had >105 parasites/μl. Among patients with P. falciparum infection, 10 (8%) had <103 parasites/μl, 42 (35%) had 103 to <104 parasites/μl, 60 (50%) had 104 to <105 parasites/μl, and 9 (7%) had >105 parasites/μl. Among patients with P. vivax infection, 8 (19%) had <103 parasites/μl, 21 (49%) had 103 to <104 parasites/μl, and 14 (33%) had 104 to <105 parasites/μl.
The sensitivity of the pLDH test for knowlesi malaria increased with increasing parasite count (Fig. 2). Among patients enrolled prior to treatment and with parasite counts of >1,000 parasites/μl, sensitivity was 97% (95% CI, 83 to 99%), but it was only 25% among patients with parasite counts of <1,000 parasites/μl. Among the 38 patients with severe knowlesi malaria, 36 had a positive result (sensitivity of 95% [95% CI, 83 to 99%]); 31 (82%) of these patients had commenced malaria treatment a median of 5.7 h prior to enrolment. The two patients with severe knowlesi malaria and negative results had parasite counts of 3,486 and 48,833 parasites/μl and were enrolled 13 and 9 h, respectively, after treatment.
The sensitivity of the pLDH test for P. falciparum was 91% (95% CI, 84 to 95%) and was not affected by the inclusion of posttreatment samples. Sensitivity of the test decreased at parasite counts of <1,000 parasites/μl, with only 6/10 (60%) positive results, compared to 104/111 (94%) among patients with parasite counts of >1,000 parasites/μl. The lowest parasite count detected was 16 parasites/μl; however, parasitemia ranged from 234 to 25,308 (median, 1,698) parasites/μl among the 11 patients with negative results. The sensitivity of the pLDH test among patients with severe falciparum malaria was 100% (95% CI, 77 to 100%), with nine (69%) patients having already commenced malaria treatment a median of 3.8 h prior to enrolment.
The sensitivity of the pLDH test for vivax malaria was 95% (95% CI, 85 to 99%) when all samples were included and 100% (95% CI, 76 to 100) among the 12 pretreatment samples, which included a parasitemia of 72 parasites/μl. The sensitivity of the pLDH test among the seven patients with severe vivax malaria was 100% (95% CI, 65 to 100%); six of these patients commenced treatment a median 9.2 h prior to enrolment.
The intensity of the pLDH band (grades 1+ to 4+) was a good indicator of parasitemia among patients with knowlesi malaria, with the median parasite count increasing with each successive grade (Spearman's correlation coefficient, 0.61; P < 0.0001) (see Table S2 in the supplemental material). Patients with knowlesi malaria and a 4+ pLDH band also had a 7.3-fold (95% CI, 2.3- to 24-fold) higher risk of severe disease (defined using modified 2010 WHO criteria [11]) than those with pLDH bands with intensities of 1+ to 3+ (P = 0.0001). Among these patients 17/24 (71%) had severe malaria, compared to 17/68 (25%) patients with knowlesi malaria and pLDH bands graded 1+ to 3+ (Table 2). The risk of severity increased further among patients of >50 years old with knowlesi malaria and a band intensity of 4+, of whom 11/13 (85%) had severe disease. The median intensity score of the pLDH band was 4+ (interquartile range [IQR], 2+ to 4+) among all patients with severe knowlesi malaria, compared to a grade of 1+ (IQR, 0 to 3+) among patients with nonsevere knowlesi malaria (P < 0.0001). This difference was less marked among patients with severe and nonsevere falciparum malaria (median intensity scores of 4 [IQR, 2 to 4] and 2 [IQR, 1 to 4], respectively; P = 0.007) and among patients with severe and nonsevere vivax malaria (median intensity scores of 4 [IQR, 3 to 4] and 4 [IQR, 2.5 to 4], respectively; P = 0.360).
Table 2.
Risk of severe malaria by intensity of First Response pLDH band
| Infecting organism | No. of severe malaria patients (%) by pLDH band intensity |
Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| 4+ | 1+–3+ | |||
| P. knowlesi | 17 (71) | 17 (25) | 7.29 (2.33–24.01) | 0.0001 |
| P. falciparum | 8 (27) | 5 (6.7) | 5.16 (1.31–22.85) | 0.005 |
| P. vivax | 4 (20) | 2 (13) | 1.75 (0.21–21.81) | 0.549 |
The sensitivity of the First Response PfHRP2 RDT for falciparum malaria was 98% overall and 100% among those with severe disease. The two patients with false-negative results were enrolled 15 and 17 h after commencing antimalarial treatment and had pretreatment parasite counts of 10,098 and 101,686 parasites/μl. No patient with knowlesi or vivax malaria had a positive HRP2 result.
Pan-aldolase-PfHRP2 (ParaHIT) RDT.
The sensitivity of the aldolase component of the ParaHIT RDT was low for all species (see Table S2 in the supplemental material). Only 22/96 (23%), 37/84 (44%), and 20/36 (56%) patients with P. knowlesi, P. falciparum, and P. vivax infections, respectively, had positive aldolase results, with similar results obtained when posttreatment samples were excluded (sensitivities of 27%, 50%, and 73% for P. knowlesi, P. falciparum, and P. vivax, respectively). The sensitivity of the aldolase test among patients with severe malaria was 39% (95% CI, 24 to 56%), 50% (95% CI, 22 to 78%) and 83% (95% CI, 44 to 97%) for patients with P. knowlesi (n = 31), P. falciparum (n = 8), and P. vivax (n = 6) infections, respectively. Sensitivity was low even among patients with higher parasite counts. Among patients with pretreatment samples and parasite counts of >10,000 parasites/μl, only 5/11 (45%) and 10/19 (56%) of those with P. knowlesi and P. falciparum infections, respectively, had positive results.
Among patients with P. falciparum infection, 79/84 (94%) had a positive PfHRP2 result, including all eight patients with severe malaria. Four of the five patients with nonsevere malaria and false-negative results were enrolled 4 to 16 h after treatment, with pretreatment parasite counts of 444 to 15,503 parasites/μl, while one patient was enrolled pretreatment with a parasitemia of 3,990 parasites/μl. One patient with P. knowlesi and two with P. vivax infections had false-positive ParaHIT PfHRP2 results.
DISCUSSION
This is the first study to systematically evaluate the sensitivities of RDTs for the diagnosis of knowlesi malaria and the first to evaluate the sensitivities of the best available RDTs for the diagnosis of nonfalciparum severe malaria. The pLDH-based RDT performed better than the aldolase-based RDT for the diagnosis of knowlesi malaria of any severity and demonstrated good sensitivity at parasite counts of >1,000 parasites/μl. This RDT performed well among patients with severe knowlesi malaria, among whom sensitivity was 95%. Sensitivity was poor, however, at low parasite counts. Given that P. knowlesi is associated with lower parasite counts than P. falciparum among patients with nonsevere malaria (11), that the parasite has a rapid replication cycle and is associated with relatively high rates of severe disease (11), and that late diagnosis has been associated with fatal outcomes (33), the poor sensitivity at low parasite counts of both pLDH- and aldolase-based RDTs limits their utility in areas of knowlesi malaria endemicity.
Despite the suboptimal sensitivity of the pLDH-based test in knowlesi malaria, we found that the intensity of the pLDH band correlated well with P. knowlesi parasitemia and risk of severe disease. In particular, a 4+ band was associated with an increased risk of complications, particularly in older patients who are at greater risk of severe disease (2, 9, 11). P. knowlesi parasite counts are strongly associated with complications, with the risk of severe disease increasing 11-fold and 28-fold with parasite counts of >20,000 parasites/μl and >100,000 parasites/μl, respectively (11). Despite this, in Sabah, malaria slides are often reported only on a scale of 1+ to 4+, with slides with >10 parasites per high-power microscopy field (or approximately 4,000 parasites/μl) reported only as 4+. Consequently, hyperparasitemia, with its associated risk of complications, may be underrecognized by clinicians, potentially leading to undertreatment and monitoring. Use of a pLDH-based RDT may assist clinicians to recognize patients at high risk of severe disease in settings where parasite counts are unavailable.
The pLDH-based RDT performed well for P. vivax, with an overall sensitivity of 95% and sensitivity of 100% among those with severe disease. False-negative results occurred in only two patients, both of whom had already commenced antimalarial treatment. This finding confirms another recent report of the excellent sensitivity of the First Response RDT for detecting P. vivax (34). In our study this RDT also performed well for P. falciparum; the sensitivity of the HRP2 component of the test was 98% overall and 100% among patients with severe disease. This provides confirmation from Southeast Asia of recent reports of the very high sensitivity of HRP2-based RDTs in severe falciparum malaria in Africa (21, 23) and India (22), despite the potential for geographic genetic variability in PfHRP2 (34–37).
The aldolase component of the aldolase-based RDT performed poorly for all species, with a sensitivity of <50% for knowlesi and falciparum malaria. Sensitivity was poor even at higher parasite densities, and less than half of patients with severe knowlesi malaria recorded a positive result. Using an aldolase-based RDT with poorer performance characteristics on WHO product testing, a recent study also reported poor sensitivity for the diagnosis of severe vivax malaria (24). The similarly poor performance of aldolase-based RDTs has also been reported in uncomplicated malaria (38, 39). Taking these results together, the use of aldolase-based RDTs for diagnosis of nonfalciparum species of any severity cannot therefore be recommended (40).
A limitation of both the RDTs evaluated in this study is that they do not differentiate between the nonfalciparum species. This is particularly problematic given the difficulties with microscopic diagnosis of P. knowlesi, the ring forms of which resemble those of P. falciparum while the more mature trophozoites are indistinguishable from those of P. malariae. Consequently, patients with knowlesi malaria continue to be misdiagnosed with the more benign P. malariae infection, leading to inappropriate management with potentially fatal outcomes (10). Furthermore, misdiagnosis of P. vivax as P. knowlesi is also common (4, 10, 41), and this has been associated with lack of antihypnozoite treatment and consequent relapses from P. vivax infection (41).
An RDT that differentiates between species is therefore urgently needed in areas of knowlesi malaria endemicity. P. knowlesi has been shown to cross-react with both P. falciparum-specific and P. vivax-specific pLDHs (17, 18, 20, 42), and RDTs that combine these antigens with HRP2 may allow differentiation between P. vivax, P. falciparum, and P. knowlesi monoinfections. Prospective evaluation of the use of these RDTs in areas of knowlesi malaria endemicity is needed.
Our study had several limitations. Most importantly, the majority of patients were referred from district hospitals and had already commenced malaria treatment prior to enrolment in the study. For these patients RDTs were done on posttreatment blood samples, possibly leading to an underestimation of sensitivity. However, the reported sensitivities of the RDTs under these circumstances reflect the real-world utility of RDTs in referral hospitals, where prior treatment is common. Moreover, we also report sensitivity calculations using only patients enrolled prior to treatment (i.e., on initial presentation to the hospital). A second limitation was that each RDT was read by a single observer; however, regular cross-checks were performed to ensure consistent reporting. The high sensitivity of the PfHRP2 component of both RDTs for diagnosing P. falciparum infection also supports the reliability of our results. Third, patients with knowlesi malaria referred to a tertiary hospital are likely to have had higher parasitemias than those diagnosed in primary care settings (2), suggesting that the suboptimal sensitivities of the pLDH and aldolase RDTs found for nonsevere P. knowlesi malaria may be even lower than reported here. Fourth, our study excluded pregnant women and children of <12 years old; the performance of RDTs for the diagnosis of P. knowlesi infection in these groups will need to be evaluated in future studies. Finally, the design of our study did not allow evaluation of the specificity of the RDTs as only patients with PCR-confirmed malaria were enrolled; further cross-sectional studies including patients with nonmalarial fevers are required to provide this information.
In conclusion, this study found that the pLDH-based First Response RDT had high sensitivity for severe malaria from all three Plasmodium species evaluated and that among patients with knowlesi malaria, the intensity of the pLDH band predicted patients at risk of complications. However, neither of the RDTs had sufficient overall sensitivity for detecting P. knowlesi. The World Health Organization (WHO) recommends a minimum sensitivity of 95% for P. falciparum densities of >100 parasites/μl (43); while there is no recommended threshold for nonfalciparum malaria, P. knowlesi has a 3-fold-greater likelihood of causing severe disease (11) and fatality rates comparable to those of P. falciparum, and hence the minimum sensitivity thresholds required for RDTs should be at least as high for P. knowlesi as for P. falciparum. Further studies are needed to develop reliable RDTs for the diagnosis of knowlesi malaria with high sensitivity and the ability to differentiate between species.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the patients enrolled in this study, all the clinical staff involved in their care, Rita Wong, Beatrice Wong and Ann Wee for their help with clinical and laboratory study procedures, Ferryanto Chalfein for performing microscopy, Jutta Marfurt and Sarah Auburn for supervising the PCR assays, the Clinical Research Centre, Sabah, for logistical support, and the Director General of Health, Malaysia, for permission to publish this study.
The study was funded by the Australian National Health and Medical Research Council (program grant 496600; scholarship to B.E.B. and fellowships to N.M.A. and T.W.Y.)
Footnotes
Published ahead of print 23 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03285-12.
REFERENCES
- 1. Barber BE, William T, Dhararaj P, Anderios F, Grigg MJ, Yeo TW, Anstey NM. 2012. Epidemiology of Plasmodium knowlesi malaria in northeast Sabah, Malaysia: family clusters and wide age distribution. Malar. J. 11:401 doi:10.1186/1475-2875-11-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daneshvar C, Davis TM, Cox-Singh J, Rafa'ee M, Zakaria S, Divis P, Singh B. 2009. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin. Infect. Dis. 49:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joveen-Neoh WF, Chong KL, Wong CM, Lau TY. 2011. Incidence of malaria in the Interior Division of Sabah, Malaysian Borneo, based on nested PCR. J. Parasitol. Res. 2011:104284 doi:10.1155/2011/104284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber BE, William T, Jikal M, Jilip J, Dhararaj P, Menon J, Yeo TW, Anstey NM. 2011. Plasmodium knowlesi malaria in children. Emerg. Infect. Dis. 17:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, Grigg MJ, Yeo TW, Anstey NM, Barber BE. 24 January 2013. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl. Trop. Dis. [Epub ahead of print.] doi:10.1371/journal.pntd.0002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantele A, Sakari Jokiranta T. 2011. Review of cases with the emerging fifth human malaria parasite, Plasmodium knowlesi. Clin. Infect. Dis. 52:1356–1362 [DOI] [PubMed] [Google Scholar]
- 8. Cox-Singh J. 2012. Zoonotic malaria: Plasmodium knowlesi, an emerging pathogen. Curr. Opin. Infect. Dis. 25:530–536 [DOI] [PubMed] [Google Scholar]
- 9. William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Fredrick C, Jilip J, Anstey NM, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary hospital, Sabah, Malaysia. Emerg. Infect. Dis. 17:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajahram G, Barber BE, William T, Menon J, Anstey NM, Yeo TW. 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as P. malariae and delayed parenteral artesunate. Malar. J. 11:284 doi:10.1186/1475-2875-11-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, Anstey NM, Yeo TW. 2013. A prospective comparative study of knowlesi, falciparum and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and P. vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. 56:383–397 doi:10.1093/cid/cis902 [DOI] [PubMed] [Google Scholar]
- 12. Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. 2008. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 21:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillet P, Bosselaers K, Cnops L, Bottieau E, Van Esbroeck M, Jacobs J. 2009. Evaluation of the SD FK70 malaria Ag Plasmodium vivax rapid diagnostic test in a non-endemic setting. Malar. J. 8:129 doi:10.1186/1475-2875-8-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillet P, Van Dijk DPJ, Bottieau E, Cnops L, Van Esbroeck M, Jacobs J. 2009. Test characteristics of the SD FK80 Plasmodium falciparum/Plasmodium vivax malaria rapid diagnostic test in a non-endemic setting. Malar J. 8:262 doi:10.1186/1475-2875-8-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmer CJ, Lindo JF, Klaskala WI, Quesada JA, Kaminsky R, Baum MK, Ager AL. 1998. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J. Clin. Microbiol. 36:203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berry A, Iriart X, Wilhelm N, Valentin A, Cassaing S, Witkowski B, Benoit-Vical F, Menard S, Olagnier D, Fillaux J, Sire S, Le Coustumier A, Magnaval JF. 2011. Case report: imported Plasmodium knowlesi malaria in a French tourist returning from Thailand. Am. J. Trop. Med. Hyg. 84:535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ta TT, Salas A, Ali-Tammam M, Martinez Mdel C, Lanza M, Arroyo A, Rubio JM. 2010. First case of detection of Plasmodium knowlesi in Spain by real time PCR in a traveller from Southeast Asia. Malar. J. 9:219 doi:10.1186/1475-2875-9-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Hellemond J, Rutten M, Koelewijn R, Zeeman A, Verweij J, Wismans P, Kocken C, van Genderen P. 2009. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg. Infect. Dis. 15:1478–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ong C, Lee S, Koh W, Ooi E, Tambyah P. 2009. Monkey malaria in humans: a diagnostic dilemma with conflicting laboratory data. Am. J. Trop. Med. Hyg. 80:927–928 [PubMed] [Google Scholar]
- 20. Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. 2009. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol. Int. 58:300–302 [DOI] [PubMed] [Google Scholar]
- 21. Hendriksen ICE, Mtove G, Pedro AJ, Gomes E, Silamut K, Lee SJ, Mwambuli A, Gesase S, Reyburn H, Day NPJ. 2011. Evaluation of a PfHRP2 and a pLDH-based rapid diagnostic test for the diagnosis of severe malaria in 2 populations of African children. Clin. Infect. Dis. 52:1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shujatullah F, Malik A, Khan HM. 2006. Comparison of different diagnostic techniques in Plasmodium falciparum cerebral malaria. J. Vector Borne Dis. 43:186–190 [PubMed] [Google Scholar]
- 23. Birku Y, Welday D, Ayele D, Shepherd A. 1999. Rapid diagnosis of severe malaria based on the detection of Pf-Hrp-2 antigen. Ethiop. Med. J. 37:173 doi:10.1097/QCO.0b013e32832f14c1 [PubMed] [Google Scholar]
- 24. Manning L, Laman M, Rosanas-Urgell A, Turlach B, Aipit S, Bona C, Warrell J, Siba P, Mueller I, Davis TME. 2012. Rapid antigen detection tests for malaria diagnosis in severely ill Papua New Guinean children: a comparative study using Bayesian latent class models. PLoS One 7:e48701 doi:10.1371/journal.pone.0048701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Price RN, Douglas NM, Anstey NM. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435 [DOI] [PubMed] [Google Scholar]
- 26. Anstey NM, Russell B, Yeo TW, Price RN. 2009. The pathophysiology of vivax malaria. Trends Parasitol. 25:220–227 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization 2010. Malaria rapid diagnostic test performance. Summary results of WHO product testing of malaria RDTs: rounds 1 and 2 (2008–2009). WHO, Geneva, Switzerland: http://www2.wpro.who.int/NR/rdonlyres/E4823BBF-69FC-45DE-B924-1A9FCDD7406C/0/RoundIII_SummaryFINAL.pdf [Google Scholar]
- 28. World Health Organization 2012. Management of severe malaria: a practical handbook, 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 29. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html [Google Scholar]
- 30. WHO 2012. Information note on recommended selection criteria for procurement of malaria rapid diagnostic tests (RDTs). WHO Global Malaria Programme, Geneva, Switzerland: http://www.who.int/malaria/diagnosis_treatment/diagnosis/RDT_selection_criteria.pdf [Google Scholar]
- 31. Padley D, Moody A, Chiodini P, Saldanha J. 2003. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 97:131–137 [DOI] [PubMed] [Google Scholar]
- 32. Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. 2009. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J. Clin. Microbiol. 47:4173–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajahram GS, Barber BE, Tan WM, Yeo TW, William T. 2013. Case report: fatal Plasmodium knowlesi malaria following an atypical clinical presentation and delayed diagnosis. Med. J. Malaysia 68:48–49 [PubMed] [Google Scholar]
- 34. Maltha J, Gamboa D, Bendezu J, Sanchez L, Cnops L, Gillet P, Jacobs J. 2012. Rapid diagnostic tests for malaria diagnosis in the Peruvian Amazon: impact of pfhrp2 gene deletions and cross-reactions. PLoS One 7:e43094 doi:10.1371/journal.pone.0043094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J. 2010. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 5:e8091 doi:10.1371/journal.pone.0008091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, Bell D, Cheng Q. 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 192:870–877 [DOI] [PubMed] [Google Scholar]
- 37. Lee N, Baker J, Andrews KT, Gatton ML, Bell D, Cheng Q, McCarthy J. 2006. Effect of sequence variation in Plasmodium falciparum histidine-rich protein 2 on binding of specific monoclonal antibodies: implications for rapid diagnostic tests for malaria. J. Clin. Microbiol. 44:2773–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh N, Shukla M, Shukla M, Mehra R, Sharma S, Bharti P, Singh M, Singh A, Gunasekar A. 2010. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malar. J. 9:191 doi:10.1186/1475-2875-9-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization 2011. Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: round 3 (2010–2011). World Health Organization, Geneva, Switzerland: http://www.who.int/tdr/publications/documents/rdt3.pdf [Google Scholar]
- 40. Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. 1999. Field evaluation of the ICT malaria Pf/Pv immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in Eastern Indonesia. J. Clin. Microbiol. 37:2412–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. 2013. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 12:8 doi:10.1186/1475-2875-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCutchan T, Piper R, Makler M. 2008. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg. Infect. Dis. 14:1750–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bell D, Peeling RW. 2006. Evaluation of rapid diagnostic tests: malaria. Nat. Rev. Microbiol. 4:S34–S38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


