Abstract
Neurons excited by stimulation of one ear and suppressed by the other, called excitatory/inhibitory (EI) neurons, are sensitive to interaural intensity disparities, the cues animals use to localize high frequencies. EI neurons are first formed in lateral superior olive, which then sends excitatory projections to the dorsal nucleus of the lateral lemniscus and the inferior colliculus (IC), both of which contain large populations of EI cells. We evaluate herein the inputs that innervate EI cells in the IC of Mexican free-tailed bats (Tadarida brasilensis mexicana) with in vivo whole-cell recordings from which we derived excitatory and inhibitory conductances. We show that the basic EI property in the majority of IC cells is inherited from lateral superior olive, but that each type of EI cell is also innervated by the ipsilateral or contralateral dorsal nucleus of the lateral lemniscus, as well as additional excitatory and inhibitory inputs from monaural nuclei. We identify three EI types, each of which receives a set of projections that is different from the other types. To evaluate the role that the various projections played in generating binaural responses, we used modeling to compute a predicted response from the conductances. We then omitted one of the conductances from the computation to evaluate the degree to which that input contributed to the binaural response. We show that the formation of the EI property in the various types is complex, and that some projections exert such subtle influences that they could not have been detected with extracellular recordings or even from intracellular recordings of postsynaptic potentials.
Introduction
The localization of a high-frequency sound is a computation made by the auditory system from interaural intensity differences (IIDs). IIDs are first “compared” by excitatory/inhibitory (EI) neurons in the lateral superior olive (LSO), where signals from one ear excite and signals from the other ear inhibit LSO neurons. The initial IID coding is then further modified in higher nuclei via the projections that the LSO sends bilaterally to both the inferior colliculus (IC) and to the dorsal nucleus of the lateral lemniscus (DNLL). The IC is of particular interest because it receives projections not only from the two LSOs and DNLLs, but also from most other lower auditory nuclei, and thus is the nexus of the auditory system. Consistent with its innervation from the LSO, many IC cells express EI properties similar to those in the LSO.
Previous studies have shown that EI cells in the IC comprise a diverse population, even though they exhibit binaural properties similar to LSO cells (Irvine and Gago, 1990; Li and Kelly, 1992; Park and Pollak, 1993, 1994; Li et al., 2010). The diversity is due to different combinations of projections from LSOs, DNLLs, and from various lower, monaural nuclei that innervate each of the EI types in the IC. The various projection patterns were inferred from the known projections to the IC and from the changes in binaural properties that were seen when inhibition was blocked at the IC or when the contralateral DNLL was reversibly inactivated.
In this study, we extend the evaluations of the various ways by which EI neurons are formed with whole-cell recordings from which we computed excitatory and inhibitory conductances evoked by monaural and binaural stimulation. The conductances provide more direct insights into monaural or binaural nature of the projections that play upon EI cells than do either postsynaptic potentials (PSPs) or spikes. Because the lower binaural nuclei that provide excitatory and inhibitory projections to the IC are known (Adams and Mugnaini, 1984; Glendenning et al., 1992; Winer et al., 1995), reasonable assumptions about the putative circuits that account for the results can be proposed. In addition, conductances can be manipulated in a model to provide an assessment of the roles that one or another of the inputs plays in shaping the sound-evoked response (Gittelman et al., 2009; Gittelman and Pollak, 2011).
By evaluating the conductances, we found that a substantially larger complement of inputs innervates each type of EI cell than those that had been proposed in previous extracellular or intracellular studies (Park and Pollak, 1994; Li et al., 2010). We show here that the basic EI property in the vast majority of IC cells is inherited from LSO, but that each of the EI cells has additional inputs, the particular complement of which differ depending upon the EI type. We show that the formation of the EI property in the various types is complex and that some projections exert subtle influences that could not have been detected with extracellular recordings or even from intracellular recordings of PSPs.
Materials and Methods
Animals.
Experiments were conducted on 20 male Mexican free-tailed bats (Tadarida brasilensis mexicana) captured from local sources in Austin, Texas. Surgical procedures were as described previously (Li et al., 2010). In brief, bats were sedated with isoflurane (inhalation) and then anesthetized with an intraperitoneal injection of ketamine/xylazine (75–100 mg/kg ketamine, 11–15 mg/kg xylazine, Henry Schein). Recordings began after recovery from the anesthetic and thus all data were obtained from awake animals. Water was presented periodically with an eyedropper. Bats typically lay quietly during the experiments. If they showed signs of discomfort, data collection was stopped and doses of the neuroleptic ketamine hydrochloride (1/40 dilution, 0.01 ml injection) were administered. All experimental procedures were in accordance with a protocol approved by the University of Texas institutional animal care committee.
Acoustic stimuli.
Auditory stimuli were tone bursts generated digitally in IGOR-PRO. Tone bursts had durations of 5–20 ms and rise-fall times of 0.2 ms and were presented at the neuron's best frequency (BF), the frequency to which it was most sensitive. The acoustic signals were sent to an Instrutech 16-bit D/A converter and were fed to custom-made electronic attenuators and then to custom-designed earphones. The frequency characteristics of each earphone was determined with a ¼ inch Bruel and Kajer microphone and the computer compensated for output fluctuations across frequency so that each earphone was flat ± 2 dB from ∼10–50 kHz. At the start of each experiment, the earphone was inserted into the funnel formed by the bat's pinnae and positioned adjacent to the external auditory meatus. The acoustic crosstalk with this arrangement was attenuated by ∼40 dB.
Tones were presented both monaurally and binaurally. Monaural tones were presented to either the contralateral or ipsilateral ear alone. Binaural signals were presented with the tone at the contralateral ear fixed at one intensity, usually at 10–20 dB above spike threshold, while tones were presented simultaneously to the ipsilateral ear over a 20-4–0 dB intensity range, from ∼10 dB below to 30 dB above the intensity at the contralateral ear.
Recording procedures and data acquisition.
Responses were recorded with patch electrodes (5–10 MΩ) pulled from thick-walled (1.65 mm outer diameter 1.1 mm inner diameter) capillary glass (#PG52165-4; WPI). The standard internal solution contained the following (in mm): 115 K-gluconate, 10 HEPES, 7 KCl, 4 MgATP, 0.3 Na2GTP, 0.5 EGTA, and 10 Na2 phosphocreatine. Membrane potentials were not corrected for liquid junction potentials.
During experiments, the electrodes were positioned over the central region of IC, a locus where there is a predominance of EI cells. Electrodes were lowered into the IC with a piezoelectric microdrive (Burleigh Inchworm; EXFO Burleigh) while under positive pressure of 2–3 PSI. Electrodes were lowered to a depth of ∼300 μ to bypass the external nucleus of the IC and to ensure that recordings were made from cells in the central nucleus of the IC. All cells were recorded at depths of 300–1000 μ from the surface of the IC. Upon entering the central nucleus, the pressure was reduced to 0.3–0.7 PSI and the electrodes were advanced in steps of 1–2 μ. Cell search was conducted in voltage-clamp mode using a −5 mV step to monitor electrode resistance. When contact with a cell was made, pressure was released and a small amount of negative pressure (<0.5 PSI) was applied to obtain a giga-ohm seal. After a seal was obtained, additional negative pressure was applied to break-in, the amplifier was switched to whole-cell current-clamp mode, the voltage offset was set to 0, and the electrode capacitance was neutralized. Recordings evoked by sound were then obtained. Responses were sent to a MultiClamp 700B Microelectrode Amplifier (Molecular Devices) and then to an A/D/A converter (200 kHz sampling rate, ITC-18/PCI; InstruTech), and stored on a Macintosh G5 computer. Analyses were done in IGOR PRO. Tone bursts were first presented to the contralateral ear and frequency was manually scanned to determine the cell's BF, the frequency at which the lowest intensity evoked discharges. BF tones were then presented to evaluate responses evoked by contralateral, ipsilateral, and binaural stimulation. Each tone was presented 8–20 times.
Estimating access resistance, membrane resistance, and membrane capacitance.
Electrode capacitance was minimized by capacity compensation. Subsequently, access resistance, membrane resistance, membrane capacitance, and membrane time constant were estimated as described previously (Gittelman et al., 2009). Briefly, voltage responses were fit to small hyperpolarizing current steps (25–100 pA, 200 ms duration) with a double exponential as follows:
where Vt was the measured change in voltage (total change) in response to the current injection at steady state, Vp was the steady-state voltage change attributed to the pipette, and Vm was the steady-state voltage change attributed to the membrane. The fast and slow time constants were attributed to the pipette (τp) and membrane (τm), respectively. Membrane resistance (Rm) was equal to the change in membrane voltage divided by the injected current (Rm = Vm/Iinj). Membrane capacitance (Cm) was then calculated as τm divided by Rm (Cm = τm ÷ Rm). Access resistance and electrode capacitance were estimated in a similar way, using the fast components of the fit (Vp and τp). In recordings judged acceptable, the fast time constant was <1 ms and the associated access resistance was less than the estimated membrane resistance.
Deriving synaptic conductances.
Synaptic conductances were derived in current-clamp mode as described previously (Priebe and Ferster, 2005; Gittelman et al., 2009). We estimated synaptic conductances using:
where Cm is the membrane capacitance, dVm/dt is the slope of the membrane potential, Im is the current across the cell membrane, and Iinj is the current injected through the electrode. We assumed three membrane currents: excitatory, inhibitory, and leak. Equation 1 can be expanded to include the conductance and driving force terms as follows:
 |
where the conductances (g) are excitatory, ge; inhibitory, gi; leak, gleak; Vm is the measured membrane potential; and the reversal potentials for gleak, ge, and gi are (respectively) Vleak, Ve, and Vi. Most of these terms can be measured or estimated; Vm and dVm/dt were measured directly. Membrane capacitance and resistance (1/gleak) were measured as described above. Ve was assumed to be 0 mV and Vi was estimated to be −68 mV from the changes in the PSP polarity while different amounts of constant current were being injected. Vleak was calculated from the steady-state Vm, membrane resistance measured at the steady-state Vm, and the Iinj.
Using the above values, there are only two unknowns in Equation 3: ge and gi. Therefore, ge and gi were estimated from tone-evoked responses while hyperpolarizing the cell to at least three different steady-state potentials. See Gittelman and Pollak (2009) for further details.
Conductances and modeling.
Recordings were made with patch electrodes from 42 IC cells after making a giga-ohm seal and breaking in. However, the conductances reported here were only from 10/42 cells because the conductances computed for most cells were either not successful or were considered to be invalid. Valid conductance estimates were based on three criteria.
The first criterion was that the access resistance of the electrodes had to remain constant throughout the recording session because changes in access resistance would change the amplitudes of the sound-evoked PSPs and thus change the values of ge and gi.
The second criterion was that the responses computed in model cells had to agree closely with the PSPs actually evoked by monaural and binaural tones. We used the estimated values for ge and gi to predict the PSPs in model cells. Model cells were “point neurons ” consisting only of excitatory, inhibitory, and leak conductances with corresponding reversal potentials and a capacitance. We made a unique model cell for each neuron from the conductance estimates based on the measured input resistance, resting membrane potential, and capacitance in each cell. Correlation analysis between the predicted PSPs and sound-evoked PSPs indicated that the conductance estimates accounted for >94% of the variance (R2 > 0.94 for all conductance sets). Predictions were good across a broad range of membrane potentials and suggest that the state of voltage-gated channels changed little during the time course of the PSPs and thus had a small effect on our conductance estimates. Therefore, the only conductances reported here were from cells in which the predicted PSPs, the PSPs computed from the conductances, agreed closely with the sound-evoked PSPs.
The third criterion was that conductances had to have positive values. We excluded analyses that found negative values for conductance. This requirement assumed that the ligand-gated channels were closed (0 nS) before sound presentation, so the conductances could only get larger. This is a reasonable assumption because there was little or no spontaneous activity in the IC cells. The absence or near absence of spontaneous activity is a common finding in the IC of bats (Vater et al., 1979; Klug et al., 1999; Bauer et al., 2000). The occasional spontaneous PSP was obvious and was excluded from analysis. In practice, negative conductance values were obtained when the access resistance was too high to determine the actual membrane potentials. These datasets were excluded.
For each of the 10 neurons, we derived three sets of synaptic conductances: one evoked by the contralateral tone alone, another evoked by the ipsilateral tone alone, and a third evoked by a binaural tone. With binaural signals, the IID was adjusted so that a complete or nearly complete spike suppression was produced. With these IIDs, the contralateral tone was at 10–20 dB above threshold and the ipsilateral intensity was 20–30 dB more intense than the contralateral tone. In two cells, we derived another set of synaptic conductances at a different ipsilateral intensity and thus at a different IID. Of particular importance, we also worked backwards and computed PSPs from the conductances for both monaural and binaural tones (we refer to the PSPs computed from conductances as the predicted PSPs). We then compared the predicted PSPs with the PSPs that were evoked by sounds. In all cases, the predicted PSPs corresponded closely with the sound-evoked PSPs, showing the validity of the conductance calculations (Fig. 3A).
Figure 3.
Conductance records of an EI cell with ipsilaterally evoked EPSPs. A, Predicted responses computed from conductances (dashed lines) provide accurate representations of sound-evoked responses (solid lines). B, Circuit suggested by excitatory and inhibitory conductances evoked by contralateral, ipsilateral, and binaural tones shown in C and E below. C, Excitatory conductances evoked by monaural and binaural tones. The binaural excitatory conductance is smaller than the contralateral excitatory conductance, suggesting that the strength of the excitation evoked by the contralateral ear was reduced when the ipsilateral tone was introduced. D, Circuitry accounting for the excitatory conductances evoked by monaural and binaural stimulation. E, Inhibitory conductances evoked by monaural and binaural tones. The binaural inhibitory conductance is also smaller than the contralateral inhibitory conductance, suggesting that the strength of the inhibition evoked by the contralateral ear was reduced when the ipsilateral tone was introduced. F, Circuitry accounting for the inhibitory conductances evoked by monaural and binaural stimulation. In this and all subsequent figures, the thickness of the lines in each circuit schematic represents the relative strength of that lower brainstem projection. Black lines are excitatory projections, red lines are GABAergic inhibitory projections, and orange lines are glycinergic inhibitory projections.
Results
We recorded spikes and PSPs with patch electrodes from 42 EI cells in the central nucleus of the IC of awake bats. After recording both the PSPs and spikes evoked by the monaural and binaural signals, synaptic conductances were computed for 10 cells that had input resistances that ranged from 150 to 250 MΩ.
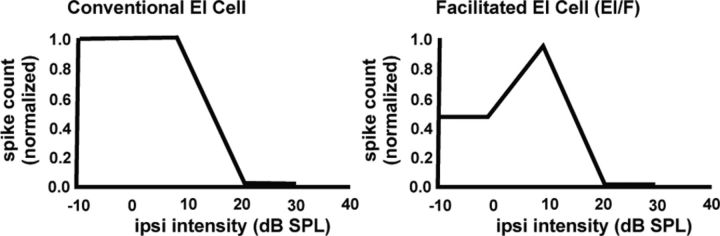
Conductances were derived from two principal types of EI cells (Fig. 1). The first type is the conventional EI cell. In these EI cells, weak ipsilateral intensities had no influence on the spike counts or PSP amplitudes evoked by the contralateral tones, where the contralateral intensity was fixed at 10–20 dB above threshold. Increasing the ipsilateral intensity in these cells caused a progressive suppression of both spikes and PSP amplitudes until the spikes were completely suppressed. The second type is the facilitated EI cell (EI/f). In these cells, low ipsilateral intensities initially caused an increase in both spike count and PSP amplitude that was at least 20% greater than that evoked by the contralateral signal alone. Higher ipsilateral intensities then progressively suppressed both spikes and reduced PSP amplitudes in the same way as in conventional EI cells.
Figure 1.
IID functions of a conventional and facilitated EI cell. Each function was generated by presenting a tone to the contralateral ear of fixed intensity at 10 dB above spike threshold. All spike-counts are normalized relative to highest spike count. Tones of progressively increasing intensity were then presented to the ipsilateral ear (ipsi intensity). The normalized spike counts evoked at each ipsilateral intensity are plotted as the IID function.
Responses evoked by contralateral and ipsilateral signals presented monaurally
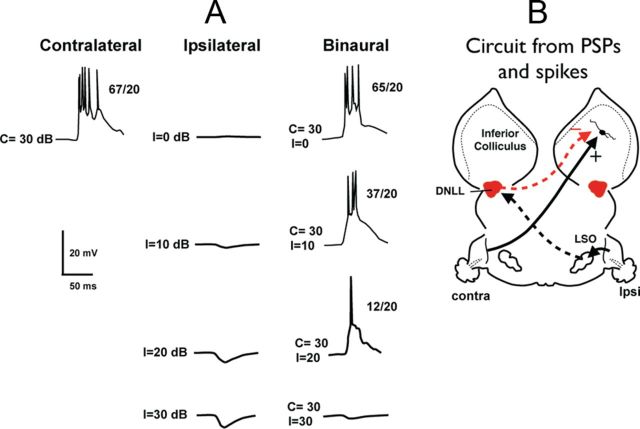
In all cells, whether conventional or facilitated, contralateral stimulation at 10–20 dB above threshold evoked strong EPSPs and discharges. In contrast, one of two different response patterns was evoked by ipsilateral tones in conventional EI cells. The first, and most common, response pattern was a subthreshold EPSP that was evoked by ipsilateral tones across all of the intensities that we presented (Fig. 2). The amplitudes of the EPSP increased with ipsilateral intensity but always were subthreshold. The second response pattern was characterized by pure IPSPs that were evoked across all the ipsilateral intensities (Fig. 4). The amplitudes of the IPSPs increased with ipsilateral intensity, as did the ipsilaterally evoked EPSPs seen in other cells.
Figure 2.
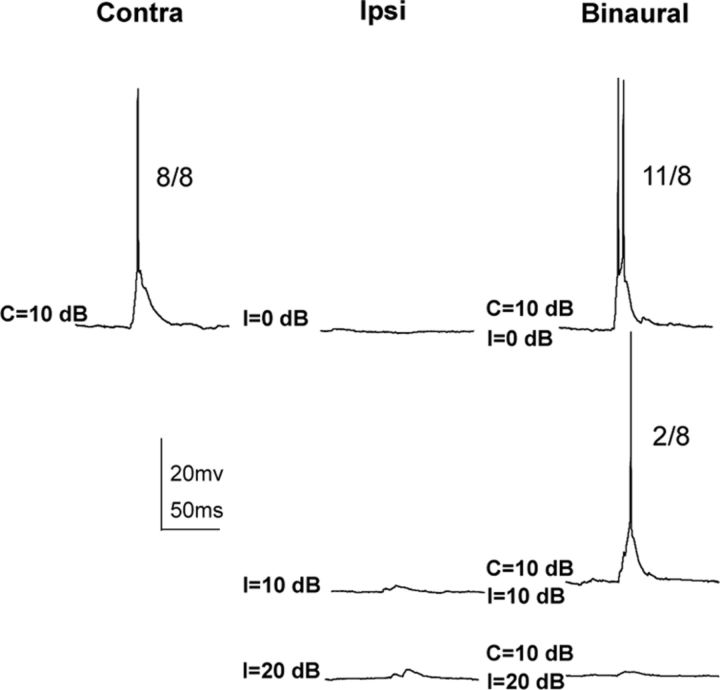
EI cell with ipsilaterally evoked EPSPs. A, Responses evoked by contralateral, ipsilateral, and binaural tones. The numbers to the right of each response show the number of discharges evoked by 15 stimulus presentations. B, Circuit proposed to account for both the spikes and EPSPs evoked by stimuli in A. C, The subthreshold EPSP evoked by an ipsilateral tone at 50 dB SPL is exactly the same as the EPSP evoked by a binaural signal with a contralateral intensity of 10 dB SPL and an ipsilateral intensity of 50 dB SPL. The 10 dB contralateral signal presented alone evoked a large EPSP and discharges.
Figure 4.
An EI cell with ipsilaterally evoked IPSPs. A, Responses evoked by contralateral, ipsilateral, and binaural tones. The numbers to the right of each response shows the number of discharges evoked by 20 stimulus presentations. B, Circuit proposed to account for both spikes and PSPs evoked by stimuli in A. Contralateral stimulus evokes excitation by a monaural excitatory projection from an unknown source. Ipsilateral stimulation drives the contralateral DNLL, which provides the inhibitory input to the IC.
In the sections below, we first present the results for conventional EI cells with ipsilaterally evoked EPSPs, the most common EI cell, and then focus on the conventional EI cells that expressed ipsilaterally evoked IPSPs. In the final section, we focus on the EI/f cells.
Conventional type 1 EI cells: EI cells with ipsilaterally evoked EPSPs
In the most common type of conventional EI cell (n = 13) contralateral tones evoked large suprathreshold EPSPs, and thus spikes, whereas ipsilateral tones only evoked subthreshold EPSPs the amplitudes of which increased with ipsilateral intensities (Fig. 2). In all of these cells, there was no evidence of hyperpolarization in the PSPs, suggesting that no local inhibition was evoked by monaural or binaural tones. Because both the contralateral and ipsilateral tones evoked only EPSPs, the surprising and paradoxical result was that binaural signals with the same ipsilateral intensities that evoked progressively larger EPSPs also generated progressively greater suppressions of the contralaterally evoked responses. When presented binaurally, ipsilateral intensities that were 30–40 dB more intense than the contralateral intensities produced complete spike suppression, as shown in Figure 2A. In all EI cells of this type, the binaural signals with the high ipsilateral intensities (C = 10, I = 50 dB for the cell in Fig. 2), evoked subthreshold EPSPs with waveforms virtually identical in latency, amplitude, and shape to the EPSPs evoked by the ipsilateral signals presented alone at that intensity (Fig. 2C). Indeed, with binaural signals, these cells behaved as if the contralateral signals were not even present, even though the contralateral signals, when presented alone, evoked suprathreshold EPSPs with discharges.
Circuit to account for responses for EI cells with ipsilaterally evoked EPSPs
In a previous study (Li et al., 2010), we proposed that two projections could account for the PSPs and their behavior to both monaural and binaural stimulation: (1) an excitatory projection from the LSO contralateral to the IC, which was activated by contralateral stimulation, and (2) a weak excitatory projection of unknown origin, which was evoked by ipsilateral stimulation (Fig. 2B). The responses evoked by monaural and binaural stimuli shown in Figure 2 are consistent with the circuit we proposed.
The key feature that accounts for the binaural responses is that ipsilateral signals not only excite the IC directly, they also inhibit the LSO via the medial nucleus of the trapezoid body. With binaural signals, increasing ipsilateral intensity increases the strength of the subthreshold ipsilateral excitation to the IC while simultaneously causing an even larger decrease in the strength of the excitatory input from the LSO (due to inhibition from the medial nucleus of the trapezoid body, as shown in Fig. 2B). An IID is reached at which the input from the LSO is completely suppressed while the ipsilateral excitatory input is still present and is unattenuated. At that IID, the binaural signal will evoke an EPSP that is the same EPSP evoked only by ipsilateral stimulation at that ipsilateral intensity (Fig. 2B,C). In this scenario, the basic EI spiking property is inherited almost entirely, if not entirely, from the LSO despite an apparent independently derived ipsilaterally evoked excitation at all intensities.
Conductances of EI cells with ipsilaterally evoked EPSPs suggest a more complex circuitry
Even though the circuit shown in Figure 2B can account for the EPSPs and discharges evoked by monaural and binaural stimulation, we computed conductances in five cells that showed that these cells received an even larger set of projections than indicated by the EPSPs and spikes. The conductances provide insights into the origins of the excitatory and inhibitory inputs evoked by each ear, whether they arise from lower monaural or lower binaural nuclei, and the degree to which binaural stimulation is a linear summation of the inputs evoked by each ear.
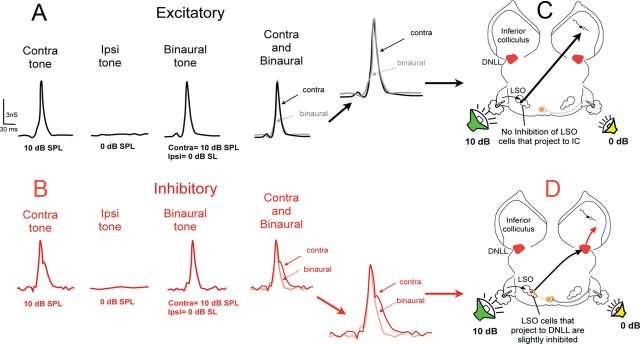
We computed conductances for five EI cells with ipsilaterally evoked EPSPs and all had the same pattern of conductances. We illustrate the conductances of these cells for the cell in Figure 3. Unlike the pure EPSPs evoked by contralateral and ipsilateral stimulation, the conductances revealed that contralateral stimulation evoked both an excitatory and an inhibitory conductance and ipsilateral stimulation also evoked both an excitatory and inhibitory conductance. Most importantly, the binaural excitatory conductance and the binaural inhibitory conductance were both smaller than the excitatory and inhibitory conductances evoked by monaural stimulation of the contralateral ear (Fig. 3C,E). In other words, a contralateral signal evoked a large excitatory and a large inhibitory conductance, but when an ipsilateral signal was presented together with the same contralateral signal, the excitatory and inhibitory conductances were reduced substantially. Because both the excitatory and inhibitory conductances were reduced with binaural stimulation, it follows that the inputs that evoked both conductances must have originated, at least in part, from lower nuclei that were themselves EI.
The circuit that can account for these features is shown in Figure 3B. The excitatory binaural nucleus is most likely the LSO, because this is the principal lower binaural nucleus where EI properties are initially formed (Caird and Klinke, 1983; Park et al., 1996) and the LSO sends a strong excitatory projection to the opposite IC (Ross and Pollak, 1989; Glendenning et al., 1992; Oliver et al., 1995). The inhibitory binaural nucleus is most likely the ipsilateral DNLL, because this is the only lower nucleus that: (1) provides inhibitory projections to the IC (Adams and Mugnaini, 1984; Shneiderman et al., 1988), (2) is binaural (EI) (Yang and Pollak, 1994; Pecka et al., 2007), and (3) is driven by stimulation of the ear contralateral to it (Fig. 3B,F). The origins of the ipsilaterally evoked excitatory and inhibitory conductances are unknown. The major point is that the minimum circuitry required to explain the conductances is substantially more complex than the circuit derived only from the PSPs and spikes, even though the simpler circuit shown in Figure 2B could fully explain the behavior of the monaurally and binaurally evoked PSPs.
Conventional type 2 EI cells: EI cells with ipsilaterally evoked IPSPs
The second type of EI cell is illustrated in Figure 4 and was characterized by ipsilateral stimulation that only evoked IPSPs (n = 3). The cell in Figure 4 has two noteworthy features. The first is that the amplitudes of the IPSPs increased with ipsilateral sound intensity. The second is that with binaural stimulation, the contralaterally evoked discharges and EPSPs were progressively reduced as the intensity at the ipsilateral ear increased. Moreover, the response evoked by the binaural signal with the strongest ipsilateral intensity was nearly flat, with only a slight indication of an IPSP. These features suggest that the ipsilaterally evoked IPSPs inhibited the contralateral excitation and the interaction of excitation and inhibition occurred in the IC, and thus the cell's EI property was formed de novo in the IC.
Circuit to account for de novo formation of EI properties in the IC
The de novo formation of EI features in the IC has also been observed in several previous extracellular studies (Li and Kelly, 1992; Park and Pollak, 1994; Burger and Pollak, 2001). Those studies assumed that the excitation evoked by the contralateral ear originated from a lower monaural nucleus of unknown origin and that the inhibitory projection was from the contralateral DNLL, a nucleus that provides GABAergic inhibition to the IC and is driven by the ear ipsilateral to the IC, as shown in Figure 4B. The reasons for proposing this circuit are that some EI cells in the IC were transformed into weakly suppressed or monaural cells when either GABAergic inhibition was blocked at the IC with the iontophoretic application of bicuculline or when the DNLL on the opposite side was reversibly inactivated (Li and Kelly, 1992; Park and Pollak, 1994; Burger and Pollak, 2001). The features observed with patch recordings described above for the EI cells with ipsilaterally evoked IPSPs are seemingly consistent with the circuitry proposed in the extracellular studies for the de novo formation of an EI property.
Conductances of EI cells with ipsilaterally evoked IPSPs
Conductances were obtained from each of the three EI cells with ipsilaterally evoked IPSPs. Like the cells with ipsilaterally evoked EPSPs, the conductance patterns were similar in all three cells and showed that the inputs that play upon these EI cells are also more complex than was proposed previously. As shown below, the conductances support the proposition that the DNLL is the nucleus that provides the ipsilaterally evoked inhibition, but the conductances do not support the idea that the contralaterally evoked excitation derives from a monaural lower nucleus. Below we first explain why the conductances support the contralateral DNLL as the major nucleus activated by stimulation of the ipsilateral ear, and then turn to the inputs evoked by stimulation of the contralateral ear.
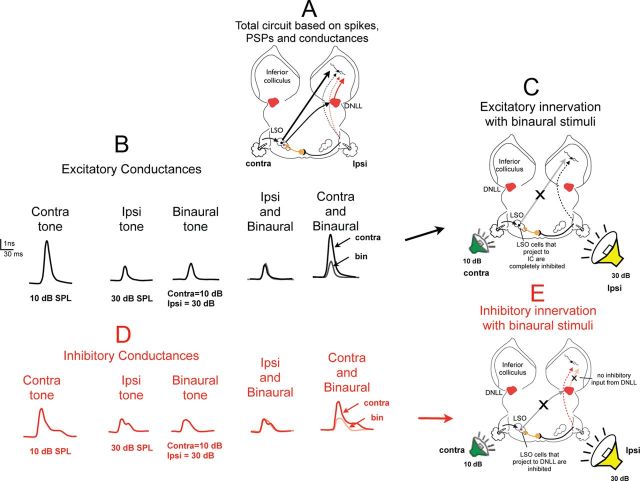
We illustrate the conductances evoked in EI cells with ipsilaterally evoked IPSPs in Figure 5, which is the same cell as the one shown in Figure 4. Conductances were computed for contralateral tones at 30 dB SPL, for ipsilateral tones at 20 dB SPL, and for binaural stimuli at the same intensities. Stimulation of the ipsilateral ear at 20 dB SPL, an intensity that when presented binaurally caused a spike reduction of 81% (Fig. 4), evoked a substantial inhibitory conductance and a small excitatory conductance (Fig. 5B,D). When the ipsilateral intensity was held constant at 20 dB and a 30 dB tone was presented simultaneously to the contralateral ear, the binaural inhibitory conductance was smaller than the inhibitory conductance evoked by monaural stimulation of the ipsilateral ear (Fig. 5D). Both the peak amplitude and total inhibitory conductance (area under the waveform) were smaller when a 30 dB tone was presented at the contralateral ear than was evoked by monaural stimulation of the ipsilateral ear alone. These features suggest that ipsilateral stimulation activated the contralateral DNLL and thereby evoked an inhibitory conductance at the IC. However, when the 30 dB tone was introduced at the contralateral ear, the contralateral tone caused a partial suppression of the DNLL output via decreased activity in the LSO that provided the driving input to the DNLL, thereby generating a smaller inhibition at the IC with binaural than with monaural stimulation (Fig. 5E). Therefore, the results from the conductance evaluations are consistent with the proposition that the ipsilaterally evoked inhibition was due to GABAergic projections from the contralateral DNLL.
Figure 5.
Conductance records of an EI cell with ipsilaterally evoked IPSPs. This is the same cell shown in Figure 4. A, Circuit suggested by excitatory and inhibitory conductances evoked by contralateral, ipsilateral, and binaural tones shown in B and D below. B, Excitatory conductances evoked by monaural and binaural tones. The binaural excitatory conductance is smaller than the contralateral excitatory conductance, suggesting that the strength of the excitation evoked by the contralateral ear was reduced when the ipsilateral tone was introduced. C, Circuitry accounting for the excitatory conductances evoked by monaural and binaural stimulation. D, Inhibitory conductances evoked by monaural and binaural tones. In these neurons, ipsilateral tones evoked a larger inhibitory conductance than did contralateral tones. The binaural inhibitory conductance is smaller than the ipsilateral inhibitory conductance, suggesting that the strength of the inhibition evoked by the ipsilateral ear was reduced when a contralateral tone was introduced. E, Circuitry accounting for the inhibitory conductances evoked by monaural and binaural stimulation. The origin of the inhibitory projection evoked by the contralateral ear is unknown and is shown generically as coming from the cochlear nucleus; however, it most likely originates from one of the monaural nuclei of the lateral lemniscus, which are innervated by cells in the cochlear nucleus.
We next show why the excitation evoked by contralateral stimulation could not have originated from a monaural nucleus. Stimulation of the contralateral ear evoked both a large excitatory conductance and a smaller inhibitory conductance (Fig. 5B,D). The source of the inhibitory conductance evoked by the contralateral tone could not be determined, but the contralateral excitatory conductance must have been evoked by projections from a nucleus that was itself EI, most likely the LSO (Fig. 5C). The reasoning is that if stimulation of the contralateral ear evoked excitatory inputs from a monaural projection to the IC, then the excitatory conductance evoked by binaural stimulation should have been a summation of the contralaterally and ipsilaterally evoked excitatory conductances and thus should have been just slightly larger than the excitatory conductance evoked by contralateral stimulation alone. However, binaural stimulation evoked an excitatory conductance that was smaller in both peak amplitude and total magnitude (area under the curve) than the excitatory conductance evoked by monaural contralateral stimulation alone (Fig. 5B). The reduced binaural excitatory conductance shows that the excitation at the IC originated, at least in part, from a lower nucleus with EI properties, presumably the LSO (Figure 5C). In short, rather than a de novo formation of the EI property in the IC, as proposed in previous extracellular and intracellular studies, the conductances suggest that suppression of contralaterally evoked responses with binaural tones is due to both a reduction in contralaterally evoked excitation at the LSO and an inhibition from the DNLL. The circuit suggested for the reduction in the binaural excitatory conductance is shown in Figure 5C.
Testing circuit predictions in a model by manipulating conductances
Because the conductances can be used to compute an accurate reproduction of the sound-evoked PSP, one or another of the conductances can be omitted from the computation of the predicted responses to reveal the contribution of that conductance or those conductances to the overall response. Therefore, we used a model to predict responses computed from the full complement of conductances evoked by the binaural tone, and then compared that response with the response predicted from only a subset of conductances. In this case, we further evaluated the proposition that the suppression of contralaterally evoked excitation with binaural stimulation was due to the dual influences of a reduced excitatory drive coupled with the ipsilaterally evoked inhibition.
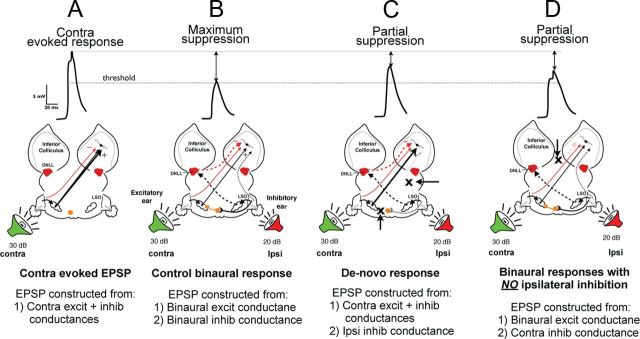
We began by investigating whether the inhibitory conductance evoked by ipsilateral stimulation alone was sufficiently strong to suppress the response evoked by monaural stimulation of the contralateral ear to the same degree as the suppression produced by binaural stimulation. To do this, we first show the computed PSP evoked by contralateral stimulation alone (Fig. 6A) and the reduction in PSP amplitude evoked by binaural stimulation (Fig. 6B, maximal suppression). We then computed a predicted PSP from the conductances evoked by contralateral stimulation together with the inhibitory and excitatory conductances evoked by ipsilateral stimulation (Fig. 6C). Although the predicted binaural PSP was substantially smaller than the EPSP evoked by the contralateral signal alone that is shown in Figure 6A, the important result is that it was not reduced to the same degree as the maximally suppressed PSP that was actually evoked by the binaural sound shown in Figure 6B. The conclusion, therefore, is that while the ipsilaterally evoked inhibition contributed to the response suppression, it was not by itself sufficient to account for the response suppression actually evoked by the binaural signal shown in Figure 6B.
Figure 6.
Predicted PSPs computed from various combinations of conductances show that the suppression of excitation with binaural signals is due to both an ipsilateral inhibition and a reduction in the contralateral excitation. Contralateral tones were 30 dB SPL and ipsilateral tones were 20 dB SPL in all panels. A, PSP evoked by monaural stimulation of the contralateral ear is far above threshold. Bottom shows the response that was computed from the contralaterally evoked excitatory and inhibitory conductances. B, Control binaural response is maximally suppressed and was generated from both the binaural excitatory and binaural inhibitory conductances. C, Simulation of conditions that would generate a de novo EI formation. The maximal excitatory response is only partially suppressed by the ipsilateral inhibition, showing that the ipsilaterally evoked inhibition does not by itself have sufficient strength to generate the maximal suppression evoked by the entire circuit in B. D, The binaural suppression in the LSO of contralaterally evoked excitation also generates a partially suppressed response in the IC. The suppressed IC response was generated without the ipsilaterally evoked inhibition from the contralateral DNLL. Threshold indicates the amplitude of the EPSP at which spikes were initiated in the response evoked by the tone at the contralateral ear.
We next evaluated the contribution of the reduced contralateral excitation to the maximally suppressed binaural response in Figure 6B by computing a predicted response from all of the conductances except the ipsilateral inhibitory conductance, which was omitted from the computation (Fig. 6D). In this case, the suppression of the binaural response could result only from the reduced excitation from the LSO. The peak amplitude of the computed binaural EPSP was suppressed and the suppression was slightly greater than the suppression produced only by the ipsilateral inhibition. However, the suppression was not as large as the suppressed binaural response shown in Figure 6B with the circuit intact. In other words, neither suppression of the predicted response based only on ipsilateral inhibition nor suppression of the predicted response based only on a reduced excitation at the LSO was as large as the suppression of the actual sound-evoked binaural response.
What this shows is that reduced excitation, which presumably occurred at the LSO, contributed to the binaural response suppression, as did the ipsilateral inhibition, but that both were required to generate the full suppression of the sound-evoked binaural response. The binaural response, therefore, could not have been formed de novo by a monaural excitatory projection evoked by the contralateral ear that was suppressed by inhibitory input from the DNLL evoked by ipsilateral stimulation.
Type 3 EI cells: EI/f cells
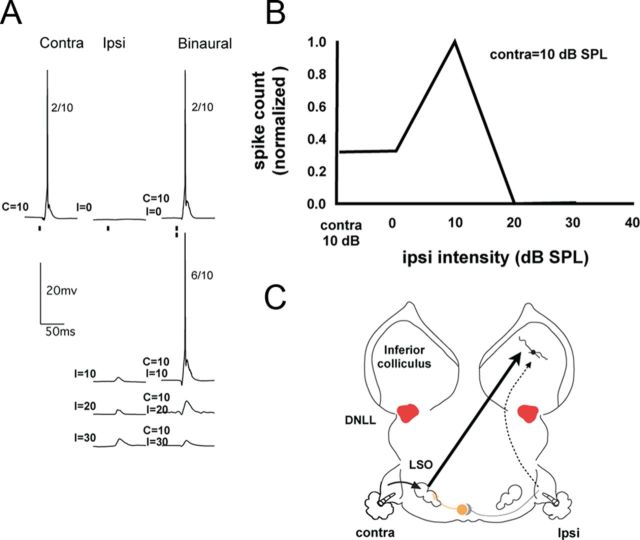
The third type of EI cell is the EI/f cell, which is distinguished by facilitated spike counts evoked over a small range of IIDs at which the ipsilateral signals were either equal to or less intense than the contralateral signals (Fig. 7 and Fig. 11). Based on the PSPs evoked by monaural and binaural tones, two subtypes of EI/f cells were observed. With the first subtype (n = 7/11), a subthreshold EPSP was evoked by a low-intensity ipsilateral tone and when the same ipsilateral tone was presented with a contralateral tone, the binaural stimulus evoked the facilitated response (Fig. 7A). With the second subtype (n = 4/11), no response, neither an EPSP nor an IPSP, was evoked by a low-intensity ipsilateral stimulus, although a facilitated response was evoked when the same ipsilateral signal was presented binaurally (Fig. 11).
Figure 7.
A: Responses of an EI/f neuron to monaural and binaural stimulation. The numbers to the right of each response show the number of discharges evoked by 10 stimulus presentations. B, IID function showing the response facilitation when the ipsilateral intensity was 10 dB SPL and the IID was 0 dB. C, Circuit that can account for the spikes and PSPs in A.
Figure 11.
EI/F cells in which there were no evoked responses at the ipsilateral intensity that evoked the facilitated response when presented binaurally. The numbers to the right of each response show the number of discharges evoked by eight stimulus presentations.
EI/f cells with an EPSP evoked at low ipsilateral intensities
We turn first to the seven EI/f cells in which the binaurally facilitated response was evoked with a low ipsilateral intensity, an intensity that also evoked an EPSP when presented monaurally to the ipsilateral ear. For these EI/f cells, the same circuitry was proposed in our previous study that was proposed for the conventional EI cells with ipsilateral EPSPs (Li et al., 2010; Fig. 7C). Specifically, two projections could account for the PSPs and spikes, one from the LSO that actually generated the EI property and a second excitatory projection driven by the ipsilateral ear. The only difference in these EI/f cells is that the circuit that generates the ipsilateral EPSP has a lower threshold than the ipsilateral excitatory circuit in the conventional EI cells. This circuitry predicts that binaural signals with low ipsilateral intensities should evoke both the excitation from the LSO and a small ipsilaterally evoked EPSP, the summation of which would generate a spike count greater than that evoked only by the LSO excitation and thereby evoke the facilitation. Binaural signals with higher ipsilateral intensities progressively inhibit the LSO and at the same time evoke a progressively larger excitation via the ipsilateral projection. The reason that spikes are suppressed at the IC is that the suppression of excitation at the LSO is greater than the increase in ipsilateral excitation, which is always subthreshold. In this way, binaural signals with larger IIDs (i.e., with stronger ipsilateral intensities) generate a progressively larger EPSP at the IC due to the increase in the subthreshold ipsilateral excitation while simultaneously suppressing discharges at the IC as a consequence of the even larger reduction in the excitatory drive from the LSO.
Conductances
The circuit outlined above accounts for both the PSPs and spikes evoked in EI/f cells, with EPSPs evoked by the ipsilateral intensity that evoked facilitation when presented binaurally. Conductances were computed in one of these EI/F cells and indicated that although the circuit proposed above is correct, it is incomplete. As we show below, contralateral and ipsilateral tones evoked both excitatory and inhibitory conductances and the facilitation was generated not only by a summation of EPSPs, but also by a reduction in ipsilaterally evoked inhibition. Moreover, the inhibition evoked by both the contralateral and ipsilateral tones requires additional inputs to those that were suggested only by the PSPs and spikes.
The conductances of the EI/f cell are shown in Figure 8 and Figure 9. Conductances were computed for monaural and binaural stimuli at two IIDs, both with the same contralateral intensity, 10 dB SPL (Figs. 8, 9). One IID, at 0 dB, had a weak ipsilateral intensity, 10 dB SPL, the same intensity as the contralateral signal. The IID with the 10 dB ipsilateral intensity generated the facilitated response. The second IID had a stronger ipsilateral intensity, 30 dB SPL. The binaural signal with the stronger ipsilateral intensity of 30 dB suppressed spikes completely but evoked a subthreshold EPSP that had the same amplitude as the 30 dB ipsilateral tone presented alone. We turn first to the IID that had the stronger ipsilateral intensity at 30 dB, because the conductances activated by the higher intensity provide insights into the inputs to the cell. We then turn to the conductances generated by the IID with the weaker ipsilateral intensity that provides insights into the circuit that generated the facilitation.
Figure 8.
Conductance records of the EI/f cell shown in Figure 7 evoked by a binaural stimulus with a 20 dB IID (10 dB contralateral and 30 dB ipsilateral tones). A, The circuit suggested by excitatory and inhibitory conductances shown in B and D. B, Excitatory conductances evoked by monaural and binaural tones. The binaural excitatory conductance is smaller than the contralateral excitatory conductance, suggesting that the strength of the excitation evoked by the contralateral ear was reduced when the ipsilateral tone was introduced. C, Circuitry accounting for the excitatory conductances evoked by monaural and binaural stimulation. D, Inhibitory conductances evoked by monaural and binaural tones. The binaural inhibitory conductance is smaller than the contralateral inhibitory conductance, suggesting that the strength of the inhibition evoked by the contralateral ear was reduced when an ipsilateral tone of 30 dB SPL was introduced. E, Circuitry accounting for the inhibitory conductances evoked by monaural and binaural stimulation.
Figure 9.
Conductance records of the EI/f cell shown in Figure 7 and Figure 8 evoked by a binaural stimulus with an IID of 0 dB that evoked facilitation (10 dB contralateral and 10 dB ipsilateral tones). A, Excitatory conductances evoked by monaural and binaural tones. The binaural excitatory conductance is equal to the summation of the contralateral and ipsilaterally evoked excitatory conductances. B, Inhibitory conductances evoked by monaural and binaural tones. The binaural inhibitory conductance is slightly smaller than the contralateral inhibitory conductance, suggesting that the strength of the inhibition evoked by the contralateral ear was reduced by a small degree when an ipsilateral tone of 10 dB SPL was introduced. C, Circuitry accounting for the excitatory conductances evoked by monaural and binaural stimulation. D, Circuitry accounting for the inhibitory conductances evoked by monaural and binaural stimulation.
Conductances with strong ipsilateral intensities
The circuit for the IID with the higher ipsilateral intensity, contralateral 10 dB and ipsilateral 30 dB in Figure 8A, is the same as that shown in Figure 3 for the EI cells with ipsilaterally evoked EPSPs, because the patterns of conductances were the same. In both cases, the contralateral tones evoked large excitatory and inhibitory conductances and the ipsilateral tones also evoked both excitatory and inhibitory conductances, although both were smaller than those evoked by the contralateral ear (Fig. 8B,D). We assume that the ipsilaterally evoked conductances originate from lower monaural nuclei, as they did in the conventional EI cells.
The contralateral excitatory conductance, however, originated in a lower binaural nucleus rather than a monaural nucleus (Fig. 8C). The rationale is that the binaural excitatory conductance was not only smaller than the contralateral excitatory conductance, but was virtually identical to the excitatory conductance evoked by monaural stimulation of the ipsilateral ear (Fig. 8B, ipsilateral and binaural). This shows that the contralateral excitation was completely suppressed in a lower nucleus, most likely in the LSO, when the 30 dB ipsilateral tone was presented together with the 10 dB contralateral tone (Fig. 8C), leaving only the ipsilaterally evoked excitatory conductance.
Similarly, the binaural inhibitory conductance was smaller than the contralaterally evoked inhibitory conductance (Fig. 8D). The binaural inhibitory conductance was nearly identical to the ipsilateral evoked inhibitory conductance (Fig. 8D, ipsilateral and binaural). Therefore, like the contralateral excitation, presenting a 30 dB tone to the ipsilateral ear completely suppressed the contralaterally evoked inhibition. That suppression must have originated in a lower nucleus with EI properties, presumably the ipsilateral DNLL (Fig. 8E). In short, the excitatory and inhibitory inputs to the cell were only from the ipsilateral ear at this IID with a 10 dB contralateral and 30 dB ipsilateral tone; the excitation and inhibition evoked by the contralateral ear were completely suppressed in lower nuclei.
Conductances with low ipsilateral intensities
The conductances evoked by the contralateral tone were the same as those shown previously, because the contralateral tone was fixed at 10 dB SPL in both binaural conditions. As described above, the excitatory conductance evoked by the contralateral tone at 10 dB SPL was large. However, when the ipsilateral intensity was 10 dB SPL, the summation of the two monaural excitatory conductances evoked by the contralateral and ipsilateral ears was equal to the excitatory conductance evoked by tones presented binaurally at the same intensities (Fig. 9A). Therefore, the behavior of the excitatory conductances is in agreement with the prediction that a linear summation of the excitatory inputs evoked by each ear generated the facilitation. The problem is that the excitatory conductance evoked by the contralateral tone is very large and predicts a PSP far above threshold that should evoke a much higher spike count than the 10 dB SPL tones presented binaurally actually evoked, only 6 spikes/10 stimuli. It is in this regard that the inhibitory conductances are significant.
As shown in Figure 9B, the contralaterally evoked inhibitory conductance at 10 dB SPL was large and partially suppressed the response that would have been evoked by the excitatory conductance alone, thereby accounting for the weak discharge evoked by the contralateral tone. The ipsilateral tone also evoked an inhibitory conductance (Fig. 9B), but one that was very small. What this shows is that the facilitation was generated by the summation of excitatory conductances and the facilitated response evoked by the binaural signal was then scaled down by inhibition, especially by the contralaterally evoked inhibition.
It is also noteworthy that the binaural inhibitory conductance was slightly smaller than the summation of the contralaterally and ipsilaterally evoked inhibitory conductances (Fig. 9B). The peak amplitudes were about the same, but the magnitude (area under the conductance waveform) of the binaural inhibitory conductance was slightly smaller than the summed inhibitory conductances. The slight difference between the summed and the binaural inhibitory conductances suggests that the binaural inhibitory conductance was reduced at a lower nucleus that was EI (Fig. 9D). The binaural source of the contralateral inhibitory conductance is confirmed by the conductances evoked at the higher IID, as was discussed above.
These features suggest that the contralateral tone at 10 dB evoked a strong excitation from the LSO, but that the 10 dB tone at the ipsilateral ear was too weak to cause any inhibition of the subset of LSO cells that project to the IC (Fig. 9C). It was for this reason that the binaural excitatory conductance was equal to the linear summation of contralateral excitation from the LSO plus the excitation evoked by the 10 dB tone at the ipsilateral ear. However, the summated excitation was then reduced substantially by the inhibition evoked by the contralateral ear and less so by the ipsilaterally evoked inhibition.
The inhibitory inputs, however, did not summate linearly because the contralaterally evoked inhibition was reduced slightly, so the binaural inhibitory conductance was slightly smaller than the summated inhibitory conductances (Fig. 9B,D). The reduction may have occurred at the LSO due to a slight inhibition of the LSO cells that project to the DNLL by the ipsilateral tone or there may have been a slight reduction of inhibition from the DNLL due to the ipsilateral tone. In either case, the net result was a small reduction in the binaural inhibitory input to the IC. Therefore, the facilitation evoked with weak ipsilateral stimulation presumably resulted from two events, the summation of contralateral and ipsilateral excitation and a small reduction in the binaurally evoked inhibition at the IC.
Manipulating conductances in modeled responses confirm the roles of excitation and inhibition for evoking facilitation
Responses computed by omitting one or another conductance from the predicted response support the hypothesis that facilitation is generated by the summation of contralateral and ipsilateral excitatory conductances coupled with a reduction of inhibition with binaural stimulation. The contribution of the excitation evoked by the ipsilateral ear is shown in Figure 10A. Three responses are shown: (1) the PSP evoked by the contralateral tone (Fig. 10A1), (2) the PSP that was actually evoked by binaural stimulation (spikes were removed by filtering; Fig. 10A2), and (3) the predicted binaural PSP when the ipsilateral excitatory conductance was omitted from the calculation (Fig. 10A3). In Figure 10A3, the predicted response was calculated using only the contralateral excitatory conductance and the binaural inhibitory conductance. The significant result is the absence of facilitation when the ipsilateral excitatory conductance was not included in the predicted response. In this case, the predicted PSP was similar in peak amplitude to the PSP evoked by contralateral stimulation alone, which is consistent with the proposition that the facilitated PSP is generated, in part, by the summation of the contralateral and ipsilateral excitatory conductances.
Figure 10.
Predicted PSPs for an EI/f cell computed from various combinations of conductances show that the facilitation with binaural signals is due to both a summation of ipsilaterally and contralaterally evoked EPSPs and to a reduction in the contralateral excitation. A, Predicted responses revealing the influence of the ipsilaterally evoked EPSP. A1 is the response to the contralateral tone, A2 is the response to the binaural tone, and A3 is the predicted response without the ipsilateral EPSP. B1 and B2 are the same as A1 and A2 above. B3 is the predicted response without the reduction of inhibition due to binaural stimulation. Conductances used to compute each predicted response are shown in the circuits below each response.
The contribution of the reduction in inhibition due to binaural stimulation is shown in Figure 10B3. Here the predicted response was computed from the binaural excitatory conductance, which is the same as the summation of contralateral and ipsilateral excitatory conductances, plus the sum of the contralateral and ipsilateral inhibitions evoked by each ear—that is, the contralaterally evoked inhibition was not reduced in this calculation. The predicted PSP in this case was slightly smaller than the PSP evoked by the contralateral tone alone and substantially smaller than the facilitated PSP evoked by binaural stimulation. Therefore, the enhanced inhibition that would result from a linear summation of the inhibitory conductances evoked by the contralateral and ipsilateral ears would suppress the summed excitation evoked by both ears and render the PSP even smaller than that evoked by contralateral stimulation alone. In summary, the binaurally evoked facilitated response required a boost in excitation from the ipsilateral ear and a reduction of the contralaterally evoked inhibition. Facilitation would not be evoked in the absence of either of these features.
EI/f cells in which no response was evoked at low ipsilateral intensities
In 4/11 EI/F cells, low-intensity ipsilateral tones presented monaurally evoked no subthreshold response even though the same ipsilateral signal generated a facilitated response when presented binaurally (Fig. 11). To confirm that no inputs were evoked by low-intensity ipsilateral signals, we evaluated responses in three cells before and after their membrane potentials were hyperpolarized and in all cases low-intensity ipsilateral signals evoked no responses. Conductances were computed in one of these cells. As we show below, the conductances indicate that the binaural facilitation in these cells was due entirely to a reduction in the contralaterally evoked inhibition, which then allowed a larger EPSP to be evoked by the contralaterally evoked excitation.
Conductances with low ipsilateral intensities
The monaural and binaural conductances evoked with a contralateral signal of 10 dB and an ipsilateral signal of 20 dB (Fig. 11), which produced a complete spike suppression when presented binaurally, were similar to those found in the previous EI/f cell in Figure 8 and are not shown again. The conductances evoked by the facilitated IID, in which the contralateral signal was 10 dB and the ipsilateral signal was 0 dB, are shown in Figure 12.
Figure 12.
Conductance records of the EI/f cell shown in Figure 11, in which ipsilateral stimulation evoked no response when presented at an intensity (0 dB) that evoked facilitation when the same ipsilateral tone was presented binaurally (10 dB contralateral and 0 dB ipsilateral tones). A, Excitatory conductances evoked by monaural and binaural tones. The contralateral tone evoked a large excitatory conductance, whereas the ipsilateral tone evoked no conductance. Because the ipsilateral tone evoked no excitatory conductance, the binaural excitatory conductance is identical to the contralateral conductance. B, Inhibitory conductances evoked by monaural and binaural tones. Similar to the excitatory conductances, a contralateral tone evoked a large inhibitory conductance, whereas an ipsilateral tone evoked no conductance. However, the binaurally evoked inhibitory conductance was slightly smaller than the contralateral inhibitory conductance, suggesting that the strength of the inhibition evoked by the contralateral ear was reduced by a small degree when an ipsilateral tone of 0 dB SPL was introduced. C, Circuitry accounting for the excitatory conductances evoked by monaural and binaural stimulation. D, Circuitry accounting for the inhibitory conductances evoked by monaural and binaural stimulation.
There are four noteworthy features of the conductances shown in Figure 12. The first is that an ipsilateral tone at 0 dB was below the threshold for both excitation and inhibition and thus did not evoke an excitatory or an inhibitory conductance (Fig. 12A,B, middle). The second feature is that the 10 dB contralateral tone evoked a strong excitatory conductance and a strong inhibitory conductance (Fig. 12A,B, far left). The third feature is that the binaural excitatory conductance was virtually identical to the excitatory conductance evoked by the contralateral tone presented monaurally—in other words, the 0 dB ipsilateral signal had no effect on the contralaterally evoked excitatory conductance (Fig. 12A, far right). The fourth feature, and the most important one, is that the total binaural inhibitory conductance, the area under the binaural, inhibitory conductance curve, was slightly smaller than the contralaterally evoked inhibitory conductance (Fig. 12B, far right). Because the excitatory conductance was unaffected by the ipsilateral tone at 0 dB, the only way that the binaural response could have been enhanced, and thereby produce the facilitation, was by a reduction of inhibition. Moreover, because the ipsilateral tone at 0 dB did not evoke any response, either excitatory or inhibitory, the inhibition must have been reduced in a lower nucleus. Assuming that the contralaterally evoked inhibition is from the ipsilateral DNLL, the suppression due to the ipsilateral tone presumably occurred in the LSO, as shown in Figure 12D, which then provided a weaker excitatory drive to the DNLL than when the contralateral tone was presented monaurally.
The LSO cells providing excitatory projections to the IC (Figure 12C) are assumed to be different from the LSO cells that provide excitatory input to the DNLL (Figure 12D). The assumption is that a signal more intense than 0 dB is required to inhibit the cells in the LSO that project to the IC, and therefore these cells are not affected by the 0 dB signal at the ipsilateral ear. In addition, we assume that the 0 dB signal at the ipsilateral ear is sufficiently strong to causes a slight inhibition of the LSO cells that project to the DNLL. These assumptions are reasonable because a wide range of ipsilateral intensities differentially affect the population of LSO cells, where some LSO cells are inhibited by low ipsilateral intensities and others require higher ipsilateral intensities (Park et al., 1996; Park et al., 1997).
Manipulating conductances in modeled responses confirms the role of a reduced inhibition for evoking facilitation
As an additional confirmation that the reduced inhibition with binaural stimulation produced the facilitated response, we compared the predicted response generated from the inhibitory and excitatory conductances with the sound-evoked responses. The contralateral tones were always 10 dB and the ipsilateral tones were 0 dB. Figure 13A, B shows the PSP evoked by the contralateral sound and the predicted binaural PSP computed from binaural excitatory conductance and the contralaterally evoked inhibitory conductance. In this case, the inhibitory conductance was not attenuated and thus was the same as that evoked only by the contralateral tone. Therefore, the predicted binaural PSP without a reduced inhibition was virtually identical to the contralaterally sound-evoked PSP. In contrast, the predicted binaural PSP shown in Figure 13D, computed from the binaural excitatory conductance and the slightly reduced inhibitory conductance, was larger than the predicted binaural PSP shown in Figure 13B, the predicted binaural PSP without a reduced inhibition. Moreover, the predicted binaural PSP shown in Figure 13D was virtually identical to the sound-evoked binaural PSP shown in Figure 13C, which evoked a spike count 37% larger than the spike count evoked by the contralateral signal alone. In short, the predicted binaural PSP with the reduced inhibition was larger than the binaural PSP computed without a reduced inhibition. These comparisons provide additional support for the hypothesis that the binaural facilitation in these EI/f cells was due to a reduction of the inhibitory drive in the IC, where the reduction was produced by introduction of an ipsilateral signal at 0 dB. In other EI/f cells, however, facilitation was produced by a combination of an ipsilaterally evoked excitation together with a reduction of inhibition.
Figure 13.
Predicted and sound-evoked conductances confirm that the binaural facilitation was due to a reduction in the contralaterally evoked inhibition by the 0 dB ipsilateral tone. A, PSP evoked by a 10 dB contralateral tone. B, Predicted PSP computed from the binaural excitatory conductance and the contralateral inhibition (the inhibition that was not reduced by the introduction of the ipsilateral tone). C, Facilitated PSP evoked by the binaural tone. D, Predicted PSP computed from the binaural excitatory and inhibitory conductances. Threshold indicates the amplitude of the EPSP at which spikes were initiated in the response evoked by the tone at the contralateral ear.
Discussion
The major finding of this study is that analyses of synaptic conductances suggest that the projections that innervate EI neurons are diverse and are more complex than was previously inferred either from extracellular or intracellular studies that recorded only PSPs and spikes. Moreover, our results show that it is not sufficient to obtain only spikes and subthreshold PSPs to obtain a view of the circuitry that innervates the EI cell from which recordings are made. Rather, it is also necessary to evaluate the conductances underlying the responses.
Our results also suggest that all EI cells receive projections from the LSO, which exert prominent and obvious influences on their IC targets. This is true even for EI cells that are not discussed in this study, which have no ipsilateral innervation and thus inherit their EI properties entirely from the LSO (Li et al., 2010). Except for those cells that inherit their EI properties from the LSO, all other EI cells also receive projections from the ipsilateral or contralateral DNLL in addition to other projections, some of which exert such subtle influences that they could not have been detected with extracellular records or even from intracellular recordings of PSPs. One example is the mechanism that generates the facilitated discharges in EI/f cells. Based on the EPSPs recorded with monaural and binaural stimuli, the conclusion that the facilitation was due entirely to a summation of the excitation evoked by both the ipsilateral and contralateral ears was seemingly obvious and complete. However, the conductances showed that the facilitation is more complex and was generated both by the summation of ipsilaterally and contralaterally evoked excitation and a reduction in the inhibition evoked by the contralateral ear. The role of a reduced inhibition was not apparent from just the PSPs and spikes, but was only revealed by the conductances that evoked the facilitation.
Comments on the sources of innervation
The innervation of IC cells that we proposed based on conductances has some limitations. We cannot, for example, distinguish GABAergic from glycinergic inhibitory inputs. Moreover, it may well be that additional inputs that synapse on the dendrites of the fusiform cells that predominate in the IC may have exerted small effects that were not detectable at the soma, where the recordings were presumably obtained.
Even with such limitations, the conductances show that the inputs that innervate EI cells derive from both lower binaural and monaural nuclei. The binaural nuclei to which we attributed the sources of the binaural excitatory and inhibitory conductances, the LSO and the DNLLs, are assumptions on our part, based on the established response properties, their connections with the IC and the neurochemistry of the cells in each nucleus. Because EI properties are first created in the LSO (Boudreau and Tsuchitani, 1968; Park et al., 1996; Tollin et al., 2008) and the LSO sends strong excitatory projections to the IC (Brunso-Bechtold et al., 1981; Ross et al., 1988; Oliver et al., 1997; Casseday et al., 2002), it is difficult to imagine that the LSO was not the source of the binaural excitatory conductances we observed. The assumption that the contralateral DNLL is the source of the binaural inhibitory conductance in the EI cells with ipsilaterally evoked IPSPs is supported by previous extracellular studies in which the contralateral DNLL was reversibly inactivated (Li and Kelly, 1992; Faingold et al., 1993; Burger and Pollak, 2001). It is also noteworthy that our results suggest how the inhibitory projections from the ipsilateral DNLL influence EI properties of IC cells, because the impact of projections from the ipsilateral DNLL is poorly understood. What all of this suggests is that every EI cell receives innervation from the LSO and, except for a small number of EI cells that inherit their EI properties entirely from the LSO, all other EI cells are also innervated by the contralateral and/or ipsilateral DNLL in addition to inputs from a variety of lower monaural nuclei.
As we pointed out previously, the sources of the monaural excitatory and inhibitory projections are unknown. This is especially true of the projections activated by the ipsilateral ear. Previous connectional studies have shown projections from the ipsilateral cochlear nucleus to the IC, which are probably at least one source of the ipsilateral excitatory conductances (Oliver, 1987). The ipsilaterally evoked inhibitory conductances are more puzzling. One possibility is the inhibitory conductances originate from the opposite IC via the commissure of the IC, which contains both excitatory and inhibitory fibers (Moore et al., 1998; Hernández et al., 2006; Malmierca et al., 2009). Because the commissure contains excitatory fibers, it may be another source of the ipsilaterally evoked excitatory conductance.
Previous studies have suggested that the binaural properties of the majority of EI cells in the IC are strongly shaped by descending cortical projections, even in anesthetized animals. Therefore, when the cortex is cooled sufficiently to block all descending inputs, the EI properties of many IC cells were shown to change markedly in guinea pigs and their activity was almost completely abolished in other EI cells (Nakamoto et al., 2008).
However, we found no evidence to suggest that descending projections were shaping the responses that we recorded. There are two features that support the hypothesis that all of the responses evoked by both monaural and binaural stimuli originate from the ascending projections of lower auditory nuclei. The first, as discussed above, is that the binaural features of the conductances that we observed are consistent with the known features of the LSO and DNLL in terms of both the binaural response properties of the two nuclei and their neurochemistry and their projections to the IC. The second feature is that the latencies of both the monaural and binaurally evoked conductances were similar. Because activation of descending projections would involve at least three additional synapses, from the IC to the medial geniculate to cortex and then back to the IC, one would have to assume that responses evoked by descending projections would have substantially longer latencies than responses evoked by ascending projections from lower nuclei. However, the inputs we recorded arrived more or less coincidentally, or at least not with substantially different latencies. In short, we see no need to postulate a role for descending projections in generating the responses evoked either by monaural or binaural stimulation in our studies.
Concluding comments
Because the basic EI property is already created in the LSO and all EI cells in the IC receive LSO projections, what functional consequences might be attributed to the various projections that innervate each of the EI types? The fundamental feature of EI cells is that an IID that evokes a criterion spike suppression is constant with static IIDs with IIDs presented one at a time, as in this study. Although the IID that evokes criterion suppression is constant with static IIDs, this is not the case with dynamic IIDs, sounds with IIDs that change over time. Previous studies have shown that when dynamic IIDs are presented, in many IC cells, there is a dramatic shift from the way the IC neuron responds to the same static IIDs that were previously presented (there is a change in the IID that evokes the criterion inhibition). These features have been reported in the IC of bats (Burger and Pollak, 2001), gerbils (Pecka et al., 2007), and rats (Sanes et al., 1998)and ferrets (Dahmen et al., 2010), and appear to be a universal feature of the IC. The responses to the dynamic stimuli used in several previous studies closely predicted the behavioral responses of humans when presented with the same stimuli (Pecka et al., 2007; Dahmen et al., 2010). Therefore, both the responses to static and dynamic stimuli observed in single-unit studies of the IC in animals appear to be critically important for acoustic perception.
In previous studies (Burger and Pollak, 2001; Pecka et al., 2007: Li et al., 2010; Pollak, 2012), we suggested that the circuitry that innervates EI cells in the IC is not simply for generating an EI property and thus encoding the location of an isolated sound source that generates a particular IID. Rather, the circuitry suggests a differential set of responses to dynamic IIDs, such as sound sources moving across space or two binaural sounds, which are presented from different regions in space but that follow each other in close temporal sequence. Ongoing work is exploring the responsiveness of EI cells in the IC to dynamic IIDs and the degree to which the circuitry suggested by conductance analysis can account for the responses evoked by dynamic IIDs.
Footnotes
This work was supported by the National Institutes of Health (Grant #DC007856). We thank Achim Klug, Michael Burger, Michael Pecka, Carl Resler, and Nace Golding for helpful comments and discussions and Carl Resler for technical support.
References
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: A nucleus of GABAergic projection neurons. Brain Res Bull. 1984;14:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Klug A, Pollak GD. Features of contralaterally evoked inhibition in the inferior colliculus. Hear Res. 2000;141:80–96. doi: 10.1016/S0378-5955(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Burger RM, Pollak GD. Reversible inactivation of the dorsal nucleus of the lateral lemniscus reveals its role in the processing of multiple sound sources in the inferior colliculus of bats. J Neurosci. 2001;21:4830–4843. doi: 10.1523/JNEUROSCI.21-13-04830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caird D, Klinke R. Processing of binaural stimuli by cat superior olivary S-segment. Exp Brain Res. 1983;52:385–399. doi: 10.1007/BF00238032. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Fremouw T., Covey E. The inferior colliculus: A hub for the central auditory system. In: Oertel D, Popper AN, Fay RR, editors. Integrative functions in the mammalian auditory pathway. New York: Springer; 2002. pp. 238–318. [Google Scholar]
- Dahmen JC, Keating P, Nodal FR, Schulz AL, King AJ. Adaptation to stimulus statistics in the perception and neural representation of auditory space. Neuron. 2010;66:937–948. doi: 10.1016/j.neuron.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Anderson CA, Randall ME. Stimulation or blockade of the dorsal nucleus of the lateral lemniscus alters binaural and tonic inhibition in contralateral inferior colliculus neurons. Hear Res. 1993;69:98–106. doi: 10.1016/0378-5955(93)90097-K. [DOI] [PubMed] [Google Scholar]
- Gittelman JX, Pollak GD. It's about time: how input timing is used and not used to create emergent properties in the auditory system. J Neurosci. 2011;31:2576–2583. doi: 10.1523/JNEUROSCI.5112-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelman JX, Li N, Pollak GD. Mechanisms underlying directional selectivity for frequency-modulated sweeps in the inferior colliculus revealed by in vivo whole-cell recordings. J Neurosci. 2009;29:13030–13041. doi: 10.1523/JNEUROSCI.2477-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319:100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- Hernández O, Rees A, Malmierca MS. A GABAergic component in the commissure of the inferior colliculus in rat. Neuroreport. 2006;17:1611–1614. doi: 10.1097/01.wnr.0000236857.70715.be. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Gago G. Binaural interaction in high-frequency neurons in inferior colliculus of the cat: effects of variations in sound pressure level on sensitivity to interaural intensity differences. J Neurophysiol. 1990;63:570–591. doi: 10.1152/jn.1990.63.3.570. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Pollak GD. Multiple components of ipsilaterally evoked inhibition in the inferior colliculus. J Neurophysiol. 1999;82:593–610. doi: 10.1152/jn.1999.82.2.593. [DOI] [PubMed] [Google Scholar]
- Li L, Kelly JB. Inhibitory influence of the dorsal nucleus of the lateral lemniscus on binaural responses in the rat's inferior colliculus. J Neurosci. 1992;12:4530–4539. doi: 10.1523/JNEUROSCI.12-11-04530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Gittelman JX, Pollak GD. Intracellular recordings reveal novel features of neurons that code interaural intensity disparities in the inferior colliculus. J Neurosci. 2010;30:14573–14584. doi: 10.1523/JNEUROSCI.2228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Hernández O, Antunes FM, Rees A. Divergent and point-to-point connections in the commissural pathway between the inferior colliculi. J Comp Neurol. 2009;514:226–239. doi: 10.1002/cne.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Kotak VC, Sanes DH. Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J Neurophysiol. 1998;80:2229–2236. doi: 10.1152/jn.1998.80.5.2229. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Jones SJ, Palmer AR. Descending projections from auditory cortex modulate sensitivity in the midbrain to cues for spatial position. J Neurophysiol. 2008;99:2347–2356. doi: 10.1152/jn.01326.2007. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol. 1987;264:24–46. doi: 10.1002/cne.902640104. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Shneiderman A. Axonal projections from the lateral and medial superior olive to the inferior colliculus of the cat: a study using electron microscopic autoradiography. J Comp Neurol. 1995;360:17–32. doi: 10.1002/cne.903600103. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382:215–229. doi: 10.1002/(SICI)1096-9861(19970602)382:2<215::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. GABA shapes sensitivity to interaural intensity disparities in the mustache bat's inferior colliculus: implications for encoding sound location. J Neurosci. 1993;13:2050–2067. doi: 10.1523/JNEUROSCI.13-05-02050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Pollak GD. Azimuthal receptive fields are shaped by GABAergic inhibition in the inferior colliculus of the mustache bat. J Neurophysiol. 1994;72:1080–1102. doi: 10.1152/jn.1994.72.3.1080. [DOI] [PubMed] [Google Scholar]
- Park TJ, Grothe B, Pollak GD, Schuller G, Koch U. Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J Neurosci. 1996;16:6554–6566. doi: 10.1523/JNEUROSCI.16-20-06554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Monsivais P, Pollak GD. Processing of interaural intensity differences in the LSO: role of interaural threshold differences. J Neurophysiol. 1997;77:2863–2878. doi: 10.1152/jn.1997.77.6.2863. [DOI] [PubMed] [Google Scholar]
- Pecka M, Zahn TP, Saunier-Rebori B, Siveke I, Felmy F, Wiegrebe L, Klug A, Pollak GD, Grothe B. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci. 2007;27:1782–1790. doi: 10.1523/JNEUROSCI.5335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD. Circuits for processing dynamic interaural intensity disparities in the inferior colliculus. Hear Res. 2012;288:47–57. doi: 10.1016/j.heares.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Ross LS, Pollak GD. Differential ascending projections to aural regions in the 60 kHz contour of the mustache bat's inferior colliculus. J Neurosci. 1989;9:2819–2834. doi: 10.1523/JNEUROSCI.09-08-02819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LS, Pollak GD, Zook JM. Origin of ascending projections to an isofrequency region of the mustache bat's inferior colliculus. J Comp Neurol. 1988;270:488–505. doi: 10.1002/cne.902700403. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Malone BJ, Semple MN. Role of synaptic inhibition in processing of dynamic binaural level stimuli. J Neurosci. 1998;18:794–803. doi: 10.1523/JNEUROSCI.18-02-00794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Koka K, Tsai JJ. Interaural level difference discrimination thresholds for single neurons in the lateral superior olive. J Neurosci. 2008;28:4848–4860. doi: 10.1523/JNEUROSCI.5421-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater M, Schlegel P, Zoller H. Comparative auditory neurophysiology of the inferior colliculus of two Molossid bats, Molossus ater and Molossus molossus. J Comp Physiol A. 1979;131:137–145. doi: 10.1007/BF00619074. [DOI] [Google Scholar]
- Winer JA, Larue DT, Pollak GD. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol. 1995;355:317–353. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD. The roles of GABAergic and glycinergic inhibition on binaural processing in the dorsal nucleus of the lateral lemniscus of the mustache bat. J Neurophysiol. 1994;71:1999–2013. doi: 10.1152/jn.1994.71.6.1999. [DOI] [PubMed] [Google Scholar]