Abstract
Tests for haemoglobinopathy carrier status are the commonest genetic screening tests undertaken internationally. Carrier screening for β-thalassaemia is not coordinated in Victoria, Australia, and is instead incorporated into routine practice where most women are screened antenatally, through a full blood examination (FBE). Little is known about how women are screened for β-thalassaemia in Australia as well as their attitudes towards the screening process. This study was conducted to explore carriers’ and carrier couples’ experiences of and attitudes towards β-thalassaemia screening in Australia. Semi-structured interviews with 26 recently pregnant female carriers and ten carrier couples of β-thalassaemia were carried out. Interviews were analysed using inductive content analysis. Unexpectedly, more than half of the women had been made aware of their carrier status prior to pregnancy, with FBEs carried out for numerous reasons other than thalassaemia screening. Most women did not recall being told about thalassaemia before notification of their carrier status and therefore did not make a decision about being screened. They were generally accepting for doctors to decide about testing; however, would have preferred to have been made aware of the screening test. Women also reported receiving insufficient information after being notified of their carrier status, leading to misconceptions and confusion. This genetic screening process, incorporated into routine care whereby informed decisions were not being made by patients, was apparently acceptable overall. Based on the results of this study, we make the following recommendations: (1) individuals should be made aware that they are being tested for thalassaemia at least before a specific thalassaemia diagnostic test is performed; (2) current understanding by known carriers of thalassaemia should be assessed and any misconceptions corrected; (3) written information should be provided to carriers; (4) referral of carrier couples to specialists in thalassaemia and genetics is strongly recommended; (5) the term ‘carrier of β-thalassaemia’ should be used rather than ‘thalassaemia minor’.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-012-0136-7) contains supplementary material, which is available to authorized users.
Keywords: Beta-thalassaemia, Carrier screening, Genetic testing, Screening program, Consent, Qualitative research
Introduction
β-Thalassaemia is a significant public health issue (Modell and Darlison 2008); therefore, carrier screening is important to identify asymptomatic carriers who have an increased risk of having a child with this condition. Where both members of a couple are found to be carriers and therefore have a one in four risk of having an affected child, informed reproductive decisions about whether or not to avoid having a child with β-thalassaemia can be made, generally through prenatal diagnosis and termination of affected foetuses or preimplantation genetic diagnosis (ACOG 2007).
The World Health Organization (WHO) guidelines on ethical issues in medical genetics released in 1998, made it clear that screening for genetic conditions as well as the actions following testing outcomes should be voluntary (WHO 1998). Informed consent is therefore considered to be an important component of most genetic screening programs requiring all participants to be appropriately educated about the condition and testing so that informed decisions can be made about undertaking the genetic test offered (Delatycki 2008). Thus, population carrier screening in Australia for genetic conditions such as cystic fibrosis and Tay–Sachs disease is usually offered through formal, co-ordinated programs (Gason et al. 2003; Massie et al. 2009). No such program exists for thalassaemia carrier screening (Cousens et al. 2010).
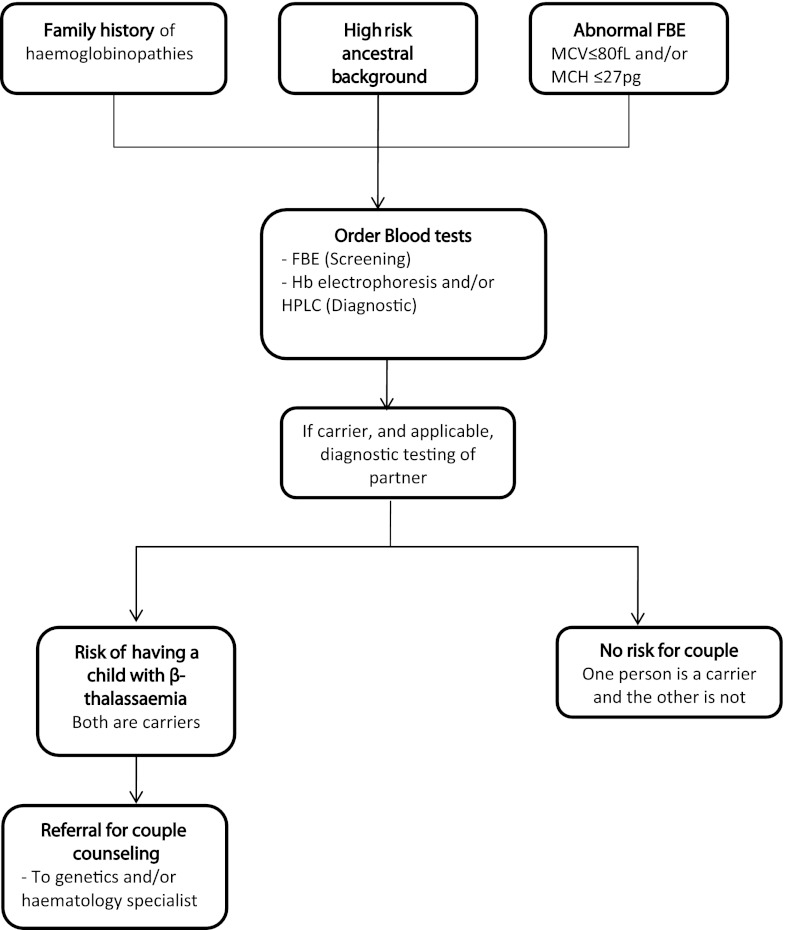
β-Thalassaemia is an autosomal recessive condition (Trent 2006). In this paper, the term β-thalassaemia is used to describe the condition that results from homozygous or compound heterozygous mutations in the β-globin gene. Affected individuals have a reduction in β-globin chain production and consequently insufficient haemoglobin (Hb), requiring regular blood transfusions to reverse the severe microcytic anaemia (Birgens and Ljung 2007) and iron chelation therapy to remove excess iron accumulated from transfusions (Metcalfe et al. 2007). Carriers of β-thalassaemia are asymptomatic, but also have somewhat reduced production of β-globin chains, and show an elevated red blood cell (RBC) count, reduced mean corpuscular volume (MCV) and mean corpuscular haemoglobin (MCH) values and high haemoglobin A2 (HbA2) (Birgens and Ljung 2007; Chui et al. 2006; Langlois et al. 2008). The identification of carriers of β-thalassaemia is generally conducted with a full blood examination (FBE) as the first step (Trent 2006). The FBE will detect people with reduced MCV and/or MCH levels. These people as well as those with a family history of haemoglobinopathies or those from a high-risk ethnic background are recommended to have further testing by Hb electrophoresis or high performance liquid chromatography to detect elevated HbA2 (Fig. 1) (Trent 2006). Partners of carriers are then recommended to have their HbA2 level ascertained, in order to determine if the couple is at risk of having a child affected by β-thalassaemia.
Fig. 1.
Recommendations for thalassaemia carrier testing in Australia (adapted from Metcalfe et al. 2007)
The overall β-thalassaemia carrier frequency in Australia is not known, but an increasing number of carriers of thalassaemia are being identified in Australia as a result of migration from countries with a high prevalence of β-thalassaemia (Metcalfe et al. 2007). In the state of Victoria, Australia (population approximately 5 million), between the years 2004 and 2008, 122 antenatal diagnostic tests were performed for β-thalassaemia. Only four babies affected with β-thalassaemia were born within that period, with two of the sets of parents being aware of their risk and deciding not to take steps to prevent the birth of an affected child (DK Bowden, personal communication).
In Australia, the identification of β-thalassaemia carriers is incorporated into routine care, with the majority of pregnant women having an FBE early in pregnancy. Although one rationale for conducting the FBE is to screen for thalassaemia carrier status, there is no centralised or co-ordinated approach to this screening (Flander et al. 2003). In contrast to structured carrier screening programs, the experiences of women notified antenatally that they are carriers of β-thalassaemia are not known and the acceptability of the processes they experienced is also unclear. Indeed, as most pregnant women have blood collected for several tests simultaneously, with thalassaemia screening only being one of them (Delatycki 2008), it is unknown whether these women are aware that they are being screened for β-thalassaemia carrier status. The aim of this study was to explore carriers’ experiences of and attitudes towards the antenatal β-thalassaemia carrier screening process in Victoria, Australia.
Materials and methods
Ethics committee approval
The study was approved by the Royal Women’s Hospital Human Research Ethics Committee (09/02), Southern Health Human Research Ethics Committee (09012B), Mercy Health Human Research Ethics Committee (R09/45) and The University of Melbourne Human Research Ethics Committee (0931582).
Study design and recruitment
A retrospective qualitative study was conducted as this is the most appropriate methodology for studying areas where there is limited knowledge available, as it explores people’s own interpretations and understandings about the phenomenon in question in a flexible way (Liamputtong and Ezzy 2005). This study drew upon the theory of phenomenology whereby “researchers study everyday events from within the life-world of the person experiencing them” (Liamputtong and Ezzy 2005).
Carrier women and carrier couples were purposively sampled for interviews. The inclusion criteria for women invited into the study were: (1) had undergone antenatal carrier screening through either the public or private health sector within 12 months prior to the interview; and, either (2) were found to be carriers of β-thalassaemia but whose partners were not; or (3) both partners were identified as carriers of β-thalassaemia or one was a carrier of β-thalassaemia and the other was a carrier of haemoglobin E (HbE). These couples were included as the clinical consequences of HbE/β-thalassaemia are similar to that of β-thalassaemia.
Between June 2009 and October 2010, carriers and carrier couples who had attended the three major public maternity hospitals in Melbourne, the capital of Victoria, or had been screened through a private pathology company during their pregnancy, were invited to participate in the study by a letter sent out by their medical practitioner or genetic counsellor, as required by the Human Research Ethics Committees and in accordance with Australian privacy legislation. Public hospital patients were followed up with phone calls made by clinic staff if there was no response to the initial invitation within 2 weeks. Carriers in the private sector were sent letters that were initially sent to their doctor by the private pathology company with a request to pass these on to the patients. Due to the recruitment process, the participation rate of women who were invited through the private pathology company cannot be determined since it is not known how many letters were subsequently forwarded to the β-thalassaemia carriers by their doctors. Forty-four female carriers and 19 carrier couples from the public sector were known to be invited to participate in the study.
Data collection
Semi-structured interviews were conducted by one researcher (NC). The interview topic guideline is shown in Table S1. Participants were asked to reflect on and describe the screening process that they had experienced in detail and were then asked to discuss their views on the β-thalassaemia screening process. Interpreters were offered when English was not a participant’s first language. The interviews lasted between 40 min and 2 h. All interviews were digitally audio-recorded and transcribed verbatim. Participants were de-identified and a code used. Data collection and analysis were performed concurrently with interviews continuing until no new themes emerged from the interviews.
Data analysis
An inductive content analysis approach was used to analyse the data, whereby categories are derived from the data which are first coded (Elo and Kyngas 2008; Hsieh and Shannon 2005). Codes were sorted into categories and sub-categories within a hierarchical system comparing similarities and differences between the codes. This continued as an iterative process to identify the emerging themes within or across all categories (Polit and Beck 2006), as an expression of the latent content (Graneheim and Lundman 2004). A sample of interviews was coded independently by the other researchers. Differences in coding were discussed and changes to the coding and categorisation was made when necessary until an agreement about the overall themes was reached. The software program NVivo 9 (QSR international, Victoria, Australia) was used to manage data analysis.
Quotes from participants are de-identified and the following notations used: carriers: ‘carriers’; couples: ‘carrier couples’. Where quotes are given, the participants’ nationalities have been indicated, including if they were born in Australia, or if English is their second language. The gender of members of carrier couples is also indicated. In instances where the notation is similar for different participants, a number is used to distinguish these, e.g., ‘Carrier, Italy, born in Australia (1)’ or ‘Carrier, Italy, born in Australia (2)’.
Results
Forty-five individuals comprising 26 female carriers of β-thalassaemia and ten carrier couples (with only the woman participating from one of the couples) were interviewed. Six of the carriers were recruited through the private system with the remaining carriers and all of the carrier couples recruited through the public hospitals. Participants were from a wide range of nationalities and ethnic backgrounds with 14 individuals migrating to Australia in recent years. Participants’ self-identified regions/countries of ancestry are shown in Table 1, illustrating the diversity of the participants. Eight of the carriers and two of the couples interviewed did not speak English as their first language, however only one woman required an interpreter and was interviewed by phone to facilitate this.
Table 1.
Self-identified regions/countries of participants’ ancestry
| Region/country of origin | Female carriers (n = 26) | Carrier couples (n = 19) | Total participants (n = 45) | Participants born in Australia (n = 28) |
|---|---|---|---|---|
| Europe | 10 | 5F 4M | 19 | 18 |
| Asia: China | 2 | 1F 1M | 4 | 3 |
| Indian subcontinent | 5 | 1F 1M | 7 | 0 |
| Southeast Asia | 5 | 2F 2M | 9 | 1 |
| Africa | 1 | – | 1 | 1 |
| Australia | 1 | – | 1 | 1 |
| Middle East | 2 | 1F 1M | 4 | 3 |
Countries of origin within Europe included: Greece, Cyprus, Italy, Macedonia and Ukraine; SE Asia included: Thailand, Philippines, Vietnam, Laos and Malaysia; Indian subcontinent included: India, Bangladesh, and Sri Lanka; Africa included: Egypt and South Africa; and the Middle East included: Lebanon and Iran
M male, F female
Four major themes emerged from these interviews, which were: (1) variability in timing of carrier identification; (2) decision-making: tensions between being informed and providing consent; (3) lack of understanding regarding thalassaemia and genetic risk; and (4) obtaining information.
(1) Variability in timing of carrier identification
“I’ve always known I was a carrier”
Although women were recruited to the study on the basis that they had been identified as a carrier when screened for β-thalassaemia during a recent pregnancy, an unexpected finding from this study was that 23 out of the 36 women interviewed had already been identified as carriers of β-thalassaemia before pregnancy. They were then re-tested during the most recent pregnancy and, in some cases, in previous pregnancies as well. These 23 women were found to be carriers at different ages ranging from early childhood through to adulthood. Three were tested as babies after their mothers were found to be carriers, and were later told about their carrier status by their mothers. Three women found out as teenagers or young adults, after requesting testing based on their family history of β-thalassaemia. Seventeen women found out incidentally when blood tests were carried out to investigate a range of different symptoms unrelated to thalassaemia.
“Important to know beforehand”
Women who had become aware of their carrier status before pregnancy as well as those who were first notified during pregnancy believe that thalassaemia screening should take place before pregnancy, as pregnancy was thought to be a stressful time. Testing before pregnancy was seen as important as reproductive options are limited if a couple are identified as carriers during a pregnancy.
…once you’re pregnant it’s either too late or you’re in a bit of an emotional state if he’s delivering bad news and it’s kind of too late to do anything. (Carrier, Greece, born in Australia (1))
Screening results were seen by some as potentially influencing choice of partners, whereas others did not think it would change their choice of partner but still believe that it is important to know before pregnancy. This importance of screening before pregnancy was particularly stressed by women who had only became aware of their carrier status after undergoing antenatal screening.
Some women, who had already been identified as carriers before pregnancy, believe that screening should occur at an early age. They feel that children, particularly teenagers, should be screened for thalassaemia as they are of child-bearing age and could have an unexpected pregnancy.
(2) Decision-making: tensions between being informed and providing consent
“He didn’t say that thalassaemia might come up”
Women were often unaware that they were being screened before being told of their carrier status, and believed that they were having a routine blood test. They reported that their doctor did not forewarn them that a possible outcome of the blood test could be identification as a β-thalassaemia carrier, or inform them of any specific thalassaemia diagnostic tests being carried out.
He didn’t say that thalassaemia might come up. Nothing like that was brought up. It was just ‘routine blood check for pregnancy, this is what we’re gonna be looking for… we’ll let you know if anything’s of concern’ and yeah that was it. (Carrier, Italy, born in Australia (1))
Some women described being asked to undergo a second blood test after the initial test results from the antenatal FBE were received. Even when women were told that they may be a carrier of thalassaemia, and were therefore aware that they were being tested for β-thalassaemia before undergoing this second blood test, they did not perceive this to be a decision left up to them to make. Rather the test was seen as a strong recommendation from their health professional.
Many women who already knew they were a carrier were re-screened at the beginning of the pregnancy and therefore re-identified as carriers. This was often due to lack of communication between the women and healthcare professionals, with the women not notifying their obstetric healthcare professionals and healthcare professionals not asking about carrier status or making women aware that the thalassaemia carrier screening was occurring.
Well the doctor didn’t say it’s for thalassaemia because had the doctor said it I would have made a comment that I know I’m a carrier. So thalassaemia wasn’t mentioned at all. (Carrier, Italy, born in Australia (2))
When asked about earlier testing experiences, the majority of women who originally found out about their carrier status before pregnancy were also not aware that they were being tested for thalassaemia at that time until they received their diagnosis.
I was either eighteen or nineteen and I wasn’t feeling too well, I was quite thin, and I went to a doctor and he just said ‘let’s get a routine blood check to see what’s going on’ you know … iron levels and so forth, and that’s when I found out that I was a carrier of thalassaemia minor. (Carrier Couple-Woman, Cyprus, born in Australia)
“I’m happy with whatever…leave it up to the doctors”
The impression that women were undergoing a routine blood test at the beginning of their pregnancy meant that they did not actively make a decision about thalassaemia screening. Women trusted their doctors to make the decisions about the tests carried out during their pregnancy, believing that their doctors knew what was best for them and therefore should decide. In some cases, this was because the women felt they lacked sufficient knowledge about medical conditions and tests in pregnancy, particularly during their first pregnancy.
Even if they have options for us to choose, we still wouldn’t know it, because it’s our first time, you know. We’re just relying on the experts to tell us. (Carrier Couple-Woman, China, born in Australia)
Nonetheless, many would have preferred to be notified at the outset that they were being screened for thalassaemia. They indicated that at least a small amount of information before testing would have helped them prepare for receiving their results. Women who had been made aware of their carrier status before pregnancy also believe that people should be made aware that thalassaemia carrier identification is a possible outcome of the blood test being conducted.
I do think it’s important to be told what they’re going to be looking for, … not just out of the blue ‘we happen to screen your blood for this and we found you’re positive’… cause if you come back with the positive results of something that you don’t know that you’ve even been screened for it, it’s quite a shock. (Carrier, South Africa, born in Australia)
By contrast, there were others who did not think it was necessary to be informed about thalassaemia screening, specifically when multiple tests were being performed during pregnancy. A few felt that knowing beforehand about the numerous potential outcomes from blood tests would be overwhelming and anxiety provoking.
Even though many would have preferred to be notified that the test was being carried out, most women did not feel that the provision of consent is necessary for the thalassaemia carrier test, whether carried out before or during pregnancy. Indeed, some of these carriers supported mandatory screening without consent, but nevertheless still believed that women should be informed of the screening. A small number, however, believed that women should make the final decision regarding tests carried out during pregnancy themselves, after recommendations are made, as well as provide consent.
(3) Lack of understanding regarding thalassaemia and genetic risk
“Not much was said about it”
Most participants had not heard about thalassaemia until they were first notified that they were a carrier and recalled being provided with little if any information about thalassaemia in antenatal or other settings. This often resulted in a lack of understanding about β-thalassaemia and thus some anxiety for many women.
When participants underwent antenatal screening, many recalled that their obstetric healthcare professional provided them with very little information about thalassaemia or genetic risk after they were informed that they are a carrier and did not understand the meaning and implications of the diagnosis.
I haven’t received any information from anyone about this condition. I haven’t. And yeah it would be nice to know what it means…so here I am two kids later… I know I’m a carrier… I was tested the first time around, wasn’t tested the second time around and I really don’t know what it means, unless I actually go and find out the information myself. (Carrier, Macedonia, born in Australia)
Women who were diagnosed as carriers before pregnancy when they were teenagers or young adults were usually told by their GP that it was not important for them at that time, therefore their attitude had been quite blasé. Some of these women were informed that thalassaemia is common amongst certain ethnic groups and they were often advised not to marry someone from the same ethnic background as themselves.
The doctor actually said when you find someone to marry, make sure he’s blonde and has blue eyes because he’s from Nordic regions and you should be fine cause it’s very rare that they’ll be thalassemic. (Carrier, Greece, born in Australia (2))
Those found to be carriers when they were young children were often told by their parents, who mentioned little else other than that they were a carrier. They commonly did not talk to a doctor about their carrier status until they had already met their partner or were pregnant, so were uninformed for many years. Sometimes the participants’ parents did not believe that being a carrier of thalassaemia had any implications for their daughter, or passed on incorrect information. This affected participants’ understanding and attitude towards being a carrier. As they matured, some of the women questioned what they had been told, realising that this information was incorrect only after speaking to a healthcare professional during their pregnancy.
I do recall someone telling me … I think my mum … whether it’s right or wrong, that … mothers pass it to their children but fathers don’t pass it to their children. I don’t know if that’s right … so that’s been one thing that I’ve thought in the past, but I don’t think that’s right, based on what the genetics person said. (Carrier, Egypt/Lebanon, born in Australia)
Women who had been identified as carriers previously usually did not recall receiving any further information about thalassaemia after being screened antenatally. Presumably doctors made the assumption that the women had already received information and understood what it means to be a carrier. This was often incorrect, as women had not received adequate information when they were first informed of their carrier status earlier in life, or during pregnancy.
She didn’t really give any information or anything and I said yeah I was aware that I was a carrier and that. And that kind of ended there…. So I don’t know that I am any more informed about it than what I initially was. (Carrier, Italy, born in Australia (3))
Some women reported being highly distressed after learning their carrier status and attributed this, in part, to the very small amount of information they received, and lack of preparedness. This was particularly seen amongst women who had been identified as carriers before pregnancy. Some women were told that their children were at risk of developing a severe disease, but did not recall being told about genetic risk or thalassaemia as a condition. Women were often warned that if their partner is also a carrier then that would cause complications, but made wrong assumptions about what their diagnosis meant for them and their children due.
…she terrified me and said ‘this is really serious. You need to have your partner screened and if you ever decide to have children this could mean that your child will die’ and needless to say I went home and was very distressed for a long time because basically she told me that any child I have would die…didn’t explain what it all meant. (Carrier, South Africa, born in Australia)
“I have thalassaemia”
Many non-genetics healthcare professionals use the terminology ‘thalassaemia minor’ rather than ‘carrier of β-thalassaemia’, and participants often misunderstood this to mean that they had been diagnosed with the medical condition ‘thalassaemia minor’, causing them to regularly make statements such as ‘I have thalassaemia’. In addition, carriers often attributed symptoms to this medical condition from which they believed they suffered. This confusion was apparently compounded because people often were not expecting this result and had not heard of thalassaemia before.
Well I thought, I’ve got a disease that I’ve gotta live with. (Carrier, Italy, born in Australia (4))
This misunderstanding was apparent in women who had been diagnosed through antenatal screening as well as those who were originally identified as carriers earlier in life and were then re-screened.
Although most women believe that ‘thalassaemia minor’ had not had a major impact on their life, it was very common for women to ascribe their own symptoms or those of family members to carrier status, especially having low iron levels and feeling lethargic. Even if family members had not undergone testing, participants made assumptions about their relatives’ carrier status based on the symptoms shown.
I’m pretty sure my brother’s got it because he’s got the same symptoms as me. My sister… I’m not sure. She sleeps a lot too, but I’m not sure if she’s got it. (Carrier, Cyprus, born in Australia)
Another area of confusion was the different types of thalassaemia — alpha and beta. Carriers were often only aware that there was thalassaemia major and minor, not that there were different types (e.g., alpha and beta).
I thought ‘oh ok alpha’s the major form and beta’s the minor form’. (Carrier, Italy, born in Australia (1))
Many participants were also unaware of the effects of β-thalassaemia, unless they knew a person affected with the condition.
“He was so sure that he didn’t have it”
A number of women reported that their partners failed to understand the importance of being tested for carrier status. Reasons for not wanting to undergo testing included disbelief that they could be a carrier, assumptions that previous blood tests would have revealed their carrier status and the belief that carriers must always have symptoms while they themselves were asymptomatic. Although most men were eventually persuaded to be tested by their partners or doctors, three had still not been tested at the time of the interviews.
Although some women were not worried about their partner being a carrier due to their partner’s low-risk ethnic background, all except one of these women’s partners still had testing. A small number of women believed their partners when they said that they were not carriers, even if they had not undergone testing.
“Is there any chance my children … could carry the blood disease?”
One group of women understood their risk of having an affected child if their partner had also been found to be a carrier. However, confusion and a lack of understanding about genetic risk was evident in other carriers, which led to these women making incorrect assumptions about the probability of having an affected child. For example, some believed that if their partner was a carrier, then there would be a 100 % chance that their children would be affected. Incorrect genetic risk assumptions were made by both women identified as carriers before pregnancy, as well as women identified as carriers through antenatal screening.
Carrier couples often recalled only being informed about the genetic aspects of β-thalassaemia when both partners were found to be carriers and had a high risk of giving birth to an affected child.
…all I knew was that if there’s a thal minor and a thal minor, you have a thal major. I didn’t know what thal major was. I didn’t know about the one in four. I didn’t know anything. (Carrier Couple-Woman, Cyprus, born in Australia)
(4) Obtaining information
“You need to be told what it means”
Participants believe that it is extremely important for people to be provided with appropriate information about thalassaemia and genetic risk, so that they are fully informed about the effects of being a carrier or carrier couple of β-thalassaemia. Some wanted this information as soon as they were identified as a carrier.
Just … a hand-out as soon as you find out, to tell you exactly what it is. (Carrier, Italy, born in Australia (4))
Written information about thalassaemia was seen as helpful in assisting recall of the verbal information provided, for future reference and as an aid when explaining thalassaemia to family members and friends.
“I went to the internet”
Due to a lack of information provided, most of the participants conducted their own research to seek more details about thalassaemia from sources other than the healthcare system, particularly the internet and to a lesser extent books.
Additional information was sought when adults and teenagers were first informed that they were a carrier, while others who found out at a younger age did so later in life. Some couples researched the topic, even after a genetic counselling session, to further their understanding about thalassaemia and genetic risk.
…when we were at home … I went through every medical site there was, you know, on thalassaemia and haemoglobin E and printed things out and started reading it. It was very confusing. (Carrier Couple-Man, China)
The internet was not always seen as a reliable, clear source of information, with some participants having trouble understanding the medical terminology used.
…just when I did the little bit of research that I did, I didn’t really understand what I was reading … you know, haemoglobin, red blood cells. (Carrier, Italy, born in Australia (4))
“She explained what it meant”
Thirteen of the 26 carrier women reported attending a session with a genetic counsellor and/or haematologist during their pregnancy when they were provided with more information. Only one of the ten carrier couples reported attending a counselling session before the male partner was identified as a carrier. The rest were only referred to specialists after both were identified as carriers. Most women found that the information provided in these sessions was easy to understand and they very much appreciated the way in which the information was presented to them.
Discussion
This study examined experiences and attitudes of carriers identified by the β-thalassaemia carrier screening process carried out in Victoria, Australia. As there are no formal carrier screening programs for thalassaemia in Australia, antenatal screening is the major point in routine healthcare where there is a specific intent to identify carriers. However, our study found that carriers are often identified before antenatal screening is carried out, as an incidental finding when an FBE is performed for other reasons. Furthermore, women do not appear to be providing informed consent for this form of genetic screening. Many women were comfortable, indeed some preferred, that their doctors make the decision about the tests conducted, trusting their doctors to ‘know best’. Thus, these women’s attitudes towards informed consent are similar to those found in two English studies (Ahmed et al. 2005; Tsianakas et al. 2012) but contrast with ‘standard’ genetic screening processes in which informed consent is considered essential (Andermann et al. 2008). The attitudes observed in our study could be a consequence of the women’s lack of knowledge about the different tests recommended during pregnancy (Ahmed et al. 2005), and/or the over-riding desire for reassurance that their baby will be healthy (Atkin and Ahmad 1998), hence their reliance on healthcare professionals’ expertise and decision-making. The attitudes towards informed consent observed illustrates that women generally accept this practice of genetic screening being incorporated into routine healthcare. There appears to be a clear difference in the requirement for informed consent between carrier screening for thalassaemia and screening for carrier status for other genetic conditions. This may relate to the fact that for other conditions, screening is by DNA testing whilst for thalassaemia, the initial test, the FBE, is one carried out for many reasons, thalassaemia screening being only one. A similar analogy would be cholesterol screening which could provide information related to inherited dyslipidaemias as well as other conditions.
There is clearly a need to improve the information available to women after they are found to be carriers, whether that is antenatally or at other times. Women were dissatisfied with the paucity of information they received when notified of their carrier status. While it is not possible to know what actually occurred when they leant they were carriers, misconceptions and uncertainty were evident in responses to interview questions. One group learned of their carrier status from parents so it is unsurprising that misconceptions arose. Women recalled being advised to avoid having children with thalassaemia by marrying men believed unlikely to be carriers such as those with blond hair. This advice is unfortunate because it may mean carriers make important life decisions such as whom they will marry in the mistaken belief that individuals of certain appearance cannot be carriers of thalassaemia and that if their partner is a carrier, that all children will be affected by the condition. It is also concerning that as a result of misunderstanding, many women from the current study believed they had been diagnosed with a medical condition, misattributed symptoms to this, and that some continued to worry about both their and their children’s future health as a carrier. Similar misunderstanding was apparent amongst carriers detected in the antenatal screening program in England due to the terminology used (Locock and Kai 2008) and, in a study by Wong and colleagues, people in Malaysia described symptoms they associated with ‘thalassaemia minor’, such as pale skin and tiredness (Wong et al. 2011). This suggests that the issue is not confined to the communication styles of Victorian healthcare practitioners, but reflects widespread practices in the education and care of those found to be carriers. Another finding of concern was that some women did not understand the genetic aspects of thalassaemia believing that they were at risk of having a child with an illness in the absence of a carrier partner. Whilst those who were referred to specialists in thalassaemia/genetics such as geneticists, genetic counsellors or haematologists, benefited from this, it is not practical to refer all carriers to such specialists. Carrier couples should always be referred and carriers who are judged to require more information about thalassaemia should also be referred to such specialists.
The qualitative design of the study means that generalisations cannot be made to other healthcare contexts or specific subgroups within the population, though it is interesting that some parallel observations have been made in two other countries and healthcare systems. As this study is retrospective, it is subject to recall biases. Therefore, while the study findings represent participants’ ongoing ‘lived experience’, they cannot be taken as an accurate description of healthcare professional practice. Nonetheless, we believe there are some implications for healthcare practice to meet the needs of those identified as carriers.
To ensure that a person is aware they could be identified as a carrier during their pregnancy, healthcare professionals should consider notifying their patients that they are being tested for thalassaemia certainly before a specific thalassaemia diagnostic test (Hb electrophoresis or HPLC) is carried out, and when there are indications such as family history or high-risk ancestry, before an FBE.
If a person is already aware that they are a carrier, there is value in checking current understanding and correct any misconceptions. Specific information provided by the practitioner about the implications of being a carrier, thalassaemia and genetic risk can be valuable before as well as during pregnancy and may reduce anxiety.
It is well documented that recall and understanding of medical information provided in medical consultations can be poor (Ong et al. 1995) and supplementary written information has been demonstrated to be beneficial (Farrell-Miller and Gentry 1989; Hoffmann and Worrall 2004; Hussey 1997). Carriers’ understanding of their test results would be enhanced by an information booklet and/or a reputable internet site that is consistent with guidelines for effective written materials (Farrell-Miller and Gentry 1989; Hoffmann and Worrall 2004; Hussey 1997) and provides simple information for carriers of thalassaemia about the meaning of being a carrier. This would also serve to reinforce the information provided by health professionals (Iverson et al. 2008). Healthcare professionals are well positioned to direct women towards good websites with reliable information about thalassaemia. Examples include www.thalassaemia.org.cy/about_thalassaemia.html and www.ukts.org/what.html?i3. The extent to which healthcare professionals who are informing carriers of their status, such as GPs and obstetricians, may themselves require further education is not known and cannot be ascertained from the present study.
Referral of carrier couples to specialists such as haematologists and genetic healthcare professionals is strongly recommended. Referral of carriers to such professionals should be considered if it is apparent that they would benefit from a more in-depth discussion about thalassaemia.
To avoid the considerable confusion and misunderstanding that use of ‘thalassaemia minor’ has been found to cause in this and other studies, we strongly endorse the use of ‘carrier of β-thalassaemia’ rather than ‘thalassaemia minor’ commonly used by healthcare professionals and often echoed by carriers themselves.
A quantitative study to determine frequency of the misconceptions and attitudes observed here and determine knowledge levels objectively would be useful to further inform practice development, and patient and community education. We are currently examining the attitudes and practice of the relevant healthcare providers to complement this study of carriers and gain a broader understanding of the screening process. Despite the misconceptions arising during thalassaemia carrier screening, this study has shown that a screening process which is incorporated into routine care and does not obtain informed consent is generally acceptable to those experiencing it and can be further improved with greater focus on information provision in some key areas. Technological advances in genomics, such as next generation sequencing, make more likely a future where carriers of genetic conditions will be identified as a result of testing for multiple purposes during routine care. Based on this study, we hypothesise that such an approach will be broadly acceptable to carriers, at least retrospectively. However, the challenge lies in preparing tested individuals for all the possible outcomes, particularly in busy clinical settings when the focus may be on other aspects of care. Our study emphasises the importance of considering how, to whom and when information needs to be disseminated, as well as the impact of medical terminology on understanding, when preparing new tests for implementation into routine care, regardless of the underlying technology.
Electronic supplementary material
Interview topic guide (DOC 67 kb)
Acknowledgments
We thank the individuals who participated in this study for their invaluable contribution. We are grateful to Prof. Don Bowden and Ms. Carolyn Cameron from Monash Medical Centre; Dr. Janine Campbell and Ms. Liz Kanellos from Royal Women’s Hospital; Ms. Melissa Graetz and Ms. Eleni Mechkaroff from Mercy Hospital for Women; and Dr. Ellen Maxwell and Ms. Irene Giovas from Melbourne Pathology for their assistance in recruiting participants into this study. Sir Mark and Lady Judy Moody Stuart as well as Thalassaemia Australia are thanked for their support for this work. MBD is a National Health and Medical Research Council Practitioner Fellow. This study was supported by the Victorian Government’s Operational Infrastructure Support Program.
Conflict of interest
All authors declare that they have no conflict of interest
References
- ACOG ACOG Practice Bulletin No. 78: hemoglobinopathies in pregnancy. Obstet Gynecol. 2007;109:229–237. doi: 10.1097/00006250-200701000-00055. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Green J, Hewison J. Antenatal thalassaemia carrier testing: women’s perceptions of “information” and “consent”. J Med Screen. 2005;12:69–77. doi: 10.1258/0969141053908258. [DOI] [PubMed] [Google Scholar]
- Andermann A, Blancquaert I, Beauchamp S, Dery V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317–319. doi: 10.2471/BLT.07.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin K, Ahmad W. Genetic screening and haemoglobinopathies: ethics, politics and practice. Soc Sci Med. 1998;46:445–448. doi: 10.1016/S0277-9536(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Birgens H, Ljung R. The thalassaemia syndromes. Scand J Clin Lab Invest. 2007;67:11–25. doi: 10.1080/00365510601046417. [DOI] [PubMed] [Google Scholar]
- Chui DH, Cunningham MJ, Luo HY, Wolfe LC, Neufeld EJ, Steinberg MH. Screening and counseling for thalassaemia. Blood. 2006;107:1735–1737. doi: 10.1182/blood-2005-09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens NE, Gaff CL, Metcalfe SA, Delatycki MB. Carrier screening for beta-thalassaemia: a review of international practice. Eur J Hum Genet. 2010;18:1077–1083. doi: 10.1038/ejhg.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatycki MB. Population screening for reproductive risk for single gene disorders in Australia: now and the future. Twin Res Hum Genet. 2008;11:422–430. doi: 10.1375/twin.11.4.422. [DOI] [PubMed] [Google Scholar]
- Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- Farrell-Miller P, Gentry P. How effective are your patient education materials? Guidelines for developing and evaluating written educational materials. Diabetes Educ. 1989;15:418–422. doi: 10.1177/014572178901500505. [DOI] [PubMed] [Google Scholar]
- Flander L, Fletcher A, Gertig D, Hopper J. Thalassaemia screening in Victoria from a public health perspective. Victoria: Department of Human Services; 2003. [Google Scholar]
- Gason AA, Sheffield E, Bankier A, Aitken MA, Metcalfe S, Barlow Stewart K, Delatycki MB. Evaluation of a Tay–Sachs disease screening program. Clin Genet. 2003;63:386–392. doi: 10.1034/j.1399-0004.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Worrall L. Designing effective written health education materials: considerations for health professionals. Disabil Rehabil. 2004;26:1166–1173. doi: 10.1080/09638280410001724816. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Hussey LC. Strategies for effective patient education material design. J Cardiovasc Nurs. 1997;11:37–46. doi: 10.1097/00005082-199701000-00007. [DOI] [PubMed] [Google Scholar]
- Iverson SA, Howard KB, Penney BK. Impact of internet use on health-related behaviors and the patient–physician relationship: a survey-based study and review. J Am Osteopath Assoc. 2008;108:699–711. [PubMed] [Google Scholar]
- Langlois S, Ford JC, Chitayat D, Desilets VA, Farrell SA, Geraghty M, Nelson T, Nikkel SM, Shugar A, Skidmore D, Allen VM, Audibert F, Blight C, Gagnon A, Johnson JA, Wilson RD, Wyatt P. Carrier screening for thalassaemia and hemoglobinopathies in Canada. J Obstet Gynaecol Can. 2008;30:950–971. doi: 10.1016/S1701-2163(16)32975-9. [DOI] [PubMed] [Google Scholar]
- Liamputtong P, Ezzy D. Qualitative research methods, vol 2nd. Melbourne: Oxford University Press; 2005. [Google Scholar]
- Locock L, Kai J. Parents’ experiences of universal screening for haemoglobin disorders: implications for practice in a new genetics era. Br J Gen Pract. 2008;58:161–168. doi: 10.3399/bjgp08X277276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie J, Petrou V, Forbes R, Curnow L, Ioannou L, Dusart D, Bankier A, Delatycki M. Population-based carrier screening for cystic fibrosis in Victoria: the first three years experience. Aust N Z J Obstet Gynaecol. 2009;49:484–489. doi: 10.1111/j.1479-828X.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe SA, Barlow-Stewart K, Campbell J, Emery J. Genetics and blood—haemoglobinopathies and clotting disorders. Aust Fam Physician. 2007;36:812–819. [PubMed] [Google Scholar]
- Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong L, Haes JD, Hoos A, Lammes F. Doctor–patient communication: a review of the literature. Soc Sci Med. 1995;40:903–918. doi: 10.1016/0277-9536(94)00155-M. [DOI] [PubMed] [Google Scholar]
- Polit D, Beck C. Essentials of nursing research. Methods, appraisal, and utilization. 6. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- Trent RJ. Diagnosis of the haemoglobinopathies. Clin Biochem Rev. 2006;27:27–38. [PMC free article] [PubMed] [Google Scholar]
- Tsianakas V, Atkin K, Calnan MW, Dormandy E, Marteau TM. Offering antenatal sickle cell and thalassaemia screening to pregnant women in primary care: a qualitative study of women’s experiences and expectations of participation. Health Expect. 2012;15:115–125. doi: 10.1111/j.1369-7625.2011.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1998) Proposed international guidelines on ethical issues in medical genetics and genetic services. World Health Organization [PubMed]
- Wong L, George E, Tan J. A holistic approach to education programs in thalassaemia for multi-ethnic population: consideration of perspectives, attitudes, and perceived needs. J Community Genet. 2011;2:71–79. doi: 10.1007/s12687-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interview topic guide (DOC 67 kb)