Abstract
Objective
Serum PIIANP and HELIXII biomarkers were evaluated for variation diurnally and with physical activity and food in participants with osteoarthritis (OA) of the knee.
Methods
Forty participants with OA of at least one knee were admitted overnight to the General Clinical Research Center for serial serum sampling. Samples were obtained on the evening (6–8pm) of day 1 (T3, n=40); prior to rising (8am) from bed (T0, n=40); one hour after rising (9am) without food consumption (T1a, n=20); one to two hours after rising (9–10am) with food consumption (T1, n=40); and additionally at noon, four hours after rising (T2, n=20). Serum PIIANP and HELIXII were measured by ELISA. Results were analyzed using non-parametric Freidman’s test with Dunn’s post-hoc multiple comparison test.
Results
Normalized mean concentrations for sPIIANP and sHELIXII increased significantly from T0 to T1 (p<0.05).
Conclusions
This is the first study to demonstrate diurnal variation of these collagen II biomarkers in individuals with knee OA. These results suggest that serum sampling for these markers should be standardized for purposes of clinical trials.
Keywords: collagen-II biomarker, osteoarthritis, diurnal variation Running title, PIIANP and HELIXII Diurnal Variation
INTRODUCTION
Osteoarthritis (OA) is the most common type of arthritis, the hallmark of which is cartilage degradation. Human cartilage matrix is primarily made-up of type-II collagen, providing cartilage tensile strength. Changes in type II collagen turnover (synthesis and degradation) are believed to play an important role in the progressive loss of cartilage in OA and provide biomarkers for the diagnostic and prognostic evaluation of OA [1, 2].
Several promising OA biomarkers are derived from type II collagen, two of which are described herein. Type II collagen is synthesized as a procollagen molecule with both amino- and carboxy-propeptide domains. Differential splicing produces two forms of the amino-propeptide [3], PIIANP and PIIBNP, containing the type IIA or type IIB exons, respectively. Type IIB is expressed in normal adult cartilage. Type IIA procollagen is expressed by pre-chondrogenic cells and osteoarthritic-adult chondrocytes, but not normal adult chondrocytes [3, 4]. Specific enzymes remove the amino-propeptides during synthetic processing of type II collagen, and therefore, serum PIIANP (sPIIANP) levels are believed to be representative of the rate of type II collagen synthesis in the setting of OA. Preliminary studies demonstrate decreased sPIIANP in patients with knee OA compared with healthy controls [1], suggesting decreased collagen synthesis in joint disease.
Collagen degradation occurs via collagenases cleaving triple-helical region of type-II collagen [5]. HELIXII is a product of collagen degradation. Urinary levels of HELIXII have been shown to be significantly elevated in OA patients compared to healthy controls [5]. Recently, a serum-HELIXII (sHELIXII) assay has been developed and found to be more precise than urinary-HELIXII, as urinary levels have limitations from dilution and creatinine correction [6].
Serum PIIANP and sHELIXII have not been evaluated previously with respect to important confounding variables, such as diurnal variation. Other biomarkers that have been shown to vary diurnally include hyaluronan (HA), MMP-3, and antigenic Keratin Sulfate (AgKS) [7–9]. HA levels in Rheumatoid Arthritis (RA) have been shown to vary significantly, especially in early morning after activity [8]. Manicourt confirmed these results, and observed that activity after rest caused markers to undergo predictable changes in RA [9]. Changes in HA and AgKS were transient, while in the case of MMP-3, the change persisted for over three hours. Similar study designs were used to assess diurnal variation of OA biomarkers [7, 10, 11]. These studies were significant in clarifying the pathophysiology of the biomarkers related to disease, and identifying diurnal variation related to non-disease influences, demonstrating that much can be learned from observing biomarker variation throughout the day. Hence, the aim of this study was to investigate the variation of sPIIANP and sHELIXII in patients with OA throughout the day, and more specifically with respect to physical activity and food consumption.
PATIENTS AND METHODS
Participants
Forty patients with knee OA were recruited through the Rheumatology and Orthopedic outpatient clinics (20 patients for each of two cohorts). Participants had symptomatic OA of at least one knee. Participants were excluded for arthritis due to RA, lupus, psoriasis, gout, or hemochromatosis. All procedures were approved by the Institutional Review Board of Duke University Medical Center (DUMC). Patients were admitted to the General Clinical Research Center of DUMC for an overnight stay, and standardized meals were provided. The standardized meals were low in collagen-content, and food containing hyaline cartilage extracts (ie. chicken broth, meat gravy, etc) were excluded. Participants were allowed to take all usual medications, including NSAIDs and dietary supplements such as glucosamine and/or chondroitin sulfate. Radiographic knee OA severity was assessed from a postero-anterior semi-flexed radiograph with SynaFlexer positioning device and quantified by Kellgren Lawrence (K-L) score for each knee[12].
Blood sampling and measurements
Samples were obtained on all patients between 6:00 and 8:00 pm on Day 1 (T3) after a day of normal activity, and prior to rising from bed (T0). In cohort-1, samples were collected one hour after arising, performing morning activities, and eating breakfast (T1). In cohort-2, this period was parsed into two intervals: sampling after an hour of morning activity (T1a); and sampling after breakfast consumption (T1b), corresponding to T1 of cohort-1. For cohort-1, blood was also obtained four hours after arising (T2). Analyses of the biomarkers were performed as previously described by ELISA technique [1, 5].
Statistical Analysis
The mean concentrations and standard deviations (SDs) of each biomarker were calculated. Due to strongly similar characteristics (see Table 1), cohorts were merged (N=40) for similar timepoints T0, T1, and T3; non-similar timepoints between cohorts (T2 and T1a for first and second cohorts, respectively) were not merged (N=20). Normalized biomarker concentrations were used to more clearly assess for individual diurnal variation and to remove interpersonal variation due to medication and supplement use. Normalization consisted of dividing each biomarker concentration by the mean concentration of the four timepoints for that individual, observing relative-concentration differences within each patient. The Bland Altman method was used to further assess whether diurnal variation was dependent on the level of the biomarker [13]. This method determines whether the observed biomarker variation trends systematically by calculating the difference between T0 and the timepoint with the greatest variation from T0 and plotting it against the mean of the 4 timepoints. Results were analyzed using non-parametric Friedman’s test, and Dunn’s post-hoc multiple comparison test. Statistical computations were performed using JMP® (SAS, Cary, NC) and Graphpad® softwares. Two-tailed p-value of less than 0.05 was considered significant for all analyses.
Table 1.
Baseline characteristics of the patients in both cohorts
| Cohort Characteristics | Cohort-1 (n=20) | Cohort-2 (n=20) | |
|---|---|---|---|
| Age | Mean (SD) | 69 (9) | 68 (13) |
| Median | 68 | 69 | |
| Range | 55–89 | 37–97 | |
| Gender | Male | 13 | 13 |
| Female | 7 | 7 | |
| BMI (kg/m2) | Mean (SD) | 33.76 (10.14) | 28.57 (8.34) |
RESULTS
Cohort characteristics are described in Table 1. The difference in mean BMI between cohorts was not statistically significant (p=0.09). The sum K-L score related to both knees ranged from 1–8. The distribution of the sum K-L scores for both knees of the 40 patients was: 15% had K-L ≤ 2, 64% had 3 ≤ K-L ≤ 6, and 21% had K-L > 6. Three individual knees were scored as zero due to 3 patients having undergone unilateral knee replacement surgery. There was no significant correlation between sPIIANP or sHELIXII and sum K-L score. Nine participants of the forty enrolled were taking oral glucosamine and/or chondroitin sulfate supplements. In addition to accounting for dietary supplementation through normalization, the differences in mean sPIIANP and sHELIXII concentrations at T1 (p>0.50 and p>0.09, respectively) and T3 (p>0.09 and p>0.50, respectively) between the supplemented and non-supplemented groups were not statistically significant.
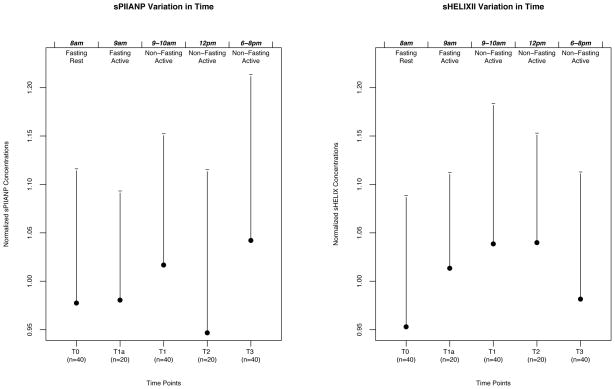
After normalization, there was a mean increase (4.5%) in sPIIANP between T0 and T1, which was statistically significant (p=0.036; see Figure 1). There was also a mean increase (9.0%) in sHELIXII from T0 to T1, which was statistically significant (p=0.038). There was no significant difference in T0 and T3 for either biomarker. Serum HELIXII also tended to increase from T0 and T2 (p=0.07), but not from T0 to T1a (p>0.10). Bland Altman analyses showed that variation was not dependent on the serum level for either PIIANP or HELIXII, and therefore variation did not trend systematically.
Figure 1.
Normalized Concentrations of sHELIX and sPIIANP vs. Timepoints. The time of collection is indicated at the tip of the figure. The status (fasting vs. non-fasting; at rest vs. active) is indicated at the bottom of the figure for each timepoint. Figure plots represent mean normalized biomarker concentrations ± one standard deviation.
DISCUSSION
Information about diurnal variation of a biomarker has the potential to improve clinical utility by clarifying whether or not strict adherence to a standardized time of collection is necessary to minimize biomarker variation from non-disease related influences. Among both cohorts, we observed significant diurnal variation in serum concentrations of PIIANP and HELIXII. Levels of sPIIANP and sHELIXII rose significantly within 1–2 hours of initiating morning activities, which included eating breakfast, however returned to baseline levels during the day. By analogy to diurnal variation of HA [7, 8], we believe this change is from the accumulation of these biomarkers in the synovium of the OA joint during rest, and mechanically transported via lymphatics to the circulation during physical activity [7]. These data suggest that utility of these biomarkers would benefit from a standardized collection regimen. Additionally, these findings are consistent with our previous report of diurnal variation of other type II collagen biomarkers, specifically sCPII, uC2C, and uCTXII [11]. In particular, sCPII, a serum biomarker for collagen synthesis, varied in a similar pattern, further validating our results.
Mechanistically, it seems plausible to assume that collagen obtained from the diet or dietary supplementation with the nutracuticals, glucosamine and chondroitin sulfate, could alter biomarker concentrations in the sera and subsequently in the urine. However, limited research has been done to analyze the effects of diet and dietary supplements on biomarker levels. The diet provided to participants was low in collagen and was therefore unlikely to be a confounder. Moreover, there are currently no data to suggest a change in biomarker concentration with dietary supplements. In a three year trial of glucosamine for knee OA, there was no significant difference in uCTXII between placebo and glucosamine-supplemented groups [14]. In a second trial studying glucosamine and biomarkers uC1,2C and uC2C over a 6-month time course, there was no significant difference in biomarker levels between placebo and glucosamine-supplemented groups [15]. Although long-term effects of glucosamine have been studied, no one has ever studied its effects on diurnal variation.
This study has limited power to detect small daily variation in biomarker concentration; however, with a larger sample size (n=40) for the timepoints T0, T1, and T3, there is a decreased likelihood of a type II error (false negative result). The more limited sample size (n=20) for T1a and T2 may have limited our power to detect a significant difference between T0 and T2 for sHELIXII. Sample size, along with the cross-sectional design of the study may have also affected correlational analysis with the sum K-L score and limited the power to detect such an association. Although there was no correlation between these biomarkers and the sum K-L score, it is nevertheless useful to clearly assess the severity of knee OA in the sample to determine the application of such findings in the OA population. Furthermore, the time intervals used may be inadequate for determining maximal variation. The half-life of biomarker clearance upon rising may be shorter than these time intervals, requiring more frequent sampling to determine the overall kinetics of diurnal variation. In this study, each patient served as their own control. Although a normal healthy reference population was not included we do not view this as a limitation since OA trials in which these biomarkers would be applied, would include only a disease cohort.
Even so, while these limitations may present biases in our study, this is the first study to evaluate diurnal variation of sPIIANP and sHELIXII and provides important information for clinical trial design, namely that standardized time of sampling would be important for these two markers to minimize variation on the basis of diurnal variation as opposed to disease-related variation.
Acknowledgments
Supported by NIH/NIAMS grant RO1 AR48769 and 1PO1-AR050245, and by the National Center for Research Resources NIH MO1-RR-30, supporting the Duke General Clinical Research Unit where this study was conducted.
References
- 1.Rousseau JC, Zhu Y, Miossec P, Vignon E, Sandell LJ, Garnero P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2004;12:440–447. doi: 10.1016/j.joca.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Kraus VB. Do biochemical markers have a role in osteoarthritis diagnosis and treatment? Best Pract Res Clin Rheumatol. 2006;20:69–80. doi: 10.1016/j.berh.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Ryan MC, Sandell LJ. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- 4.Aigner T, Zhu Y, Chansky HH, Matsen FA, 3rd, Maloney WJ, Sandell LJ. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999;42:1443–1450. doi: 10.1002/1529-0131(199907)42:7<1443::AID-ANR18>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52:1081–1090. doi: 10.1002/art.20930. [DOI] [PubMed] [Google Scholar]
- 6.Garnero PTN, Juillet F, Whittles RC, Sharif M. A new serum-based assay for type II collagen helical peptide (serum HELIX-II) is associated with long-term radiological progression in knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:C49–C50. [Google Scholar]
- 7.Criscione LG, Elliott AL, Stabler T, Jordan JM, Pieper CF, Kraus VB. Variation of serum hyaluronan with activity in individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2005;13:837–840. doi: 10.1016/j.joca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lindqvist U, Engstrom-Laurent A, Laurent U, Nyberg A, Bjorklund U, Eriksson H, et al. The diurnal variation of serum hyaluronan in health and disease. Scand J Clin Lab Invest. 1988;48:765–770. doi: 10.3109/00365518809088758. [DOI] [PubMed] [Google Scholar]
- 9.Manicourt DH, Poilvache P, Nzeusseu A, van Egeren A, Devogelaer JP, Lenz ME, et al. Serum levels of hyaluronan, antigenic keratan sulfate, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 change predictably in rheumatoid arthritis patients who have begun activity after a night of bed rest. Arthritis Rheum. 1999;42:1861–1869. doi: 10.1002/1529-0131(199909)42:9<1861::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Gordon CDST, Kraus VB. Variation in Serum and Urine Biomarkers from Activity not Food Consumption in Participants with Radiographic Knee Osteoarthritis. CCA. Durham Duke University; 2008. [Google Scholar]
- 11.Kong SY, Stabler TV, Criscione LG, Elliott AL, Jordan JM, Kraus VB. Diurnal variation of serum and urine biomarkers in patients with radiographic knee osteoarthritis. Arthritis Rheum. 2006;54:2496–2504. doi: 10.1002/art.21977. [DOI] [PubMed] [Google Scholar]
- 12.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 14.Christgau S, Henrotin Y, Tanko LB, Rovati LC, Collette J, Bruyere O, et al. Osteoarthritic patients with high cartilage turnover show increased responsiveness to the cartilage protecting effects of glucosamine sulphate. Clin Exp Rheumatol. 2004;22:36–42. [PubMed] [Google Scholar]
- 15.Cibere J, Thorne A, Kopec JA, Singer J, Canvin J, Robinson DB, et al. Glucosamine sulfate and cartilage type II collagen degradation in patients with knee osteoarthritis: randomized discontinuation trial results employing biomarkers. J Rheumatol. 2005;32:896–902. [PubMed] [Google Scholar]