Abstract
Early-bactericidal-activity (EBA) studies measure the change in mycobacterial load in sputum over time to evaluate antituberculosis drugs. We investigated whether a delay in sputum processing influences the quantitative results of sputum mycobacterial culture. We identified pretreatment smear-positive sputum samples collected overnight and processed at a single laboratory. Sputum volume, time from sputum collection to processing, CFU counts/ml of sputum, and time to culture positivity (TTP) data were retrieved. We obtained 817 TTP and 794 CFU results from a total of 844 sputum samples. Contamination did not occur more frequently with prolonged storage (TTP, 2.0%; CFU, 2.4%). Sample volumes were <5 ml in 5%, 5 to 10 ml in 46%, and >10 ml in 49%. Delays to processing were 0, 1, 2, and 3 days in 696 (43.2%), 722 (44.8%), 128 (7.9%), and 65 (4.0%) samples, respectively. TTP and CFU did not significantly differ between days of delay to processing (P = 0.098 and P = 0.908, respectively), but there was a nonsignificant trend toward a prolonged TTP over time (P = 0.052, Jonckheere-Terpstra trend test). Sputa of <5 ml in volume showed a significantly prolonged TTP compared to sputum of >5 ml (113 h versus 99 h; P < 0.01) but no significant decrease in CFU. Sputum can be stored under refrigerated conditions for deferred processing for at least 3 days. This means that central laboratories can be used for quantitative mycobacterial study endpoints when delays to processing are not expected to exceed a few days. Care should be taken to collect sputum of sufficient volume.
INTRODUCTION
Sputum culture for Mycobacterium tuberculosis remains a cornerstone of clinical mycobacteriology. The detection of viable M. tuberculosis in sputum establishes the diagnosis of pulmonary tuberculosis (TB), and sustained sputum culture conversion is a marker of clinical improvement and cure. Storage of sputum for deferred culture may be considered where decentralized health care centers rely on a central laboratory service, but this may result in loss of viability and false-negative cultures (1–4) or overgrowth of contaminants, particularly if sensitive liquid media are used (5–7). Beyond its clinical purpose, sputum culture can quantify the viable M. tuberculosis sputum load expressed as CFU per ml of sputum counted on agar plates or as times to culture positivity (TTP) of a volume of decontaminated sputum inoculated in liquid mycobacterial culture (8). High sputum mycobacterial loads at diagnosis have been associated with treatment failure and recurrence (9, 10). Early-bactericidal-activity (EBA) studies are considered a key step in the clinical evaluation of individual antituberculosis agents and new combination regimens and describe the change in log CFU and TTP on consecutive sputum samples collected over up to 14 treatment days (2, 11).
EBA studies commonly include small groups of sputum smear-positive, treatment-naïve patients who are given investigational treatments under controlled conditions in hospital. The change of the bacterial sputum load from baseline values obtained before treatment is determined on consecutive overnight sputum collections. To detect small differences between treatments, it is imperative that the variation of quantitative sputum bacterial load measurements is kept to a minimum (12). It has previously been shown that higher sputum volumes increase the precision of CFU measurements (12). Other potential sources of variation include delay to sputum processing, which might happen if a central laboratory is used, and between-laboratory variation, which might occur if multiple laboratories are used to avoid delays to processing. It is unclear whether short-term sputum storage influences mycobacterial viability and the quantitative results of sputum culture. This study was a retrospective analysis of five serial EBA studies to investigate if delays in sputum processing influence the viable mycobacterial sputum load.
MATERIALS AND METHODS
Background and setting.
EBA studies quantify the effect of drug treatments over a period of no longer than 2 weeks by calculating the fall in log10 CFU/day per ml of sputum and the increase in TTP in hours per day of study. Baseline CFU counts and baseline TTP are averaged from two samples collected before drug treatment is initiated. We retrospectively identified pretreatment sputum samples collected for baseline sputum bacterial load measurements in EBA studies done at our center for which quantitative microbiology data and collection and processing times as well as volume data had been collected. Inclusion criteria for patient recruitment and laboratory methodologies relevant to this analysis were identical for all studies. Briefly, sputum smear-positive patients (≥1+ on the WHO International Union Against Tuberculosis and Lung Disease [IUATLD] scale [13]) without other significant underlying medical conditions were recruited from outpatient clinics and hospitalized at two centers in Cape Town, South Africa (Task Applied Science, Bellville, and the Centre for Tuberculosis Research Innovation, University of Cape Town [UCT] Lung Institute). Sputum collection was commenced midafternoon and continued overnight for 16 h at ambient temperature. From completion of collection until processing, sputum samples were refrigerated at 2 to 8°C. Samples were transferred from the collection site to the laboratory by car in cooler boxes maintaining the cold chain. Quantitative microbiology was performed centrally at the Medical Research Council [MRC] Centre for Molecular and Cellular Biology at the Faculty of Medicine and Health Sciences, Stellenbosch University. Processing was done on the day of sample collection if possible. Samples collected on weekends or public holidays or during scheduled laboratory closures for maintenance or periods of limited processing capacity were carried over to the next day. For this study, samples processed on the day when collection ended were labeled “day 0” and samples whose processing was delayed were labeled according to the processing delay in days.
Sputum processing.
Before processing, sputum volume was estimated visually as <5 ml, 5 ml to 10 ml, or >10 ml by comparing the collection container to identical containers filled with a reference amount of water. The methods of processing sputum for culture endpoint determination in EBA studies have been described previously (11). In summary, sputum is homogenized and digested using magnetic stirring and 0.1% dithiothreitol (Sputasol; Oxoid, Cambridge, United Kingdom) (1:20 dilution). For CFU counting, two series of 10-fold dilutions (100 to 10−5) of digested sputum are prepared and 100 μl is inoculated in quadruplicate onto 7H11 agar plates (Becton, Dickinson, Franklin Lakes, NJ) enriched with oleic acid-albumin-dextrose-catalase (OADC) (Becton, Dickinson) and made selective by addition of polymyxin B, amphotericin B, ticarcillin, and trimethoprim (Selectatab; Mast, Merseyside, United Kingdom). CFU are counted after 3 to 4 weeks of incubation at 37°C at the dilution that yields 20 to 200 visible colonies. If counts cannot be made in this range, higher numbers of colonies are counted as accurately as possible. Counts are averaged and corrected for dilution factors to result in a CFU count per ml sputum. In case of contaminated plates, the average value determined for the countable plates is used; if all are contaminated, the sample is scored as contaminated. For culture in Bactec MGIT 960 (Becton, Dickinson), digested sputum is decontaminated with a final concentration of 1% NaOH (Mycoprep; Becton, Dickinson) for 15 min, neutralized by the addition of phosphate-buffered saline (PBS; pH 6.8), concentrated by centrifugation (3,000 × g for 15 min at 4°C), and inoculated into 2 volumes of PANTA (Becton, Dickinson)-containing Mycobacterium Growth Indicator Tubes (MGIT; Becton, Dickinson) that are incubated in duplicate in the MGIT 960 system (Becton, Dickinson). When cultures are flagged as positive, the TTP is recorded automatically. The presence of mycobacteria in positive tubes is confirmed by microscopy and contamination excluded by blood agar culture. In cases of a single contaminated tube, the value for the remaining tube is used; if both are contaminated, the sample is scored as contaminated.
Statistical analyses.
TTP and CFU values are presented as median with interquartile range (IQR) as they were not normally distributed (Kolmogorov-Smirnov). The Pearson X2 test was used to compare sputum volume categories and the Kruskal-Wallis test to determine whether TTP or CFU values differed significantly for the different days of storage. The change of TTP and CFU over time during storage was estimated with the Jonckheere-Terpstra (JT) trend test (14). The JT test is comparable to the Kruskal-Wallis test, but it differs in testing an a priori ordering of the populations from which the samples are drawn. Linear regression was calculated with the method of least squares. P values < 0.05 were considered significant. All statistical analyses were performed using SPSS version 19 (SPSS Inc., Chicago, IL).
RESULTS
Sputum samples.
We identified 844 pretreatment specimens from 412 patients participating in 5 studies between August 2009 and April 2012 (Table 1 and Table 2). Growth was detected in all samples incubated for TTP, but 17 (2.0%) were contaminated (day 0, 2.5%; day 1, 1.9%; day 2, 1.5%; day 3, 0%). For CFU, 21 (2.5%) samples remained negative and 20 (2.4%) were contaminated (day 0, 3.3%; day 1, 2.1%; days 2 and 3, 0%). For the final analysis, we excluded 14 samples with processing delays of 4, 5, and 6 days brought about by various circumstances (thereby excluding small group sizes) and 63 samples that were contaminated, showed no growth, or were not processed. This resulted in 817 valid TTP results (96.8% of sputa submitted) and 794 valid CFU results (94.1% of sputa submitted). The delays to processing were 0, 1, 2, and 3 days for 696 (43.2%), 722 (44.8%), 128 (7.9%), and 65 (4.0%) samples, respectively.
Table 1.
Time to culture positivity of sputum samples grouped by storage time from collection to processinga
| Processing day | No. (%) of sputum samples with indicated TTP | Total no. of contaminated sputum samples | No. of samples with no growth | TTP (h) (median IQR) |
|---|---|---|---|---|
| 0 | 348 (42) | 9 | 0 | 99 (36) |
| 1 | 366 (45) | 7 | 0 | 100 (34) |
| 2 | 67 (8) | 1 | 0 | 103 (36) |
| 3 | 36 (4) | 0 | 0 | 104 (52) |
| Total | 817 (100) | 17 | 0 | 100 (36) |
Abbreviations: IQR, interquartile range; TTP, time to culture positivity. The times to culture positivity were not significantly different for days 0, 1, 2, and 3 (P = 0.098, Kruskal-Wallis test). A total of 10 samples with a delay to processing of ≥4 days were excluded.
Table 2.
CFU counts of Mycobacterium tuberculosis per milliliter of sputum in sputum samples grouped by storage duration (days) from collection to processinga
| Processing day | No. (%) of sputum samples with indicated log10 CFU/ml | Total no. of contaminated sputum samples | No. of sputum samples with no growth | Log10 CFU, median (IQR) |
|---|---|---|---|---|
| 0 | 348 (43) | 12 | 6 | 5.97 (1.53) |
| 1 | 356 (44) | 8 | 10 | 6.06 (1.45) |
| 2 | 61 (8) | 0 | 3 | 6.18 (1.15) |
| 3 | 29 (4) | 0 | 2 | 6.19 (1.47) |
| Total | 794 (100) | 20 | 21 | 6.05 (1.45) |
Abbreviation: IQR, interquartile range. Log CFU data were not significantly different for day 0, day 1, day 2, and day 3 (P = 0.908, Kruskal-Wallis test). A total of 9 samples with a delay to processing of ≥4 days were excluded.
Sputum volumes.
Sputum volumes were equally distributed across the days of delay to processing (P = 0.897 and P = 0.970 for TTP and CFU, respectively). Volumes of <5 ml, 5 ml to 10 ml, and >10 ml were found in 5%, 46%, and 49% for TTP and in 4%, 46%, and 50% for CFU, respectively. Samples with <5-ml volume had a significantly increased median TTP compared to larger volume samples. A trend to decreased CFU was not seen (Table 3).
Table 3.
The relationship between time to culture positivity and viable CFU counts per milliliter of sputum and sputum volume
| Parametera | Values for sputum samples with indicated volume |
Values for total no. of sputum samples | P | ||
|---|---|---|---|---|---|
| <5 ml | 5–10 ml | >10 ml | |||
| Median TTP (h) (IQR) | 113 (70–267) | 98 (58–401) | 100 (49–544) | 100 (49–544) | <0.01b |
| Median log10 CFU/ml (IQR) | 5.97 (2.85–7.82) | 5.96 (2.30–7.88) | 6.11 (2.59–7.78) | 6.05 (2.30–7.88) | 0.144 |
Abbreviations: IQR, interquartile range; TTP, time to culture positivity.
TTP significantly differed between volume <5 ml and larger volumes (P < 0.01, Kruskal-Wallis test).
Time to processing.
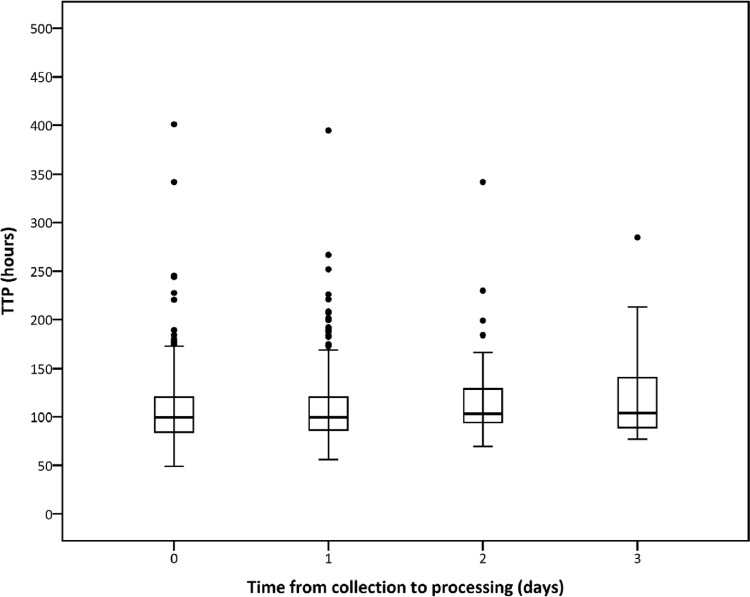
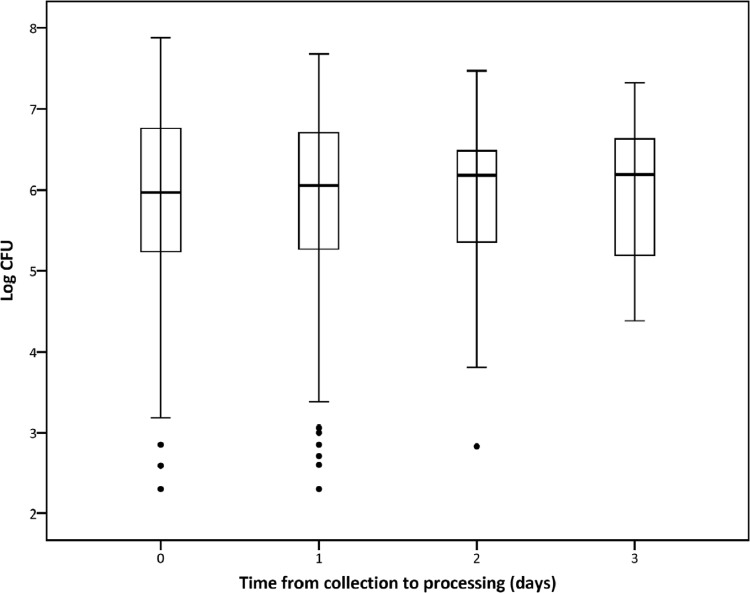
The median TTP derived from 817 samples was 100 ± 36 (IQR) h. TTP values were not significantly different between the days to processing (P = 0.098, Kruskal-Wallis test, Table 1), but a trend toward prolonged TTP was noted over time (P = 0.052, Jonckheere-Terpstra test, Fig. 1) that could be estimated by linear regression as 2.9 h per day of storage. The median CFU count on 794 samples was 6.05 ± 1.45 log CFU/ml sputum. There was no difference between log CFU values at different days of processing (P = 0.908, Kruskal-Wallis test, Table 2), and no significant trend was found over time (P = 0.551, Jonckheere-Terpstra test, Fig. 2) when storage time increased.
Fig 1.
Box plots of TTP (in hours) of 817 samples grouped by the numbers of days from collection to processing. TTP were not significantly different between days to processing (P = 0.098, Kruskal-Wallis test), but a trend toward increased TTP over time was observed (P = 0.052, Jonckheere-Terpstra test). With linear regression, this increase is estimated as 2.9 h of TTP per day of storage.
Fig 2.
Box plots of log CFU of 794 samples grouped by the numbers of days from collection to processing. Log CFU were not different between days to processing (P = 0.908, Kruskal-Wallis test).
DISCUSSION
This study has shown that the determination of the M. tuberculosis load in sputum samples collected overnight by CFU counts on agar plates was unaffected by sputum volume and by storage for up to 3 days under refrigerated conditions. TTP in liquid media were not statistically different between discrete days of storage, but they tended to increase slowly over time and were prolonged when sputum volumes were <5 ml. Contamination rates were low and did not increase with storage time. This suggests that central laboratories can be used for studies that include quantitative mycobacteriological endpoints if requirements for refrigerated sputum storage and transport conditions can be met and the time to processing does not exceed a few days.
The trend showing a mild and linear increase in TTP while log CFU results remained stable is an interesting observation. This may be explained by the use of the underlying two culture systems. TTP in liquid culture is chiefly driven by the most metabolically active subpopulations present in the culture, while on agar plates any colony visible after 3 to 4 weeks of incubation is accounted for. Delays in processing may induce subtle downshifts in bacterial metabolism. This is likely to directly affect TTP as an essentially metabolic measurement. Colonies on agar plates might be imperceptibly smaller, but this does not affect the overall colony count. The effect of delayed processing might be larger in samples stored over a longer period of time or in samples collected during treatment, because exposure to antituberculosis drugs could shift the relative proportions of bacterial subpopulations according to their respective bactericidal or sterilizing properties. This study did not include samples collected from patients under treatment, nor was a meaningful number of samples available that were stored over a longer period. There was no indication from the sparse data that the trends observed would not continue over longer periods of storage.
How relevant would a TTP prolongation of 2.9 h per day of delay to processing be from the perspective of a clinical trial? With standard four-drug anti-TB treatment (weight-adjusted isoniazid, rifampin, pyrazinamide, and ethambutol), TTP increases by 37 h per day over the first 2 days and on average by 13.4 h per day over the first 14 days of therapy (15). This is a considerably larger daily change than the 2.9 h that storage might induce. An additional consideration is the technical variation of the TTP measurement, which we estimated to be 4.3 h from 2,198 duplicate TTP measurements from the same sputum sample (8). This duration is, again, greater than the increase expected from a day of storage.
Low-volume samples were infrequent, but we noted a significantly prolonged TTP in sputum volume samples of <5 ml whereas CFU counts were unaffected. The reasons for this are unclear. Storage times were equally distributed, implying that sputum volumes cannot be the reason for the mild increase in TTP over time. It has previously been documented that CFU counts from samples collected over a shorter period of time and thus of smaller volume have a significantly higher variation (12). Tessema et al. found that low-volume sputa were particularly prone to loss of viability when frozen for storage (7). The minimum sputum volume that should be targeted in studies with quantitative mycobacteriology endpoints deserves further investigation.
In summary, this report provides evidence that pooled sputum samples can be stored under refrigerated conditions for deferred processing for at least 3 days without increased contamination rates. CFU counts on solid media can be expected to be stable. Time to positivity in liquid culture might be prolonged, if at all, by a small and predictable margin. This means that central laboratories can be considered for quantitative mycobacterial study endpoints when delays to processing are not expected to exceed a few days. Care should be taken to collect sputum samples of sufficient volume.
ACKNOWLEDGMENTS
We thank the Global Alliance for TB Drug Development (Pretoria, South Africa, and New York, NY) and Pfizer (Groton, CT) for samples collected during their studies.
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Bhat J, Selvakumar N, Rao VG, Gopi PG, Yadav R, Wares DF. 2011. Yield of culture of Mycobacterium tuberculosis complex in sputum samples transported from tribal areas. Int. J. Tuberc. Lung Dis. 15:478–482 [DOI] [PubMed] [Google Scholar]
- 2. Banda HT, Harries AD, Boeree MJ, Nyirenda TE, Banerjee A, Salaniponi FM. 2000. Viability of stored sputum specimens for smear microscopy and culture. Int. J. Tuberc. Lung Dis. 4:272–274 [PubMed] [Google Scholar]
- 3. Rao KP, Nair SS, Cobbold N, Naganathan N. 1966. Some operational factors influencing the utility of culture examination in the diagnosis of pulmonary tuberculosis. Bull. World Health Organ. 34:589–604 [PMC free article] [PubMed] [Google Scholar]
- 4. Sula L, Sundaresan TK, Langerova M. 1960. Effects of storage and transport on the cultivability of mycobacteria. Bull. World Health Organ. 23:635–651 [PMC free article] [PubMed] [Google Scholar]
- 5. Paramasivan CN, Narayana AS, Prabhakar R, Rajagopal MS, Somasundaram PR, Tripathy SP. 1983. Effect of storage of sputum specimens at room temperature on smear and culture results. Tubercle 64:119–124 [DOI] [PubMed] [Google Scholar]
- 6. Piersimoni C, Scarparo C, Callegaro A, Tosi CP, Nista D, Bornigia S, Scagnelli M, Rigon A, Ruggiero G, Goglio A. 2001. Comparison of MB/BacT ALERT 3D system with radiometric BACTEC system and Löwenstein-Jensen medium for recovery and identification of mycobacteria from clinical specimens: a multicenter study. J. Clin. Microbiol. 39:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. 2011. Rate of recovery of Mycobacterium tuberculosis from frozen acid-fast-bacillus smear-positive sputum samples subjected to long-term storage in Northwest Ethiopia. J. Clin. Microbiol. 49:2557–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diacon AH, Maritz JS, Venter A, van Helden PD, Dawson R, Donald PR. 2012. Time to liquid culture positivity can substitute for colony counting on agar plates in early bactericidal activity studies of antituberculosis agents. Clin. Microbiol. Infect. 18:711–717 [DOI] [PubMed] [Google Scholar]
- 9. Hesseling AC, Walzl G, Enarson DA, Carroll NM, Duncan K, Lukey PT, Lombard C, Donald PR, Lawrence KA, Gie RP, van Helden PD, Beyers N. 2010. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int. J. Tuberc. Lung Dis. 14:560–570 [PubMed] [Google Scholar]
- 10. Visser ME, Stead MC, Walzl G, Warren R, Schomaker M, Grewal HM, Swart EC, Maartens G. 2012. Baseline predictors of sputum culture conversion in pulmonary tuberculosis: importance of cavities, smoking, time to detection and W-Beijing genotype. PLoS One 7:e29588 doi:10.1371/journal.pone.0029588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donald PR, Diacon AH. 2008. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb) 88(Suppl 1):S75–S83 [DOI] [PubMed] [Google Scholar]
- 12. Hafner R, Cohn JA, Wright DJ, Dunlap NE, Egorin MJ, Enama ME, Muth K, Peloquin CA, Mor N, Heifets LB. 1997. Early bactericidal activity of isoniazid in pulmonary tuberculosis. Optimization of methodology. The DATRI 008 Study Group. Am. J. Respir. Crit. Care Med. 156(Pt 1):918–923 [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization 1998. Laboratory services in tuberculosis control. (WHO/tuberculosis/98.258). WHO, Geneva, Switzerland: http://www.who.int/tb/dots/lab.pdf [Google Scholar]
- 14. Jonckheere AR. 1954. A distribution-free k-sample test against ordered alternatives. Biometrika 41:133–145 [Google Scholar]
- 15. Diacon AH, Dawson R, du Bois J, Narunsky K, Venter A, Donald PR, van Niekerk C, Erondu N, Ginsberg AM, Becker P, Spigelman MK. 2012. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56:3027–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]