Abstract
We report the first case of Neosartorya laciniosa invasive sinusitis involving the orbit in an immunocompromised male with aplastic anemia. Treatment included surgical debridement with enucleation of the eye and combination voriconazole and micafungin therapy followed by voriconazole alone. The fungus was identified using sequencing of partial benA and calmodulin genes.
CASE REPORT
A 66-year-old male was diagnosed with aplastic anemia in March 2010 and subsequently treated with bone marrow transplant (BMT) followed by immunosuppressive therapy with lymphocyte immune globulin antithymocyte globulin (Atgam; Pfizer Canada Inc., Kirkland, Quebec, Canada), steroids, and cyclosporine. On 19 May 2011, he was referred from a community hospital to the University of Alberta Hospital with a history of pancytopenia (absolute neutrophil count [ANC] of <500 cells/μl) beginning in February 2011 and intermittent fevers since mid-April. Additional findings included increased left-sided periorbital swelling and radiographic evidence of pneumonia, but no respiratory symptoms were evident. He had not received antifungal prophylaxis. On admission, he was hemodynamically stable and febrile (temperature of 39.1°C). A complete blood count (CBC) revealed pancytopenia with a white blood cell count of 400 cells/μl (ANC, 0 cells/μl) and a creatinine level of 112 μmol/liter (estimated glomerular filtration rate, 57 ml/min). Electrolytes and liver enzymes were normal. Urine and blood cultures were negative.
The patient was treated empirically with vancomycin and ciprofloxacin for presumed periorbital cellulitis. A computed tomography (CT) scan of the head showed left-sided protposis and progression of fluid and high-density material causing opacification within all of the visualized paranasal sinuses on the left side. This finding was interpreted to represent probable chronic paranasal sinus inflammation. A prior head CT scan done at the time of the BMT was normal. The patient deteriorated clinically with adverse changes, including advancing opacification of frontal, ethmoidal, and maxillary sinuses, with erosions of bony septations of ethmoidal air cells on the left side seen on repeat CT scan on day 6 postadmission. An abscess along the wall of the left medial orbit was documented on CT scan on day 13, and the patient was sent to the operating room the next day for drainage of a subperiosteal-enhancing fluid collection. Intraoperative tissue samples were sent for pathological analysis as well as for bacterial and fungal cultures. Bacterial culture yielded only coagulase-negative Staphylococcus (CoNS). No fungal elements were seen on direct smear with calcofluor white (BD Diagnostic Systems, Sparks, MD), and fungal cultures were negative. However, the pathology report documented hemorrhagic necrotic sinus mucosal tissue with numerous branched, septate hyphae and focal vascular invasion consistent with invasive fungal infection (Fig. 1A). The patient was promptly started on liposomal amphotericin B (5 mg/kg body weight/day). Tissue from a second debridement done on day 16 was again negative for hyphae by direct microscopy, but culture yielded growth of a mold within 3 days that was suspected later to be a possible “white, nonsporulating Aspergillus” species. An exoantigen immunodiffusion test, in which a filtrate from the culture was tested against commercially available antibody reagents (Pulse Scientific Inc., Burlington, Ontario, Canada), gave a positive result for Aspergillus fumigatus. The isolate was identified as a Neosartorya species when characteristic ascospores were produced 2 weeks later. A serum galactomannan enzyme immunoassay (Platelia Apergillus EIA; Bio-Rad Laboratories Ltd., Montreal, Quebec, Canada) performed following the manufacturer's protocol on day 21 was also positive, with an index value of 2.8 (≥0.5 used as the cutoff value) confirming the diagnosis of invasive aspergillosis. Antifungal therapy was switched to 300 mg voriconazole twice daily, and the patient continued on vancomycin, ciprofloxacin, and cyclosporine and acyclovir for immunosuppression and antiviral prophylaxis, respectively.
Fig 1.
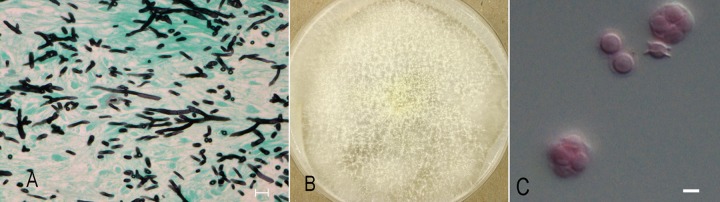
(A) Gomorri methanamine silver-stained section of sinus mucosal tissue showing numerous branched, septate hyphae (bar = 20 μm); (B) colony on OAT showing ascomata after 7 days of growth at 35°C; (C) ascospores in face and side view showing two crests (bar = 5 μm).
Despite the antifungal and broad antimicrobial coverage, the patient continued to be pancytopenic and was persistently febrile. A CT scan on day 26 showed suggestion of dehiscence of the sphenoid sinus, and possible communication with the anterior cranial fossa could not be excluded. No intracranial venous thrombosis was noted on magnetic resonance imaging of the brain. A repeat CT scan on day 33 revealed evidence of gas within a large collection of fluid infraorbitally measuring 5.6 cm in coronal diameter and again extensive mucoperiosteal thickening within frontal and ethmoidal regions as well as the right maxillary sinus. The patient was taken for surgical enucleation of the left eye on day 35, and further debridement was performed 3 days later. Fungal hyphae accompanied by tissue necrosis were again seen on histopathology of tissue and cortical bone samples, and bacterial cultures were positive for Enterococcus species and CoNS. The patient was continued on vancomycin for a total of 6 weeks of therapy. Unfortunately, fungal culture was not requested at surgery, but a repeat serum galactomannan on day 40 remained positive with an index value of 2.075.
Based on our patient's tenuous clinical picture, we elected to continue him on 300 mg oral voriconazole twice daily and to add 150 mg intravenous micafungin daily based on reported uniform susceptibility to this drug among Neosartorya and related species classified within Aspergillus section Fumigati (1, 2). A final CT scan done on day 40 documented mucosal thickening within the remainder of paranasal sinuses and no fluid collections. He remained transfusion dependent for his pancytopenia but defervesced and had gradual clinical improvement, resulting in his transfer to a community hospital 13 days later. The patient completed a total of 6 months of antifungal therapy (7 weeks of combination voriconazole and micafungin and 4 months of monotherapy with voriconazole). He was discharged from the hospital 7 months after his initial presentation. He continued on cyclosporine for his aplastic anemia, and his blood counts stabilized. He was scheduled to have reconstructive surgery to his left orbital area. There was no evidence of recurrence of his fungal infection 2 months after stopping voriconazole therapy.
Mycology.
As per review of the literature, which indicated variable susceptibility to voriconazole depending on species of Neosartorya, the isolate was submitted for further identification to the University of Alberta Microfungus Collection and Herbarium (UAMH), where it was accessioned as UAMH 11627. Subcultures on potato dextrose agar (PDA) (BD) and oatmeal salts agar (OAT; prepared in-house) were incubated at 30°C and 35°C. Growth was faster at 35°C, with the colony on PDA reaching a diameter of 6 cm after 3 days of incubation. The submitting laboratory reported growth at 45°C but no growth at 50°C. Colonies remained yellowish white with no blue-green surface coloration after 14 days. Conidial heads were sparse. The vesicle was about 15 μm wide and bore few phialides on the upper surface. Characteristic yellowish-white, thin-walled ascomata were produced on both media within 7 days but were more profuse on OAT (Fig. 1B). Ascospores were broadly lenticular with two prominent equatorial crests and measured 4.5 to 5 μm long (Fig. 1C). Sequences of the beta-tubulin and calmodulin gene regions were obtained for species identification. DNA was extracted using the EZNA SP fungal DNA kit (United Bioinformatica Inc., Saskatoon, Saskatchewan, Canada). The partial benA and calmodulin genes were sequenced using primers previously described (3, 4). Sequences were edited using Sequencher version 5.0 (Gene Codes Corp., Ann Arbor, MI) and compared with available sequences in the GenBank nucleotide database using a BLAST search. Results were similar for both searches, with the case isolate showing 99 to 100% benA similarity and 98 to 100% calmodulin similarity with several sequences of Neosartorya spinosa and Neosartorya laciniosa, including the ex-type strain KACC 41657. To further assess the genetic relationship between the case isolate and these Neosartorya species, a data set of combined benA and calmodulin sequences was obtained and a parsimony analysis was performed using PAUP* version 4.0b10 (http://paup.csit.fsu.edu/) and with Neosartorya fischeri as the outgroup. The robustness of the trees obtained was evaluated by 1,000 bootstrap replications. In the resultant tree, the case isolate is shown grouping with isolates of N. laciniosa with 100% bootstrap support, and the group is sister to N. spinosa (Fig. 2).
Fig 2.
One of 19 equally parsimonious trees (consistency index, 0.891; retention index, 0.867; homoplasy index, 0.109) inferred from maximum parsimony analysis of combined partial benA and calmodulin gene sequences showing the placement of the case isolate. Bootstrap values above 50% are shown. For each isolate, GenBank accession number and culture collection number are listed. Culture collection acronyms are as follows: CBS, Centraalbureau voor Schimmelcultures, Utrecht, Netherlands; IBT, Culture Collection of Fungi, Technical University of Denmark, Lyngby, Denmark; KACC, Korean Agricultural Culture Collection, Suwon, South Korea; NRRL, USDA Agricultural Research Service Culture Collection, Peoria, IL; SUM, Medical Mycology Research Center, Chiba University, Chiba, Japan; UAMH, University of Alberta Microfungus Collection and Herbarium, Edmonton, Canada.
Susceptibility testing performed retrospectively on isolate UAMH 11627 yielded MICs (mg/liter) of 0.5 for amphotericin B, 8 for 5-fluorouracil, >64 for fluconazole, 1 for itraconazole, 0.25 for posaconazole, 1 for voriconazole, and 0.5 for caspofungin.
Invasive aspergillosis (IA) is the most common filamentous fungal infection among immunocompromised patients, with very high mortality rates. Although the majority of infections are attributed to Aspergillus fumigatus, there is increasing recognition of the role of related species in causing invasive disease, especially since the advent of molecular methods allowing for more precise identification of the etiologic agent involved. There are currently nine Aspergillus species and 27 Neosartorya teleomorphs included within the Aspergillus section Fumigati (2) (Mycobank; http://www.mycobank.org/). Neosartorya species are generally recognized by their rapidly growing, poorly sporulating white to pale-green colonies in primary culture, by their thermotolerance (all growing at 37°C and a few up to 50°C), and by formation of ascomata containing ascospores on sporulation media. Currently, Neosartorya hiratsukae, Neosartorya pseudofischeri, and Neosartorya udagawae are confirmed as opportunistic pathogens, with the latter species gaining recent attention (5–13). Although N. fischeri and N. spinosa have been identified in some reports, their roles in causing infection have not been reliably confirmed. Isolates from some older cases have been reidentified as N. pseudofischeri, and isolates from more recent cases have not been identified using sequencing of genes appropriate for Neosartorya identification (14–17).
Neosartorya infections in humans may include invasive infections of eye, ear, or lung; endocarditis; peritonitis; multifocal brain abscesses; and osteomyelitis (5–16). We report the first case of N. laciniosa invasive sino-orbital aspergillosis (SOA) in a patient with aplastic anemia. How our patient acquired his infection is unknown. He did not live on a farm or acreage and had not worked for some time due to illness, so no specific exposures could be identified. Neosartorya laciniosa was described in 2006 for 12 isolates mainly from agricultural soils with a single isolation from strawberry fruit pulp (4). The species has a broad geographic distribution in Korea, Dominican Republic, Kenya, Pakistan, Netherlands, Surinam, and the United States. N. laciniosa is genetically closest to N. spinosa, and these species could not be reliably distinguished based on results of blast search. However, the differentiation between the species is clearly supported in the phylogenetic tree based on the combined data set (Fig. 2).
Neosartorya species are uncommonly isolated in our hospital, so there was concern 4 months after this patient's presentation when a pediatric patient with acute lymphocytic leukemia presented with a necrotic nasal lesion from which a Neosartorya species was again cultured. However, sequencing of the isolate from the second patient determined its identity as N. pseudofischeri.
SOA is a relatively uncommon form of aspergillosis, occurring in both immunocompetent and immunosuppressed humans, as well as in canines and felines. Most cases have been described based on clinical and histopathological features, and the etiologic agent has been infrequently identified to the species level (18). A recent study of the etiology of feline SOA in Australia determined that Neosartorya species were the cause of all 17 cases documented (19). Although sequencing of the internal transcribed spacer regions was insufficient to identify the isolates to the species level, the high percentage of similarity, the production of ascospores in some mating studies, and the frequent treatment failure suggest that N. udagawae was the main species involved. N. udagawae differs from N. laciniosa in being heterothallic, in failing to grow above 42°C, and in being refractory to antifungal therapy (10, 12, 13, 19).
Our patient was successfully treated with voriconazole and micafungin together with surgical debridement. Antifungal susceptibility testing done retrospectively on the case isolate showed that N. laciniosa is similar to N. hiratsukae in showing low MICs to amphotericin, itraconazole, voriconazole, and caspofungin (1). In contrast, both N. udagawae and N. pseudofischeri show high MICs to voriconazole and itraconazole (1, 10). It is therefore clinically important to not only differentiate Neosartorya isolates from the related Aspergillus species within the section Fumigati but also to identify Neosartorya isolates to the species level.
This case confirms that Neosartorya species should be suspected when a white thermotolerant Aspergillus species with sparse conidiation is isolated and that identification to the species level can be obtained by sequencing of partial benA and calmodulin genes. Accurate identification is necessary to elucidate clinical or therapeutic differences between Neosartorya species.
Nucleotide sequence accession numbers.
Sequences for N. laciniosa UAMH 11627 were deposited in GenBank under accession no. JX845619 for the partial calmodulin gene and JX845620 for the partial benA gene.
ACKNOWLEDGMENTS
We thank the Mycology Unit, Laboratory Medicine and Pathology, University of Alberta Hospitals, for providing results of susceptibility testing.
L. Sigler acknowledges financial support from the Natural Sciences and Engineering Research Council of Canada (G121160004).
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Alcazar-Fuoll L, Mellado E, Alastruey-Izqulerdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samson RA, Hong S, Peterson SW, Frisvad JC, Varga J. 2007. Polyphasic taxonomy of Aspergillus section Fumigati and its teleomorph Neosartorya. Stud. Mycol. 59:147–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA. 2006. Novel Neosartorya species isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 56:477–486 [DOI] [PubMed] [Google Scholar]
- 5. Shivaprakash MR, Jain N, Gupta S, Baghela A, Gupta A, Chakrabarti A. 2009. Allergic fungal rhinosinusitis caused by Neosartorya hiratsukae from India. Med. Mycol. 47:317–320 [DOI] [PubMed] [Google Scholar]
- 6. Koutroutsos K, Arabatzis M, Bougatsos G, Xanthaki A, Toutouza M, Belegraki A. 2010. Neosartorya hiratsukae through continuous ambulatory peritoneal dialsysis. J. Med. Micro. 59:862–865 [DOI] [PubMed] [Google Scholar]
- 7. Lehtmaa JH, Summerbell RC, Hoekstra ES, Samson RA, Naaber P. 2004, Isolation of Neosartorya pseudofischeri from blood: first hint of pulmonary aspergillosis. J. Clin. Microbiol. 42:925–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghebremedhin B, Bluemel A, Neumann KH, Koenig B, Koenig W. 2009. Peritonitis due to Neosarotrya pseudofischeri in an elderly patient undergoing peritoneal dialysis successfully treated with voriconazole. J. Med. Microbiol. 58:678–682 [DOI] [PubMed] [Google Scholar]
- 9. Balajee AS, Gribskov J, Brandt M, Ito J, Fothergill A, Marr KA. 2005. Mistaken identity: Neosartorya pseudofischeri and its anamorph masquerading as Aspergillus fumigatus. J. Clin. Microbiol. 43:5996–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vinh DC, Shea YR, Sugui JA, Parrilla-Castellar ER, Freeman AD, Campbell JW, Pittaluga S, Jones PA, Zelazny A, Kleiner D, Kwon-Chung KJ, Holland SM. 2009. Invasive aspergillosis due to Neosartorya udagawe. Clin. Infect. Dis. 49:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sugui JA, Vinh DC, Nardone G, Shea YR, Chang YC, Zelazny AM, Marr KA, Holland SM, Kwong-Chung KJ. 2010. Neosartorya udagawe (Aspergillus udagawe), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Posteraro B, Mattei R, Trivella F, Maffei A, Torre A, De Carolis E, Posteraro P, Fadda G, Sanguinetti M. 2011. Uncommon Neosartorya udagawe fungus as a causative agent of severe corneal infection. J. Clin. Microbiol. 49:2357–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gyotoku H, Izumikawa K, Ikeda H, Takazono T, Morinaga Y, Nakamura S, Imamura Y, Nishino T, Miyazaki T, Kakeya H, Yamamoto Y, Yanagihara K, Yasuoka A, Yaguchi T, Ohno H, Miyzaki Y, Kamei K, Kanda T, Kohno S. 2012. A case of bronchial aspergillosis caused by Aspergillus udagawae and its mycological features. Med. Mycol. 50:631–636 [DOI] [PubMed] [Google Scholar]
- 14. Lonial S, Wiliams L, Carrum G, Ostrowski M, McCarthy P., Jr 1997. Neosartorya fischeri: an invasive fungal pathogen in an allogeneic bone marrow transplant patient. Bone Marrow Transplant. 19:735–755 [DOI] [PubMed] [Google Scholar]
- 15. Summerbell RC, de Repentigny L, Chartrand C, St Germain G. 1992. Graft-related endocarditis caused by Neosartorya fischeri var. spinosa. J. Clin. Microbiol. 30:1580–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padhye AA, Godfrey JH, Chandler FW, Peterson SW. 1994. Osteomyelitis caused by Neosartorya pseudofischeri. J. Clin. Microbiol. 32:2832–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krockenberger MB, Swinney G, Martin P, Rothwell TR, Malik R. 2011. Sequential opportunistic infection in two German Shepherd dogs. Aust. Vet. J. 89:9–14 [DOI] [PubMed] [Google Scholar]
- 18. Pushker N, Meel R, Kashyap S, Bajaj MS, Sen S. 2011. Invasive aspergillois of orbit in immunocompetent patients: treatment and outcome. Ophthalmology 118:1886–1891 [DOI] [PubMed] [Google Scholar]
- 19. Barrs VR, Halliday C, Martin P, Wilson B, Krockenberger M, Gunew M, Bennett S, Koehlmeyer E, Thompson A, Filegner R, Hocking A, Sleiman S, O'Brien C, Beatty JA. 2012. Sinonasal and sino-orbital aspergillosis in 23 cats: aetiology, clinicopathological features and treatment outcomes. Vet. J. 191:58–64 [DOI] [PubMed] [Google Scholar]