Abstract
Poly lactic acid (PLA) ultrasound contrast agents (CA) have been previously developed in our laboratory for ultrasound (US) imaging, as well as surface coated with doxorubicin to create a potential targeted platform of chemotherapeutic delivery using focused US. However, we have previously found it impossible to sterilize these agents while at the same time maintaining their acoustic properties, a task that would probably require fabrication within a clean facility. The purpose of this paper is to investigate the feasibility of using plasma to sterilize these CA while maintaining maximum echogenicity, a step that would greatly facilitate in vivo investigations. Effects of plasma exposure time (1, 3 and 6 minutes) and intensity (low- 10 mA, 6.8 W; medium- 15 mA, 10.5 W; and high- 25 mA, 18W) on the CA’s acoustic properties, surface morphology, zeta potential, capacity to carry chemotherapeutics, and overall sterility are described. Both increases in plasma intensity and exposure time increased CA zeta potential and also significantly increased drug payload. High intensity plasma exposure for three minutes was found to be an optimal sterilization protocol for maximal (100%) preservation of CA echogenicity. Plasma exposure resulted in sterile samples and maintained original CA enhancement of 20 dB and acoustic half-life over 75 minutes, while increasing CA zeta potential by 11 mV and doxorubicin loading efficiency by 10%. This study not only shows how a highly temperature and pressure sensitive agent can be sterilized using plasma, but also that surface modification can be used to increase surface binding of drug.
Keywords: Ultrasound contrast agents, poly lactic acid, plasma sterilization, surface modification, drug delivery
Introduction
Ultrasound contrast agents (CA) generally consist of a gas core stabilized by a surfactant, phospholipid, or polymer shell. These agents must be non-toxic, intravenously injected and smaller than 8 μm in order to freely pass through the capillaries (Forsberg et al. 1998). The gas within these agents provides a near perfect acoustic impedance mismatch between the CA and surrounding tissue, backscattering ultrasound (US) waves and providing image contrast. Although currently only approved for cardiac use by the FDA, CA-aided US has been shown to provide comparable imaging capabilities to magnetic resonance imaging (MRI) in numerous applications (Hohman et al. 2003; Magarelli et al. 2001; Malm et al. 2004) while at the same time being significantly less expensive, portable, and providing imaging in real time. Additionally, the use of CA-aided US is frequently used in Europe, Japan and China for a variety of imaging applications and is expected to expand with the development of therapeutic and targeted agents (Goldberg et al. 2001). CA research has begun to move from diagnostic to therapeutic, applications and to focus on the development of both drug loaded agents, in which encapsulated drug can circulate throughout the body and only released at a desired location through activation from external, focused US, and the development of targeted CA, surface coated with targeting ligands for both targeted diagnostic imaging and drug delivery.
Within our laboratory, we have developed and optimized a method for creating both poly lactic acid (PLA) and poly lactic-co-glycolic acid (PLGA) CA (Narayan and Wheatley, 1999) These CA have a mean diameter of 1.8 μm, show resonance behavior close to 5 MHz, and provide up to 20 dB of US contrast both in vivo and in vitro (El-Sherif and Wheatley, 2003; Wheatley et al. 2006). These agents have shown potential for both drug delivery applications and targeting (Eisenbrey et al. 2008a; Wheatley et al. 2006). Doxorubicin (Dox), an extensively used chemotherapeutic has been electrostatically attached to the surface of these CA, while having a minimal impact on the acoustic properties of the agent (Eisenbrey et al. 2008a). These agents have also been modified through surface attachment of the integrin-binding peptide sequence GRGDS (Gly-Arg-Gly-Asp-Ser), and show an increased affinity for attachment to integrins on breast cancer cells in vitro (Wheatley et al. 2006a). Seeman and colleagues have used a similar PLGA CA to both encapsulate and successfully deliver plasmid DNA using focused US (2002). Similar drug delivery and targeting strategies are also being developed using lipid based CA (Klibanov, 2006).
No techniques for sterilizing polymer CA post fabrication have been reported, making long term in vitro and in vivo studies difficult, especially on a laboratory scale. While polymer CA have successfully been fabricated in clean facilities (Straub et al. 2005), this solution is cost intensive and may not be feasible for research efforts. Previous sterilization attempts in our laboratory using autoclave, dry heat, ethylene oxide, ultraviolet light, filtering, and washing with diluted isopropyl alcohol were all unsuccessful at either sterilizing the agent or maintaining the CA’s reactivity to US. This failure is attributed to the need for the gas-filled capsules to remain intact combined with the agent’s susceptibility to alcohol dissolution and a high sensitivity to heat and pressure. Hence the motivation for the inquiry described in this paper was to develop an effective method of CA sterilization that both preserved the echogenicity of the CA, and was feasible on a laboratory scale, thus facilitating long term in vivo studies of drug-delivery efficacy in this small but potentially translatable area of theranostics.
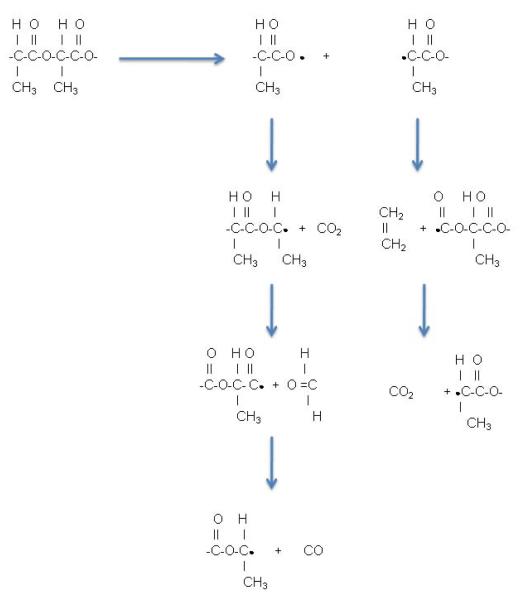
Plasma sterilization relies on glow discharge plasma created by evacuating a vessel, replacing it with a low pressure gas (generally oxygen, or argon), then energizing the gas. The energized gas results in ions, electrons, free radicals, and photons in the ultraviolet range which interact with exposed surfaces and transfer as deep as 10μm into a surface (roughly 50 times our CA shell thickness) without changing the material’s bulk properties (Wise et al. 1996). This process can result in both sterilization and surface modification by oxidation at the material’s surface (Wise et al. 1996). Considerable research efforts have been invested in modifying polymer surfaces using gas plasma. In a study using PLA rods, Gogolewski et al. (1996) used 15 and 30 minute treatments and reported that sterility was achieved in only 70% of the samples, perhaps not surprising since this is a surface phenomenon that might not penetrate into the thickness of the rods, a result that is consistent with the report that mechanical properties did not change. Changes that were limited to the skin of the rods included a slight increase in molecular weight and polymer branching compared to the interior, a change that was less noticeable in the more crystalline samples. Huang and colleagues (2007) showed that oxidation reactions occurring with oxygen plasma on a PLGA cell scaffold resulted in improved laminin and eventually increased Schwann cell attachment without compromising the scaffold. Inagaki et al. (2002) examined surface modification of PLA films using argon plasma with a view to establishing hydrophilic modification of the surface. Although surface modification was demonstrated, due to C-C and C-H bond scission, followed by post treatment oxidation of the free radicals that were produced in the plasma, no increases in surface hydrophilicity was detected. They attribute this degradation to depropagation and transfer reactions along the chains due to the instability of the carbon radicals that are formed at the PLA surface. The scheme that they propose is outlined in Fig. 1 (Inagaki et al. 2002). The resulting cascade deposits small molecules from degradation of the chain ends on the surface of the polymer. These molecules are easily washed off, restoring the surface to an almost identical hydrophobicity. However, Wan et al. (2008) showed surface modification of PLGA films using oxygen plasma resulted in increases of hydroxyl, carboxyl, and peroxyl groups at the surface, and that the magnitude of these changes could be controlled by both the plasma power and exposure time. It is notable that this report did not indicate that the films were rinsed in any way before being analyzed, a step that proved crucial according to Inagaki et al. (2002). Increases in these groups on the surface of a CA may result in increased binding points for therapeutics or targeted ligands for targeted drug delivery applications. These results demonstrate how plasma sterilization may be ideal for PLA CA sterilization as well as surface modification.
Figure 1.
Degradation of a poly lactic acid surface from depropagation and transfer reactions along the chains due to the instability of the carbon radicals formed during plasma treatment. Adapted from Inagaki et al. 2002.
This paper will investigate the effects of both exposure time and intensity on generating sterile PLA CA using an RF- generated O2-plasma. Effects of these procedures on the acoustic performance of the agent are discussed. Surface modification and changes in morphology are examined through zeta potential, differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). Finally, surface adsorption of Dox is used as an example of how surface modification of an existing CA through plasma treatment can be used to provide an improved vehicle for US-triggered, drug delivery.
Materials and Methods
Materials
100 DL Low IV Poly-lactic-acid (PLA) (MW = 83 KDa) was purchased from Lakeshore Biomaterials (Birmingham, AL). Poly (vinyl alcohol), 88% mole hydrolyzed, with a MW of 25 KDa, Dox, and camphor were reagent grade from Sigma-Aldrich (St.Louis, MO). Ammonium carbonate was purchased from J.T. Baker (Phillipsburg, NJ). All other chemicals were reagent grade from Fisher Scientific (Waltham, MA).
CA preparation
PLA CA were fabricated using a previously optimized double emulsion method (W/O)/W developed in our lab (El-Sherif and Wheatley, 2003). Using this method, 0.5 g of PLA combined with 0.05 g of camphor is dissolved in 10 ml of methylene chloride during continuous stirring. One ml of ammonium carbonate (4% w/v) is then added and the mixture sonicated in an ice bath at 110 Watts for 30 seconds at 3 seconds on, 1 second off using a Misonix Inc. sonicator probe (Farmingdale, NY). After sonication, the resulting (W/O) emulsion is then poured into 50 ml of 4°C, 5 % polyvinyl alcohol and homogenized for 5 minutes at 9000 rpm (Brinkmann Instruments, Westbury, NY). After homogenization, CA is collected by centrifugation and washed with hexane. Hexane is then evaporated, after which the capsules are flash frozen and lyophilized for 48 hours. During freeze drying, ammonium carbonate and camphor sublime out of the capsule, leaving a void in their place. This void later fills with the gas of choice, in this case air after being exposed to atmospheric pressure. The gas within a CA is primarily responsible for backscattering enhancement. After fabrication, dry samples are stored in a desicator at −20°C until used.
Plasma sterilization
Plasma processing was done with oxygen using a Harrick PDC-32G Plasma Sterilizer (Ithaca, NY). Seventy mg of agent was sterilized for t=1, 3, and 6 minutes on the machine’s low, medium, and high plasma settings. These power settings correspond to powers applied to the RF coil of: Low- 10 mA, 6.8 W; Medium- 15 mA, 10.5 W; and High- 25 mA, 18W. Three samples were run at a time and the results averaged. Each condition is the product of three separate processing runs (9 samples total, n=3). A thin (< 1 mm) layer of sample was sterilized on a flat surface while in covered well plates 6 cm from the RF coil. Prior to processing, the chamber was filled with pure oxygen and sealed. After sealing, the chamber was pumped down to a pressure of roughly 200 mTor and the RF coil used to generate oxygen plasma. After processing, the chamber was backfilled with unfiltered air and samples immediately transferred to a sterile fume hood using sterile technique. No changes in temperature were noticed during plasma processing.
Determination of agent sterility
After exposure to plasma, CA sterility was determined by adding 2 mg of dry CA to 2 ml of RPMI-1640 cell culture media (ATCC, Manassas, VA). Media was then incubated at 37°C for 48 hours and presence of bacteria was determined using the pH sensitive nature of the media combined with microscopy. Both the opacity and color of the media change in the presence of bacteria. Bacteria can also be seen at magnifications over 40X. All values were based on an average of three readings from three individual samples (9 total samples). The optimal condition (18 W for 3 mins) was also confirmed to be sterile by adding 9 separate samples each from a separate sterilization run to blood agar cultures (Sigma-Aldrich, St.Louis, MO.) over a period of 60 hours as outlined by Geze et al. (2001). Additionally, sterility within the core of the CA using optimal settings was demonstrated by ultrasonic rupture of the agent (post plasma processing) in culture media within an Opticell (Thermo Fisher Scientific, Rochester, NY) with no detection of bacteria after 60 hours (results not shown), by which time the capsules had started to disintegrate.
Acoustic testing
US enhancement and stability during insonation were both measured to judge the CA’s ability to provide clinically relevant US contrast, as well as its sensitivity to US for possible US triggered drug release. The pulse-echo setup shown in Fig. 2 was used. A Panametrics (Waltham, MA) 5 MHz transducer with a 12.7 mm diameter, −6 dB bandwidth of 91%, and focal length of 50.8 mm was placed in a 37°C water bath filled with 18.6 MΩ-cm deionized water. Previous studies have shown a similar polymer shelled agent displays resonance behavior (maximal contrast enhancement) within the 6 dB bandwidth of the 5 MHz transducer (Wheatley et al. 2006b). The beam of the transducer was focused through the acoustically transparent window at a depth of roughly 5 cm from the surface. This results in a peak positive pressure amplitude of 0.69 MPa, peak negative pressure amplitude of 0.45 MPa at the focus and pulse length of roughly 1 μs. While it is acknowledged that this setup lacks many in vivo features, the system does provide a direct measurement of the parameter of interest, acoustic activity and has been shown to correlate well to in vivo results (Wheatley et al. 2006b).
Figure 2.
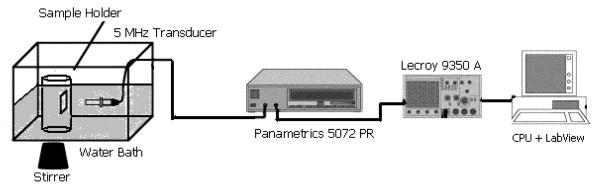
Acoustic setup used to test ultrasound enhancement and contrast agent stability under ultrasound prior to and post plasma exposure.
A pulser/receiver (5072 PR Panametrics ,Waltham, MA) was used to generate an acoustic wave with pulse repetition frequency equal to 100 Hz. Reflected signals were then amplified 40 dB before being read by an oscilloscope (Lecroy 9350 Chestnut Ridge, NY). Data acquisition and processing was done using LabView 7 Express (National Instruments, Austin, TX) and stored on a Dell CPU for later calculation.
Backscattering enhancement was measured as a function of sample dosage. The sample holder shown in figure 2 was filled with 50 mL of phosphate buffered saline (PBS) pH 7.4, at 37 °C and continually stirred. Three mg of dry CA was then suspended in 800 μl of PBS by vortexing briefly. Samples were then pipetted into the sample holder in incremental dosages and allowed to mix for 10 seconds to ensure a homogenous media. Enhancement in relationship to a baseline reading was then measured for each dosage incrementally to a maximal dosage of 16 μg/ml, after which noticeable shadowing begins to take place within the setup. All values were based on an average of three repetitions of sixty readings from three individual samples and expressed as dB relative to the baseline value.
CA stability under ultrasonic conditions was measured to assess the effect of ultrasound on the stability of the CA and in order to determine the response of the agents for the duration of a typical ultrasound imaging session (up to 15 minutes). Four micrograms per milliliter of CA (a dose chosen on the rise of the dose response curve to give an accurate measure of stability) was added to 50 ml of 37°C PBS and continually stirred and insonated with the setup described above. Enhancement was measured every minute for 15 minutes and the results normalized with respect to initial enhancement. All values were based on an average of three readings from three individual samples.
SEM imaging
CA was imaged using an environmental SEM (FEI XL30, Hillsboro, OR). CA was sputter coated with platinum for 30 seconds prior to imaging. Images were taken at varying magnifications with an accelerating voltage of 10.0 kV. All SEM imaging was done at the Drexel University Materials Characterization Facility.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was done on a DSC 7 (Perkin Elmer, Waltham, MA) using an intracooler 2 in nitrogen atmosphere to measure changes in the glass transition temperature of the polymer. Calibration was done beforehand using indium. Two milligrams of sample was added to the sample holder with the rate of heating/cooling fixed at 20° C per min. All samples were analyzed in triplicate.
Zeta potential
Zeta potential was measured using a Malvern Zetasizer (Worcestershire, United Kingdom) in order to gauge changes in surface charge on the surface of the agent. One mg of agent was suspended in 1 ml deionized water (pH=4.7) and measured using Malvern’s zeta potential capillary cuvette. Results represent the average of three readings for three independent samples at each plasma power level and exposure time.
Doxorubicin surface loading and determination of encapsulation efficiency
Ten mg of prefabricated CA were suspended in 1 ml of Dox in deionized water (1 mg/ml). CA-Dox solution was then stirred end over end at 4°C for 24 hours. After which surface coated CA were collected by centrifugation, washed to remove free Dox, and freeze dried for 48 hours (Freeze Dryer, VirTis, Gardiner, NY). This process has been previously optimized and shown to create echogenic, drug coated, CA (Eisenbrey et al. 2008a). This process can also be done under sterile conditions by filtering the Dox solution with a 0.22 μ filter prior to bathing sterile CA. Encapsulation efficiency was defined by:
and measured by dissolving 3 mg of Dox loaded CA in 2 ml dimethylesulfoxide (DMSO). Fluorescence was then measured using an Infinte M200 fluorimeter (Tecan, Switzerland). Dox concentration was then calculated using the linear portion of a calibration curve of known Dox quantities, constructed in DMSO.
Statistical analysis
Statistical significance was determined using a One-way ANOVA using Prism 3.0 (GraphPad, San Diego, CA). Statistical significance was determined using α = 0.05. A Newman-Keuls test was performed as a post test to determine significant variance between groups and p values calculated using a Student’s t-test.
Results
Plasma processing parameters: effects on CA sterility
Sterility of the CA samples was first examined. Effects of both plasma exposure time (1, 3 and 6 minutes) and power level (unsterilized, i.e. 0 W and 6.8, 10.5 and 18.0 W), on the CA sterility were examined. These results are summarized in Fig. 3. Samples treated on high power (18.0 W) for times of 3 and 6 minutes showed complete sterilization of all three samples in both culture media and on blood agar. Samples treated for shorter time periods or using lesser power levels showed either no or inconsistent sterilizations. Samples treated using 18 W power at all time points and samples sterilized with 10.5 W power for times of 3 and 6 minutes showed statistically significant higher chances of sterility than the unsterilized samples (p<0.001, 9 samples/condition, n=3).
Figure 3.
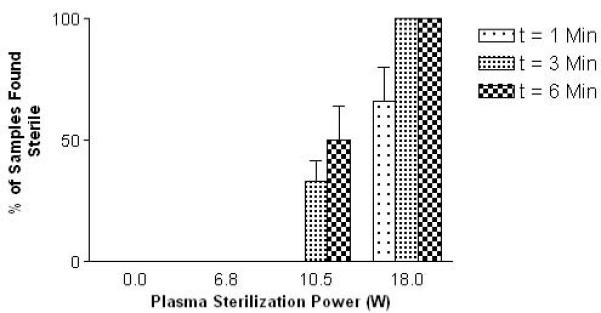
Effects of exposure time and plasma intensity on contrast agent sterility. No sterility was seen in untreated samples and samples treated with a low power.
Effects on acoustic properties of CA
Acoustic performance of plasma processed CA was examined to determine how well the sterilized CA would provide contrast during an US scan, and how sensitive the agent was to US for potential US triggered, drug delivery applications. Surprisingly, processing power (unsterilized or 0 W, 6.8, 10.5 and 18.0 W) had no differential effect on the measured profiles (data not shown), whereas time did. As a consequence, only 18 W data are presented. Fig. 4 shows the effects of plasma processing time using 18.0 W on US enhancement in vitro. Even though no significant effects on enhancement were seen between CA sterilized at varying plasma powers, a statistically significant (p-0.0045) drop off in acoustic enhancement was seen in samples sterilized for t=6 mins compared with 0 and 3 mins (18.0 W data shown).
Figure 4.
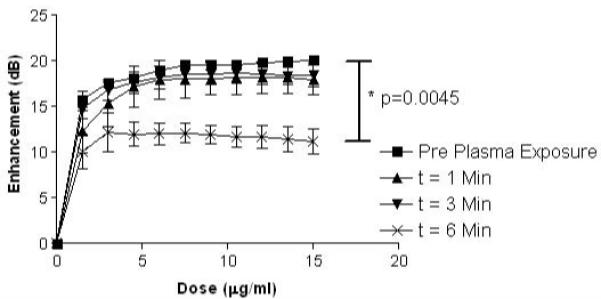
Effect of exposure times to plasma at 18.0 W on in vitro ultrasound enhancement of poly lactic acid ultrasound contrast agents. (n=3, error bars= standard error about mean, p=0.0045)
Enhancement during continuous US was measured to determine if the plasma sterilization significantly affected the agent’s stability or ability to provide contrast over the course of a typical ultrasound scan. Data was normalized to account for small inter-sample variation in starting values. As with dose response curves, the processing power (unsterilized or 0 W, 6.8, 10.5 and 18.0 W) had no differential effect on the stability of the agent for a given sterilization time (data not shown). For a power of 18.0 W, the effects on stability are shown in Fig. 5 for various processing times. Unsterilized samples and samples processed for 1 and 3 min lost a relative enhancement of 10% after 15 mins of insonation. However, samples sterilized for 6 mins were significantly less stable, losing roughly 35% of their original enhancement.
Figure 5.
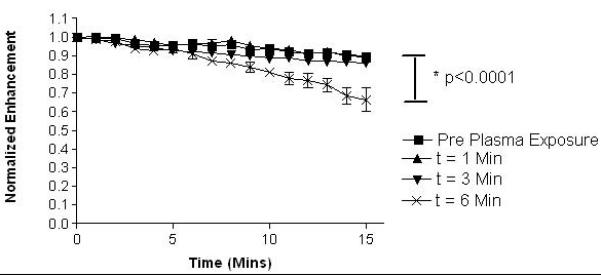
Effect of exposure times to plasma at a power of 18.0 W on normalized in vitro ultrasound enhancement over time. (n=3, error bars= standard error about mean, p< 0.0001)
Surface morphology
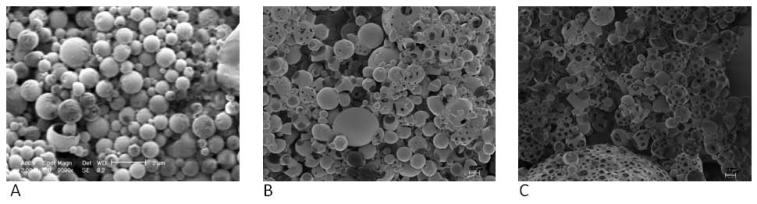
CA samples were examined using SEM to determine changes in morphology or noticeable deformation of the CA surface. Increased surface pitting was observed on the shell of the agent as plasma exposure time and intensity increased. It was also noticed that increases in exposure time resulted in a more dramatic increase in surface pitting than increases in plasma intensity. An example of pre and post processing SEM images is shown in Fig. 6 for 0, 3 and 6 minute treatments at 18 watts. In light of these changes, differential scanning calorimetry (DSC) was performed on samples to study the effect on bulk properties of the PLA during plasma exposure. The glass transition temperature (Tg) was used as an indication of bulk change in polymer. Although all samples showed hysteresis, presumably due to the CA fabrication process, all samples showed a consistent Tg of 59.8 +/− 0.9 °C with no trends among plasma intensity or exposure time (data not shown).
Figure 6.
Scanning electron micrographs of contrast agents (A) pre-plasma sterilization (magnification=9000X, size bar= 2 μm), (B) post-sterilization at 18.0 W for 3 mins (magnification=2500X, size bar= 2 μm), and (C) post-sterilization at 18.0W for 6 mins (magnification=2500X, size bar= 2 μm).
Zeta potential
Zeta potential of treated CA was measured in order to judge changes in surface chemistry due to surface modification of the agent while interacting with plasma. Zeta potential was seen to increase with both plasma sterilization power and exposure time. These trends are counterintuitive to what would be expected from generation of –COOH groups and are summarized in Fig. 7. These results show that exposure to plasma has significantly altered the surface chemistry of CA.
Figure 7.
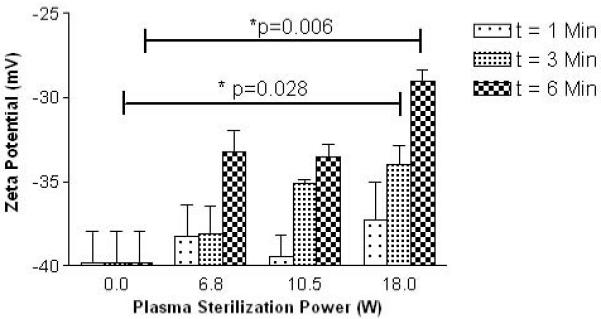
Changes in zeta potential of contrast agents treated with plasma at varying powers and exposure times. (n=3, error bars= standard error about mean, p=0.028 for optimal processing conditions (3 mins at 18.0 W))
Dox surface loading
A method of loading Dox to the surface of the CA was used as an example of how modification of the agent’s surface during sterilization can be used to alter potential drug and surface ligand attachment for targeted drug delivery applications. CA sterilized with varying plasma power levels and exposure times were drug loaded as described in the methods section. A positive correlation between encapsulation efficiency with both plasma exposure time and sterilization power was seen. These results are summarized below in Fig. 8. A general trend of increasing drug attachment is seen for both increases in sterilization power and plasma exposure times. Samples sterilized using 18W showed a statistically significant increase in encapsulation efficiency for exposure times of 3 mins (5% increase, p=0.023) and 6 mins (10% increase, p=0.023).
Figure 8.
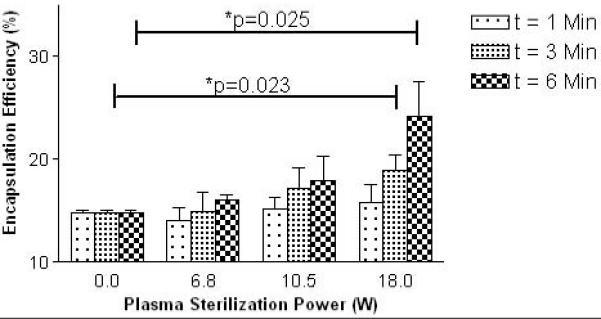
Effect of plasma conditions on ability of contrast agents to adsorb doxorubicin. (n=3, error bars= standard error about mean, p=0.023 for optimal processing conditions (3 mins at 18.0 W), p=0.025 for maximal drug payload conditions (6 mins at 18.0 W))
Discussion
To date, there have been no successful reports on methods of sterilizing polymeric CA other than by asceptic/clean room fabrication, which is also a chosen method for solid polyester capsules (Felder et al. 2003). A filtration method has been described for drug-loaded nanocapsules by Konan et al (2003), but this is only suitable for submicron particles. Due to the multiple steps that are usually involved in microencapsulation processes, terminal sterilization is generally viewed as the only economical and efficient method (EMEA, 2000). Steam and dry heat destroy even solid capsules, and are disastrous methods for thin-walled, hollow CA, rendering them collapsed and melted (unpublished observations). Ethylene oxide is not acceptable due to toxic residues, and we observed that use of the standard hospital ethylene oxide sterilization unit (Anprolene, Andersen Products, Haw River, NC) produced the same results as autoclaving (unpublished observation) (Swarbrick and Boyland, 2002; Zislis et al. 1998). By far the most frequently reported method for solid capsules involves γ–irradiation (Igartua et al. 2008). Most reports highlight unfavorable dose-dependent polymer degradation, and the effects increase as glycolide content increases (Bittner et al. 1999; Claybourn et al. 2003; Hausberger et al. 1995; Williams et al. 2006).
We describe here a method that consistently sterilized PLA CA using plasma exposure of 18.0 W for times of 3 and 6 minutes, as demonstrated in both culture medium and blood agar. The exact mechanism of biological decontamination by low temperature plasma is not known, but the two prevailing mechanisms are thought to be electrostatic disruption of cell membranes and lethal oxidation of membrane or cytoplasmic components (Gaunt 2006). In general as would be expected spores are the most resistant, and Gram-positive and Gram-negative bacteria respond differently, with Gram-negative organisms demonstrating cell lysis and fragmentation while Gram-positive organisms are subject to cell leakage (Montie 2000). The substrate has also been shown to play a crucial role (Gadri 2000). In our case we are interested in not only surface sterilization, but also inactivation of any organisms that are within the CA shells, and a lack of time for penetration and in depth sterilization might explain the fact that the one minute treatment time was ineffective for complete sterilization at all power levels (Fig 3).
The effects of both plasma sterilization power and exposure time on PLA CA were found to be crucial for successful preservation of echogenicity. While sterilization power did not affect either the agent’s ability to provide contrast or its acoustic stability, exposure times of over 3 minutes did reduce these properties and thus the agent’s overall function as a CA. These findings are consistent with our SEM findings in which surface pitting was seen to greatly increase with increased plasma exposure time, while only moderately increasing with increases in plasma intensity. While increased shell pitting was noticed with increased plasma intensity, this increase was not detectable in terms of acoustic performance, indicating a potential threshold of shell integrity. It is believed that as extensive and deeper pitting occurs, the shell becomes more porous and is eventually breached, at which point the CA loses the ability to entrap gas for extended periods of time. This decrease in shell integrity explains both decreases in in vitro enhancement and stability during insonation.
Both increased sterilization power and exposure time lead to increased zeta potential (less negative) of the agent, although no changes in the agent’s ability to re-disperse were noticed. This is somewhat surprising if the proposed interaction between the plasma and the PLA is the production of –COOH groups. Generation of a carboxyl groups, with pKa’s below 3.0 would normally result in an increase in negative surface charge. It is still unclear why the zeta potential of treated CA becomes less negative, but this is consistent with the proposed mechanism of Inagaki et al. (2002) referred to in the introduction, the scheme of which is shown in Fig. 1, and to their observation that the surface hydrophilicity of plasma treated PLA surfaces did not increase as originally expected. We have reported on anomalous zeta potential data of PLGA CA in an earlier study in which fabrication of PLGA CA using polymer with lauryl ester end-capped groups resulted in CA with an average zeta potential of −36.06 mV, while agents fabricated using PLGA with free –COOH end groups resulted in CA with an average zeta potential of −28.49 mV (an increase of 7.57 mV) (Eisenbrey et al. 2008b). However, this is more likely to do with forces that affect the actual conformation if the polymer at the surface of the capsule during fabrication, than with any post fabrication modification. At this point in time, the exact mechanisms of shifts in zeta potential are not well understood and may equally well be from changes in surface morphology altering the particles’ electrophoretic mobility.
Modification at the CA surface provides increased flexibility for surface attachment in both drug delivery applications, as was demonstrated by a 10% increase in encapsulation efficiency using a surface adsorption technique. This process of surface modification is also expected to increase ligand attachment for targeting applications (more active groups are available for ligand conjugation). This later property is significant in our application of tumor-targeted CA. The plasma sterilization process is therefore ideal for our agents, enabling CA fabrication using an end-capped polymer, followed by surface modification during sterilization for potential drug delivery and targeting applications, without sacrificing the acoustic properties of the agent.
While this sterilization process is ideal for polymer shelled agents, other agents that are already in the clinic are either amenable to sterilization by other methods or problematic for plasma exposure. For example Imagent®, which was formerly named Imavist™, (Alliance Pharmaceuticals) composed of phospholipid, hydroxyethyl starch and a poloxamer surfactant, is prepared de novo as a heat-sterilized spray-dried powder. Optison™ (GE Healthcare) composed of an albumin shell and octafluoropropane gas core, is prepared in sterile facilities and may be problematic for plasma sterilization due to plasma’s destructive interaction with proteins (Moreau et al. 2008). In terms of polymer shelled agents, Imagify™ (Perflubutane PLGA microspheres developed by Acusphere) is prepared under sterile conditions by spray drying an emulsion containing the polymer, a phospholipid and a pore-forming agent (all pre-sterilized by heat). While these methods are viable on the commercial scale, the vast majority of research looking at polymer shelled CA for imaging, targeting, and drug delivery is currently being done bench side with limited resources. A rapid, cost effective method of sterilization should provide future opportunities for in vivo research, and ultimately faster translation to the clinic.
Conclusions
We have shown that exposure to oxygen plasma reliably sterilizes PLA CA without sacrificing their ultrasonic properties as long as the parameters are chosen with care. Additionally, surface modification during this process can be used to improve drug loading for ultrasound triggered drug delivery. Parameters have been identified (18.0 W sterilization for t=3 mins) that reliably sterilizes the CA, do not affect the agent’s sensitivity to ultrasound, and result in significant surface modification that can be used to increase drug adsorption. These conditions have been repeated 9 times and shown to also be sterile in blood agar cultures. Surface modification could well prove to be highly significant, firstly in improving targeting potential of ligated CA, and secondly facilitating use of end-capped PLGA, a polymer that produces more stable CA, but which to date had generated few active groups on the CA surface. These possibilities are being actively pursued by us. While numerous methods of sterilization have proven to destroy PLA CA, plasma sterilization appears to both sterilize and beneficially modify the agent for increased Dox adsorption. These results pave way for future targeting and drug delivery applications, while also making long term cellular and in vivo studies feasible without the need for production within a clean facility.
Acknowledgements
The authors would like to thank Dr. Wei Sun and Eda Yildirim of Drexel University’s Department of Mechanical Engineering and Mechanics for the training and use of their plasma sterilization setup and Dr. Chris Li and Mathew Hood of Drexel University’s Department of Material Science and Engineering for DSC measurements. Electron Microscopy was done at Drexel University’s Materials Characterization Institute with the help of Kelleny Oum. Funding was provided through NIH grants HL 52901, and The Coulter Foundation. Jennifer Hsu was supported by the Research Experience for Undergraduates Program sponsored by the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bittner B, Mader K, Kroll C, Borchert HH, Kissel T. Tetracycline–HCl-loaded poly(DL-lactide co-glycolide) microspheres prepared by a spray drying technique: influence of gamma-irradiation on radical formation and polymer degradation. J Control Release. 1999;59(1):23–32. doi: 10.1016/s0168-3659(98)00170-9. [DOI] [PubMed] [Google Scholar]
- Claybourn M, Gray H, Murphy DM, Purnell IJ, Rowlands CC. Electron magnetic resonance study of gamma-irradiated poly(lactideco-glycolide) microspheres. J Control Release. 2003;91:431–438. doi: 10.1016/s0168-3659(03)00269-4. [DOI] [PubMed] [Google Scholar]
- Eisenbrey JR, Huang PN, Soulen MC, Wheatley MA. Doxorubicin loaded contrast agents for ultrasound triggered drug delivery: importance of process parameters. Pharmaceutical Engineering. 2008a;5:70–8. [Google Scholar]
- Eisenbrey JR, Mualem-Burstein O, Wheatley MA. Effect of molecular weight and end capping on poly (lactic-co-glycolic acid) ultrasound contrast agents. Polymer Eng Sci. 2008b;48:1785–92. [Google Scholar]
- El-Sherif DM, Wheatley MA. Development of a novel method of synthesis of a polymeric ultrasound contrast agent. Journal of Biomed Mat. 2003;66A:347–55. doi: 10.1002/jbm.a.10586. [DOI] [PubMed] [Google Scholar]
- EMEA (European Agency for the Evaluation of Medicinal Products) Decision Trees for the Selection of Sterilization Methods. CPMP (Committee for Proprietary Medicinal Products); London: Apr 5, 2000. CPMP/QWP/O54/98 Corr. [Google Scholar]
- Felder AH, Blanco-Príeto MJ, Heizmann J, Merkle HP, Gander B. Ultrasonic atomization and subsequent polymer desolvation fo peptide microencapsulation into biodegradable polyesters. J Microencap. 2003;20(5):553–567. doi: 10.1080/0265204031000148059. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Merton DA, Liu JB, Needleman L, Goldberg BB. Clinical applications of ultrasound contrast agents. Ultrasonics. 1998;36:695–701. doi: 10.1016/s0041-624x(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Forsberg F, Wu Y, Makin IRS, Wang W, Wheatley MA. Quantitative acoustic characterization of a new surfactant-based ultrasound contrast agent. Ultrasound in Med & Biol. 1997;23:1201–1208. doi: 10.1016/s0301-5629(97)00078-1. [DOI] [PubMed] [Google Scholar]
- Gadri BR, Roth JR, Montie TC, Kelly-Wintenberg K, Tsai PPY, Helfritch DJ, Feldman P, Sherman DM, Karakaya F, Chen ZY. Sterilization and plasma processing of room temperature surfaces with a one atmosphere uniform glow discharge plasma (OAUGDP) Surf Coat Technol. 2000;131:528–542. [Google Scholar]
- Gaunt LF, Beggs CB, Georghiou GF. Bactericidal Action of the Reactive Species Produced by Gas-Discharge Nonthermal Plasma at Atmospheric Pressure: A Review. IEEE Trans Plasma Sci. 2006;34:1257–1269. [Google Scholar]
- Geze A, Venier-Julienne MC, Cottin J, Faisant N, Benoit JP. PLGA microsphere bioburden evaluation for radiosterilization dose selection. J Microencpasulation. 2001;18:627–36. doi: 10.1080/02652040010019424. [DOI] [PubMed] [Google Scholar]
- Gogolewski S, Mainil-Varlet P, Dillon JG. Sterility, mechanical properties, and molecular stability of polylactide internal-fixation devices treated with low-temperature plasmas. J Biomed Mater Res. 1996;32:227–35. doi: 10.1002/(SICI)1097-4636(199610)32:2<227::AID-JBM12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Goldberg BB, Raichlen JS, Forsberg F. Ultrasound contrast agents; basic principles and clinical applications. 2nd ed Martin Dunitz; London: 2001. [Google Scholar]
- Hausberger A, Kenley R, De Luca PP. Gamma irradiation effects on molecular weight and in vitro degradation of poly(D,Llactide-co-glycolide) microparticles. Pharm Res. 1995;12:851–856. doi: 10.1023/a:1016256903322. [DOI] [PubMed] [Google Scholar]
- Hohman J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147–59. doi: 10.1016/s0720-048x(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Huang YC, Huang CC, Huang YY, Chen KS. Surface modification and characterization of chitosan or PLGA membrane with laminin by chemical and oxygen plasma treatment for neural regeneration. Journal of Biomed Mat Res Part A. 2007;82A:842–51. doi: 10.1002/jbm.a.31036. [DOI] [PubMed] [Google Scholar]
- Igartua M, Hernández RM, Rosas JE, Patarroyo ME, Pedraz JL. c-Irradiation effects biopharmaceutical properties of PLGA microspheres loaded with SPf66 synthetic vaccine. Euro J Pharma Biopharm. 2008;69:519–526. doi: 10.1016/j.ejpb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Narushima K, Tsutsui Y, Ohyama Y. Surface modification and degradation of poly(lactic acid) films by Ar-plasma. J Adhesion Sci Technol. 2002;16:1041–54. [Google Scholar]
- Klibanov A. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug delivery applications. Inv Radiol. 2006;41:354–62. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- Konana YN, Cerny R, Favet J, Berton M, Gurnya R, Allémann E. Preparation and characterization of sterile sub-200 nm meso-tetra(4-hydroxylphenyl)porphyrin-loaded nanoparticles for photodynamic therapy. Euro J Pharm Biopharm. 2003;55:15–124. doi: 10.1016/s0939-6411(02)00128-5. [DOI] [PubMed] [Google Scholar]
- Magarelli N, Guglielmi G, de Matteo L, Tartaro A, Mattei PA, Bonomoa L. Diagnostic utility of an echo-contrast agent in patients with synovitis using power Doppler ultrasound: a preliminary study with comparison to contrast-enhanced MRI. Eur Radiol. 2001;11:1039–46. doi: 10.1007/s003300000650. [DOI] [PubMed] [Google Scholar]
- Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography-a comparison with magnetic resonance imaging. J Am Coll Cardiol. 2004;44:1030–35. doi: 10.1016/j.jacc.2004.05.068. [DOI] [PubMed] [Google Scholar]
- Montie TC, Kelly-Wintenberg K, Roth JR. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans Plasma Sci. 2000;28:41–50. [Google Scholar]
- Moreau M, Orange N, Feuilloley MGJ. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnology Advances. 2008;26:610–17. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Narayan P, Wheatley MA. Preparation and characterization of hollow microcapsules for use as ultrasound contrast agents. Polymer Eng & Sci. 1999;39:2242–55. [Google Scholar]
- Seeman S, Hauff P, Schultze-Mosgau M, Lehmann C, Reszka R. Pharmaceutical evaluation of gas-filled microparticles as gene delivery system. Pharm Res. 2002;19:250–57. doi: 10.1023/a:1014430631844. [DOI] [PubMed] [Google Scholar]
- Straub JA, Chickering DE, Church CC, Shah B, Hanlon T, Bernstein H. Porous PLGA microparticles: AI-700, and intravenously administered ultrasound contrast agent for use in echocardiography. J Control Release. 2005;108:21–32. doi: 10.1016/j.jconrel.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Swarbrick J, Boyland BC. Second ed encyclopedia or pharmaceutical technology. vol 3. Marcel Dekker Inc.; New York: 2002. [Google Scholar]
- Wan Y, Qu X, Lu J, Zhu C, Wan L, Yang J, Bei J, Wang S. Characterization of surface property of poly(lactide-co-glycolide) after oxygen plasma treatment. Biomaterials. 2004;25:4777–83. doi: 10.1016/j.biomaterials.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Wheatley MA, Lathia JD, Oum KL. Polymeric ultrasound contrast agents targeted to integrins: Importance of process methods and surface density of ligands. Biomacromolecules. 2006a;8:516–22. doi: 10.1021/bm060659i. [DOI] [PubMed] [Google Scholar]
- Wheatley MA, Forsberg F, Oum K, Ro R, El-Sherif DM. Comparison of in vitro and in vivo acoustic response of a novel 50:50 PLGA contrast agent. Ultrasonics. 2006b;44:360–67. doi: 10.1016/j.ultras.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Williams HE, Huxley J, Claybourn JM, Booth J, Hobbs M, Meehan E, Clark B. The effect of Gamma-irradiation and polymer composition on the stability of PLG polymer and microspheres. Polym Degrad Stabil. 2006;91(9):2171–2181. B. [Google Scholar]
- Wise DL, Trantolo DJ, Altobelli DE, Yaszemski MJ, Gresser JD, Schwartz ER. Encyclopedic handbook of biomaterials and bioengineering, part A. Materials. Marcer Dekker; New York: 1996. Biomaterials surface modification using plasma gas discharge process; pp. 865–94. [Google Scholar]
- Zislis T, Martin SA, Cerbas E, Heath JR, Mansfield JL, Hollinger JO. A scanning electron microscopic study of in vitro toxicity of ethylene-oxide-sterilized bone repair materials. J Oral Implant. 1989;15:41–6. [PubMed] [Google Scholar]