Abstract
Typically, vaccines distributed through the Expanded Program on Immunization (EPI) use a 2–8 °C cold chain with 4–5 stops. The PfSPZ Vaccine comprises whole live-attenuated cryopreserved sporozoites stored in liquid nitrogen (LN2) vapor phase (LNVP) below −140 °C and would be distributed through a LNVP cold chain. The purpose of this study was to model LNVP cold chain distribution for the cryopreserved PfSPZ Vaccine in Tanzania, estimate the costs and compare these costs to those that would be incurred in distributing a ‘conventional’ malaria vaccine through the EPI. Capital and recurrent costs for storage, transportation, labor, energy usage and facilities were determined for the birth cohort in Tanzania over five years. Costs were calculated using WHO/UNESCO calculators. These were applied to a 2–8 °C distribution model with national, regional, district, and health facility levels, and for the cryopreserved vaccine using a ‘modified hub-and-spoke’ (MH-S) LNVP distribution system comprising a central national store, peripheral health facilities and an intermediate district-level transhipment stop. Estimated costs per fully immunized child (FIC) were $ 6.11 for the LNVP-distributed cryopreserved vaccine where the LN2 is generated, and $ 6.04 with purchased LN2 (assuming US $ 1.00/L). The FIC costs for distributing a conventional vaccine using the four level 2–8 °C cold chain were $ 6.10, and with a tariff distribution system as occurs in Tanzania the FIC cost was $ 5.53. The models, therefore, predicted little difference in 5-year distribution costs between the PfSPZ Vaccine distributed through a MH-S LNVP cold chain and a conventional vaccine distributed through the more traditional EPI system. A LNVP cold chain provides additional benefits through the use of durable dry shippers because no refrigerators, freezers or refrigerated trucks are required. Thus strain at the cold chain periphery, vaccine wastage from cold chain failures and the environmental impact of distribution would all be reduced.
Keywords: Vaccine, Cold chain, Liquid nitrogen vapor phase, Malaria, Distribution cost, Model
1. Introduction
Whole organism vaccines targeting Plasmodium falciparum, currently in Phase I clinical development, comprise live, metabolically active (but non-replicating) sporozoites [1]. The immunogens are cryopreserved and stored in liquid nitrogen vapor phase (LNVP) below −140 °C and would be distributed through a LNVP cold chain. A LNVP cold chain is required because sporozoites must be cryopreserved to retain viability and stability over time – no methods have yet been developed to thermostabilize living sporozoites by alternate means. There are different scenarios under which these vaccines might be used, including adoption into the Expanded Program for Immunization (EPI). Typically 6–9 vaccines [2,3], varying in number between countries, are included in the EPI and are distributed through a 2–8 °C cold chain. Additional new, often expensive, vaccines are being considered for the EPI [4], and new models of vaccine distribution are being evaluated to improve efficiency and reduce costs [5–9]. The standard EPI distribution model has received most attention in the literature (including for a hypothetical conventional malaria vaccine [5]), and this is the only model currently available that provides a base for distribution cost comparisons. Even if never distributed through the EPI, it is useful to compare the relative costs of a LNVP cold chain with the costs of distributing a new vaccine through the EPI.
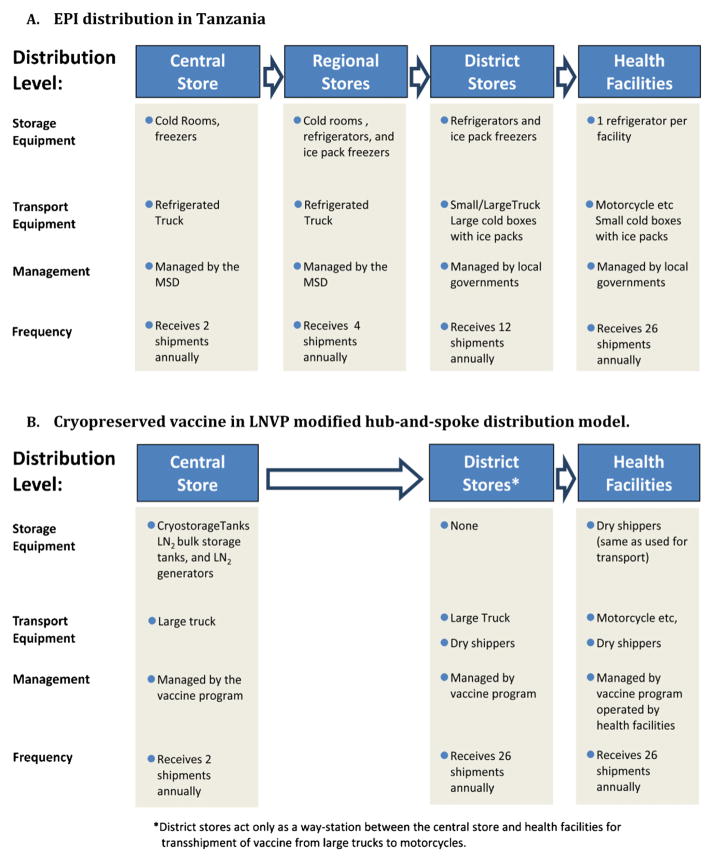
The EPI model comprises 3–4 levels: a national/central store [8–13], intermediate or regional stores (occasionally, zonal stores), second-level intermediate stores/district stores, and service points/health facilities. Considerable effort is expended to ensure 2–8 °C is maintained, however, temperature excursions occur, particularly at the periphery [13–18], resulting in vaccine potency loss and wastage. Reducing the number of cold chain steps to improve efficiency and speed, and reduce stockouts and wastage [9,19–21] has been recommended by WHO/PATH Project Optimize. Such modifications to the EPI, implemented in Thailand, are being evaluated in Senegal, Tunisia and other countries. The Centers for Disease Control and Prevention (CDC) has introduced a ‘hub-and-spoke’ (H-S) distribution system for vaccines in the USA [22,23]. This program, managed by McKesson Corp., distributes vaccines directly from two hubs to ~40,000 health facilities. Preliminary analyses by us together with Air Liquide (personal communication) and by LEK Consulting [24] (and personal communication), indicate that a LNVP cold chain would operate most efficiently as an H-S network. The LNVP distribution model here is a modified H-S (MH-S) system that includes transshipment at the district level. In Tanzania the EPI is atypical, with a Medical Stores Department (MSD) tariff structure operating from the central store, through distribution, to the district stores, and has been studied previously [25] for vaccine distribution costing without capacity enhancement. Here we have compared the LNVP MH-S to the standard EPI, a MH-S EPI, and the MSD-tariff models.
No human vaccines manufactured at scale currently use liquid nitrogen (LN2) or LNVP for storage and distribution. However, LN2 is in widespread use for storage and distribution of several veterinary vaccines [26], in livestock reproduction (particularly artificial insemination; AI) [27,28], for transfusion blood products, and for the newer cell therapies and anti-cancer vaccines [29,30]. The East Coast fever (ECF) vaccine, comprising an infection-treatment regimen of Theileria parva sporozoites injected concomitantly with oxytetracycline, is a commercial product distributed through a LN2 cold chain in rural areas of Malawi, Tanzania and Kenya [31]. A similar infection–treatment sporozoite-based approach for P. falciparum (PfSPZ-CVac) has just entered phase 1 trials – this would also be distributed using a LNVP cold chain.
Many locations throughout Africa have LN2 plants, though LN2 availability and cost varies widely. Tanzania is relatively well served – LN2 is produced commercially in Arusha (used for ECF vaccine distribution), Dar es Salaam (and soon Bagamoyo) and bulk distributed to Mwanza and Mbeya. Numerous smaller plants throughout the country also generate LN2 principally to support research. Thus, a nascent LNVP cold chain network already exists in Tanzania.
The principal purpose of this study was to generate comparative analyses of the cold chain marginal costs for adding a new malaria vaccine to the EPI under different scenarios. Costs were estimated for distributing a hypothetical lyophilized (conventional) malaria vaccine through (1) a standard 2–8 °C cold chain, (2) a MH-S 2–8 °C network or (3) the Tanzanian MSD tariff-based distribution network, and these were compared to the costs of distributing the cryopreserved malaria vaccine using a MH-S LNVP cold chain where LN2 is (4) purchased, or (5) generated.
2. Materials and methods
2.1. Overview
The WHO EPI calculators and available commercial pricing were used to estimate vaccine distribution costs, including capital equipment and recurrent operating costs (energy usage, LN2, maintenance, insurance, rent, labor), transport and storage of vaccine, vaccine diluent, injection devices and safety boxes [32–36]. Not included were the costs of the vaccines (currently unknown), vaccine inoculation personnel, training costs and social mobilization costs. The 4-stop cold chain model used in the EPI and MSD-managed systems [37] for the hypothetical conventional lyophilized malaria vaccine is indicated in Fig. 1A. The MH-S distribution model for the cryopreserved LNVP-distributed vaccine is indicated in Fig. 1B.
Fig. 1.
Overview of vaccine distribution cold chain models. (A) EPI distribution in Tanzania. (B) Cryopreserved vaccine in LNVP modified hub-and-spoke distribution model.
Estimates of the annual birth cohort for Tanzania vary [38–40]; we used a mid-range estimate of 1,662,925 (provided by FM, [39]) for 2011. 100% coverage was assumed with a fully immunized child (FIC) requiring three doses. For vaccine forecasting the estimated total number of annual doses needed ranged from 5,537,540 for year 1 to 6,232,550 in year 5 (including wastage and 3% annual population growth). The Tanzania EPI consisted, in 2011 of 1 national store in Dar es Salaam, 20 regional stores, 131 district stores, and 4,571 health facilities (dispensaries, health centers, and hospitals) [39].
Cost estimates for the conventional vaccine, diluent and safe injection equipment were based on the single dose MMR vaccine and included packaging and storage at 2–8 °C for the vaccine and ambient for other items. Volume estimates for the LNVP-distributed cryopreserved vaccine were based on using cryovials similar to those currently utilized; diluent and safe injection equipment volumes were as for the conventional vaccine. The efficacies and shelf-lives for the cryopreserved and hypothetical conventional vaccines were, for the purpose of this analysis, assumed to be equivalent.
2.2. Wastage rates
Vaccine wastage rates vary: we used an overall vaccine wastage rate of 10%, based on WHO recommended rates for conventional vaccines [41,42], which includes wastage in the vial and injection equipment, and loss due to non-use and to cold chain temperature excursions in line with observed wastage rates [13]. Wastage rates for LNVP-distributed vaccine would likely be lower, but were assumed here to be equivalent to those for the conventional vaccine.
2.3. Vaccine storage costs
Cold rooms were specified for national and regional stores, and 2–8 °C refrigerators and ice-pack freezers for district stores, and 2–8 °C refrigerators at health facilities. All equipment for 2–8 °C storage was selected from the WHO/UNICEF Product Information sheets of approved EPI cold chain equipment [33–36], standardized by manufacturer to reduce repair costs, and selected based on the minimum storage volume needed and the energy source used. The life span of this equipment was taken as 5 years [36] (10 years for cold rooms). Power for 2–8 °C cold chain equipment was electricity, except for liquefied petroleum gas (LPG) or kerosene for refrigerators at health facilities. Current prices in effect in Tanzania in February 2011 [43] were used for electricity, LPG and kerosene. Backup generators were included for the national and regional stores. MSD tariffs were applied at February 2011 rates to distribution model number 3. The LNVP cold chain included purchased or generated LN2, bulk storage containers for LN2, and LNVP cryostorage tanks for vaccine storage only at the national store. The costs for these items, as well as dry shippers for transport, were estimated based on current prices from commercial suppliers.
2.4. Vaccine transport costs
Estimated vehicle requirements were based on vaccine volume (including diluent, safe injection equipment), numbers of stores, distances and the frequency of shipments [44]. Shipment and storage at 2–8 °C has been included for diluent, however if no diluent is needed (a possibility) this would reduce these costs for the cryopreserved vaccine. In the MH-S models, transit from national store to district stores (2–8 °C) or district transshipment points (LNVP) was estimated at 1–2 days, with one round-trip/week serving 10 district stores/points. Each district would serve up to 18 health facilities/week. For 2–8 °C transport, refrigerated trucks were included from national to regional stores, and for MH-S, from national to district stores. Pick-up trucks or vans were factored for regional to district store transport, and motorcycles between district stores/points and health facilities. A 20% annual replacement rate for cold boxes was assumed. Vehicle purchase, maintenance, insurance, and other costs including gasoline, diesel and driver pay were obtained from commercial vendors in Tanzania current at February 2011.
For the LNVP distribution, vaccine would be loaded into dry shippers at the national store and would remain in these shippers through to administration at the health facilities or until returned to the national store (Fig. 1B). Non-refrigerated large trucks would transport dry shippers to each district level transshipment point every two weeks, with onward transport by motorcycles to health facilities. To ensure a continuous supply of cryopreserved vaccine, each health facility would require 3 dry shippers on six-week rotations (n = 13,713 total; 8.3 round trips/dry shipper/year). An annual replacement rate of 10% was assumed for dry shippers.
2.5. Labor costs
Personnel costs were included at all levels based on EPI estimates, including managers, surveillance staff, and general staff as well as truck and motorcycle drivers. Technicians at each distribution level were assumed to devote 10% of time to monitoring the 2–8 °C vaccine and 10% to repackaging and preparing onward shipments. Similar costs were assumed for the LNVP distribution models.
2.6. Other costs
Costs for training, immunization (doctors, nurses, and village health workers) and social mobilization activities were not included. Costs for these components were considered to be equivalent for each model, nevertheless, once information becomes available to estimate these costs, they should be added to the models.
3. Results
3.1. Overall costs
Total costs, from vaccine receipt at the national store to administration in the health facility, with 100% coverage for the birth cohort for five years, was estimated for the LNVP-distributed cryopreserved vaccine to be $ 54.0 million using generated LN2 and $53.4 million using purchased LN2 (Table 1). Comparable costs for an EPI-distributed conventional malaria vaccine were estimated to be $ 53.8 million, and $ 53.4 million for a MH-S model. Costs for MSD-managed conventional vaccine distribution were $ 48.8 million. These translate to per-FIC costs of $ 6.04 and $ 6.11 for the cryopreserved vaccine using purchased or generated LN2, respectively (Table 1), $ 6.10 and $ 6.04 for the conventional vaccine delivered using the standard EPI or MH-S EPI, respectively, and $ 5.53 for the MSD-managed EPI.
Table 1.
Estimated capital, recurrent and total costs for a five year vaccine introduction program in Tanzania: cost per fully immunized child. Capital investment and recurrent costs for a conventional malaria vaccine distributed under the standard EPI model, through an EPI modified hub-and-spoke model, or the EPI MSD-tariff model, and also for the cryopreserved vaccine distributed under two versions of the modified hub-and-spoke model where LN2 is either purchased or generated by the program. Cost percentages for each year of the five year periods are also indicated.
| Standard EPI model | EPI modified hub-and-spoke model | EPI MSD tariff model | LNVP modified hub-and-spoke model (purchase LN2) | LNVP modified hub-and-spoke model (generate LN2) | ||
|---|---|---|---|---|---|---|
| Year 1 | Capital | $ 9,633,090 | $ 8,853,461 | $ 5,892,461 | $ 10,245,707 | $ 13,445,819 |
| Recurrent | $ 7,587,726 | $ 8,115,418 | $ 7,353,738 | $ 7,831,908 | $ 6,885,724 | |
| Total | $ 17,220,816 | $ 16,968,878 | $ 13,246,199 | $ 18,077,615 | $ 20,331,543 | |
| Total as % of 5 year | 32.0% | 31.8% | 27.2% | 33.9% | 37.7% | |
| Year 2 | Capital | $ 21,544 | $ 19,958 | $ 19,958 | $ 69,955 | $ 69,955 |
| Recurrent | $ 7,939,675 | $ 7,899,606 | $ 7,721,500 | $ 7,611,643 | $ 7,232710 | |
| Total | $ 7,961,219 | $ 7,919,563 | $ 7,741,457 | $ 7,681,598 | $ 7,302,666 | |
| Total as % of 5 year | 14.8% | 14.8% | 15.9% | 14.4% | 13.5% | |
| Year 3 | Capital | $ 99,332 | $ 107,303 | $ 20,955 | $ 73,453 | $ 73,453 |
| Recurrent | $ 8,348,496 | $ 8,296,643 | $ 8,107,753 | $ 7,992,294 | $ 7,594,892 | |
| Total | $ 8,447,828 | $ 8,403,946 | $ 8,128,709 | $ 8,065,747 | $ 7,668,345 | |
| Total as % of 5 year | 15.7% | 15.7% | 16.7% | 15.1% | 14.2% | |
| Year 4 | Capital | $ 1,895,892 | $ 2,189,941 | $ 2,189,941 | $ 2,245,064 | $ 2,245,064 |
| Recurrent | $ 9,054,758 | $ 8,711,476 | $ 8,513,260 | $ 8,391,971 | $ 7,975,199 | |
| Total | $ 10,950,650 | $ 10,901,417 | $ 10,703,201 | $ 10,637,034 | $ 10,220,263 | |
| Total as % of 5 year | 20.5% | 20.4% | 21.9% | 19.9% | 18.9% | |
| Year 5 | Capital | $ 36,352 | $ 23,103 | $ 23,103 | $ 80,982 | $ 80,982 |
| Recurrent | $ 9,206,954 | $ 9,151,347 | $ 8,939,047 | $ 8,811,634 | $ 8,374,551 | |
| Total | $ 9,243,306 | $ 9,174,451 | $ 8,962,151 | $ 8,892,616 | $ 8,455,533 | |
| Total as % of 5 year | 17.2% | 17.2% | 18.4% | 16.7% | 15.7% | |
| Total | Capital | $ 11,686,211 | $ 11,193,766 | $ 8,146,419 | $ 12,668,343 | $ 15,915,273 |
| % of total | 21.7% | 21.0% | 15.7% | 23.7% | 29.5% | |
| Recurrent | $ 42,137,609 | $ 42,174,490 | $ 40,635,299 | $ 40,639,449 | $ 38,063,076 | |
| % of total | 78.3% | 79.0% | 83.3% | 76.2% | 70.5% | |
| Total | $ 53,823,819 | $ 53,368,256 | $ 48,781,718 | $ 53,354,610 | $ 53,978,349 | |
| % of total | 100% | 100% | 100% | 100% | 100% | |
| Cost/child | $ 6.10 | $ 6.04 | $ 5.53 | $ 6.04 | $ 6.11 |
3.2. Capital and recurrent costs
Capital investment and recurrent costs for each model for the five year period are indicated in Table 1. Capital costs accounted for 55.9% of year 01 total costs (21.7% over 5 years) for the conventional vaccine standard EPI model and 56.7% for the cryopreserved vaccine (23.7% over 5 years) when purchasing LN2, and 66.1% (29.5% over 5 years) with generated LN2. Overall, capital investment was calculated to be $ 1.0 million more for the LNVP-distributed vaccine using purchased LN2 and $ 4.2 million more for generated LN2 than for the standard EPI model although total overall costs were similar ($ 53.4, $ 53.8 and $ 54.0 million, respectively). Recurrent costs for the LNVP model using purchased and generated LN2 were, over five years, $ 1.5 million and $ 4.1 million lower, respectively, than for the standard EPI-delivered conventional vaccine, and were similar or lower, respectively, than for the conventional vaccine delivered through the EPI-MSD or EPI-MH-S models.
3.3. Distribution levels
Total costs over five years apportioned to national, regional, district and health facility levels are indicated in Table 2. Proportionally, the cost distribution as a percentage of the total was greater at the national level for the cryopreserved vaccine (48.9–50.6%) than for the conventional vaccine (10%). Conversely, for all distribution models for the conventional vaccine, costs at the district level (31.3–37.9%) and health facilities level (40.9–46.7%), were proportionally greater than for either cryopreserved vaccine distribution model (23.9% and 25.3% for district, and 25.7% and 25.4% for health facilities, respectively). For the LNVP-distributed vaccine models, dry shippers were included at the national level.
Table 2.
Vaccine distribution costs by distribution level. Estimated costs at each level of the distribution network for a five year vaccine introduction program in Tanzania assuming a 10% wastage rate, for a conventional malaria vaccine distributed either under the standard EPI model, through an EPI modified hub-and-spoke model, or the EPI MSD-tariff model, and also for the cryopreserved vaccine distributed under two versions of the modified hub-and-spoke model where LN2 is either purchased or generated by the program. Cost percentages for each distribution level are also indicated.
| Distribution level | Standard EPI Model | EPI modified hub-and-spoke model | EPI MSD tariff model | LNVP modified hub-and-spoke model (purchase LN2) | LNVP modified hub-and-spoke model (generate LN2) |
|---|---|---|---|---|---|
| Nationala | $ 5,408,732 | $ 11,146,694 | $ 1,895,304 | $ 26,111,845 | $ 27,323,652 |
| % of total | 10.0% | 20.9% | 3.9% | 48.9% | 50.6% |
| Regionalb | $ 9,591,454 | – | $ 7,273,608 | – | – |
| % of total | 17.8% | 14.9% | |||
| Districtc | $ 16,827,636 | $ 20,225,812 | $ 16,846,631 | $ 13,514,796 | $ 12,926,631 |
| % of total | 31.3% | 37.9% | 34.5% | 25.3% | 23.9% |
| Health Facilities | $ 21,995,997 | $ 21,995,750 | $ 22,766,175 | $ 13,727,969 | $ 13,727,969 |
| % of total | 40.9% | 41.2% | 46.7% | 25.7% | 25.4% |
| Total | $ 53,823.819 | $ 53,368,256 | $ 48,781,718 | $ 53,354,610 | $ 53,978,349 |
| % | 100% | 100% | 100% | 100% | 100% |
Costs at the National level include those transport costs for moving vaccine and supplies out to the next level – either the regional storage depots for the conventional malaria vaccine, or to the district level in the modified hub-and-spoke models. The costs of dry shippers for transporting the PfSPZ Vaccine in LNVP have been included at the national level.
Costs at the regional level include those transport costs for moving vaccine and supplies to the district level.
Costs at the district level include the costs of moving vaccine and supplies to the health facilities.
3.4. Cost categories
Cost breakdowns into the main component categories are indicated in Table 3. Three components with notable differences were: storage, transport and facilities rent. Storage costs over 5 years were lower for cryopreserved vaccine models, absolutely ($ 5.5 and $ 8.7 million vs. $ 11.9 million) and proportionally (10.2% and 16.3% vs. 22.0%) compared to the standard EPI model. Non-labor transportation costs were higher for both cryopreserved vaccine models ($19.7 million; at 36.4% and 36.9% of total costs) than for the conventional EPI vaccine model ($ 15.4 million, equivalent to 28.6%), largely reflecting inclusion of dry shippers in this category. Facilities rental costs were moderately lower ($ 11.3 million, 21.2%) for cryopreserved vaccine models using purchased LN2 and higher ($ 15.1 million, 28.0%) using generated LN2, compared to the conventional EPI model ($ 12.3 million, 23.0%).
Table 3.
Vaccine distribution costs by major cost category: estimated costs apportioned to the major categories of a five year vaccine introduction program in Tanzania assuming a 10% wastage rate for a conventional malaria vaccine distributed under the standard EPI model, through an EPI modified hub-and-spoke model, or the EPI MSD-tariff model, and also for the cryopreserved vaccine distributed under two versions of the modified hub-and-spoke model where LN2 is either purchased or generated by the program. Cost percentages for each component of the totals are also indicated.
| Distribution component | Standard EPI model | EPI modified hub-and-spoke model | EPI tariff modela | LNVP modified hub-and-spoke model (purchase LN2) | LNVP modified hub-and-spoke model (generate LN2) |
|---|---|---|---|---|---|
| MSD storage tariffsa | – | – | $ 2,014,639 | – | – |
| % of total | 4.1% | ||||
| Storagea | $ 11,853,363 | $ 11,222,496 | $ 10,705,372 | $ 8,723,260 | $ 5,523,746 |
| % of total | 22.0% | 21.0% | 21.9% | 16.3% | 10.2% |
| MSD distribution tariffsa | – | – | $ 7,154,272 | – | – |
| % of total | 14.7% | ||||
| Transport | $ 15,390,562 | $ 15,131,461 | $ 7,980,602 | $ 19,673,591b | $ 19,673,591b |
| % of total | 28.6% | 28.4% | 16.4% | 36.9% | 36.4% |
| Transport labor | $ 5,487,965 | $ 5,207,353 | $ 4,736,589 | $ 4,915,137 | $ 4,915,137 |
| % of total | 10.2% | 9.8% | 9.7% | 9.2% | 9.1% |
| Facilities rent | $ 12,300,404 | $ 13,148,752 | $ 7,177,350 | $ 11,291,096 | $ 15,114,348 |
| % of total | 22.9% | 24.6% | 14.7% | 21.2% | 28.0% |
| Facilities laborb | $ 8,791,526 | $ 8,658,195 | $ 9,012,893 | $ 8,751,526 | $ 8,751,526 |
| % of total | 16.3% | 16.2% | 18.5% | 16.4% | 16.2% |
| Total cost | $ 53,823,819 | $ 53,368,256 | $ 48,781,718 | $ 53,354,610 | $ 53,978,349 |
| % | 100% | 100% | 100% | 100% | 100% |
MSD tariffs apply to storage at the national store and regional stores and also to transport from the national store to the regional stores, and from the regional stores to the district stores.
The purchase cost of dry shippers used to transport the cryopreserved vaccine in LNVP from the national store through the transshipment point at the district level to the health facilities and that act as temporary storage for 2 weeks at the health facilities have been included in the costs of transport.
4. Discussion
A LNVP cold chain designed to distribute a cryopreserved malaria vaccine would require new capital investment and incur new recurrent costs. Realistically, LNVP distribution would likely be most efficient operating outside the EPI, nevertheless, the EPI is the best comparator for costing: additional costs would be incurred by adding any new vaccine into the EPI whether distributed through a LNVP or a 2–8 °C cold chain.
Preliminary analyses indicated that a MH-S design is optimal for a LNVP cold chain. The MH-S model, by comparison to a true hub-and-spoke model, incorporates a transshipment point at the district level (Fig. 1B); this functions as an exchange point for dry shippers. The central component of LNVP MH-S distribution is the dry shipper. Dry shippers would circulate on overlapping 6-week schedules: week 1 – transport from the national store to the health facility; weeks 2 and 3 – temporary storage at the health facility for immunizations; week 4 – return to the national store; weeks 5 and 6 – cleaning, recharging and restocking. The ability to provide a significantly extended storage time in dry shippers also reduces the risk of vaccine wastage resulting from transport delays and storage, which is a relatively frequent event with 2–8 °C cold chains. At the district transshipment point during its return journey, the courier with the empty dry shipper exchanges this for a new, stocked, dry shipper that is conveyed to the health facility (outbound dry shippers remain sealed after loading at the central store until they arrive at each health facility). Thus, each health facility is kept continuously supplied with vaccine through new shipments arriving every two weeks.
Dry shippers are triple walled vessels that contain an insulating vacuum between the outer and central walls and porous material that absorbs LN2 by capillary action to provide the refrigerant, between the central and inner walls. They are highly resilient, have an additional outer protective casing, are continuously reusable, and would optimally transport 100–200 cryovials each per shipment. No cold chain storage equipment is needed at regional or district levels nor at health facilities. Dry shippers are independent of electricity and require no or minimal maintenance, and incur a minimal space (rent) requirement. With no added equipment (or equipment maintenance) at the periphery, strain on this normally weaker portion of the cold chain is reduced. LNVP distribution also does not need refrigerated trucks. By contrast, the 2–8 °C cold chain uses refrigerated trucks, and trucks with cold boxes that have a limited holding time from ~3 days to a few hours [36], for transportation. Collectively, costs peripheral to the national store are considerably lower in the LNVP distribution models ($ 26.7 million and $ 27.2 million) vs. the standard EPI model ($48.4 million).
The LNVP distribution models potentially have a lower environmental impact due to (a) the longer life span of dry shippers compared to 2–8 °C cold boxes and ice packs, (b) the non-requirement for refrigerated trucks with their higher fuel consumption and maintenance requirements, and, (c) the independence, after LN2 has been generated, from electricity or LPG. The projected overall non-transport energy needs (including energy used in LN2 generation, which comprises 5.6% of National level storage costs) of the LNVP distribution models were 90–92% lower than for the conventional vaccine models.
Overall, costs were more centralized with the LNVP distribution models, with national level costs comprising 48.9–50.6% of total costs compared to 10.0% for the conventional EPI vaccine distribution model. This was accounted for by greater capital investment in the LNVP distribution models. The least expensive model was the MSD-tariff distribution model used with the conventional vaccine, likely due to spare capacity in the tariff structure, while for all other models, overall total costs were similar.
Either purchased or generated LN2 would only be required at the central National store. We factored a bulk purchase cost for LN2 of $ 1.00/L. The current market for LN2 in Tanzania is relatively small and commercially-sourced LN2 is currently (2011/2012) approximately Sh3,000 TZ ($ 1.90)/L. Typically, as LN2 markets grow, LN2 cost falls (<$ 0.25/L in the USA). Overall total cost equivalence for the two LNVP models would occur with a LN2 purchase cost of $ 1.09/L. With LN2 purchased at the current price in Tanzania, the overall distribution costs would be $ 1.6 million higher and the per-FIC cost $ 0.96 higher. Conversely, with LN2 costs comparable to those in the USA, the per-FIC cost would decline by $ 0.80 to $ 5.24.
There is a general misconception [45] that LNVP cold chain distribution is untested and would be novel and expensive. However, LN2-based cold chains are in widespread use [26–30], particularly in the anti-cancer, regenerative medicine/cell therapy and veterinary fields – the latter distributing >600 M units/year globally, including within sub-Saharan Africa. Six recent clinical trials in the USA, The Netherlands, United Kingdom, Tanzania and Germany ([46] and Lyke et al.; Bijker et al.; Sheehy et al.; Abdulla et al., al, and Mordmüeller et al., publications pending), and successful tests of the cold chain to clinical sites in Burkina Faso and Ghana, using cryopreserved PfSPZ, have demonstrated LNVP cold chain reliability, that LNVP-stored and distributed PfSPZ are stable and that cryopreserved non-attenuated PfSPZ are highly infectious to human volunteers.
We show here that the costs associated with a LNVP distribution system are very competitive with those of 2–8 °C cold chain distribution models. Incorporating additional vaccines or therapies into the same LNVP distribution network could provide further economies of scale. For the purpose of this cost analysis, we assumed equal efficacies and stabilities for both the conventional and cryopreserved vaccines and looked only at distribution costs. As additional information becomes available on the cryopreserved vaccine and particularly protective efficacy, other economic analyses, including cost-effectiveness, cost-utility, and cost-benefit, should be performed. The licensure and introduction of a highly effective malaria vaccine has the potential to save millions of lives from malaria, significantly impact the economies of affected countries, and be a key tool in malaria eradication.
Acknowledgments
This study was performed in part in partial fulfillment of the requirements for the degree of MHS awarded to Cristina Reyes Garcia, from the Bloomberg School of Public Health, Johns Hopkins University. Sanaria acknowledges support from the PATH Malaria Vaccine Initiative (MVI) with funds from the Bill and Melinda Gates Foundation (BMGF), the Institute for OneWorld Health (funds from BMGF), and Small Business Innovation Research grants from NIAID/NIH for work to produce the PfSPZ Vaccine.
Contributor Information
Cristina Reyes Garcia, Email: crgarcia@jhsph.edu.
Fatuma Manzi, Email: fatuma.manzi@gmail.com.
Fabrizio Tediosi, Email: Fabrizio.Tediosi@unibas.ch.
Stephen L. Hoffman, Email: slhoffman@sanaria.com.
Eric R. James, Email: ejames@sanaria.com.
References
- 1.Hoffman SL, Billingsley PF, James ER, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccine. 2010;38:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Expanded Programme on Immunization (EPI) http://who.int/immunizationdelivery/en/
- 3.World Health Organization. WHO/EPI/TRAM/98.02 REV.1, Module 2: EPI vaccines. http://whqlibdoc.who.int/hq/1998/WHO_EPI_TRAM_98.02.pdf.
- 4.International AIDS Vaccine Initiative, PATH. HPV vaccine adoption in developing countries: cost and financing issues. 2007 http://screening.iarc.fr/doc/IAVI_PATH_HPV_financing.pdf.
- 5.Maire N, Shillcutt SD, Walker DG, Tediosi F, Smith TA. Cost-effectiveness of the introduction of a pre-erythrocytic malaria vaccine into the expanded program on immunization in sub-Saharan Africa: analysis of uncertainties using a stochastic individual-based simulation model of Plasmodium falciparum malaria. Value Health. 2011;8:1028–38. doi: 10.1016/j.jval.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Clemens J, Jodar L. Introducing new vaccines into developing countries: obstacles, opportunities and complexities. Nat Med. 2005;11:S12–5. doi: 10.1038/nm1225. [DOI] [PubMed] [Google Scholar]
- 7.PATH. Summary of stability data for commonly used vaccines and novel vaccine formulations. PATH Vaccine Stabilization Team; 2008. http://www.path.org/publications/files/TS_vaccine_stability_table.pdf. [Google Scholar]
- 8.World Health Organization. Immunization service delivery: Project Optimize. http://www.who.int/immunization_delivery/optimize/en/index.html.
- 9.World Health Organization. Guidelines for establishing or improving national, regional and district vaccine stores. (WHO/EPI/LHIS/96.03). http://whqlibdoc.who.int/hq/1996/WHO_EPI_LHIS_96.03.pdf.
- 10.World Health Organization. Guideline for establishing or improving primary and intermediate vaccine stores. (WHO/V&B/02.34). http://www.who.int/vaccines-documents/DocsPDF02/www715.pdf.
- 11.UNICEF. Supplies and logistics: vaccine price data. Pneumoccocal vaccine. http://www.unicef.org/supply/index_60990.html?p=printme.
- 12.WHO Optimize Project in Tunisia 2010–2012. Collaborative agreement between WHO Tunisia and the Ministry of Public Health, project proposal. http://www.who.int/immunization_delivery/systems_policy/Tunisia_Optimize_ProjectProposal_15Jan10_EN.pdf.
- 13.World Health Organization. EPI logistics: cold chain. 2009 Available from: http://www.unicef.org/immunization/files/CCL_Workshop_Report_Nov_2009.pdf [cited 23.09.09]
- 14.Weir E, Hatch K. Preventing cold chain failure: vaccine storage and handling. CMAJ. 2004;171:1050. doi: 10.1503/cmaj.1041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berhane Y, Demissie M. Cold chain status at immunisation centres in Ethiopia. East Afr Med J. 2000;77:476–9. doi: 10.4314/eamj.v77i9.46692. [DOI] [PubMed] [Google Scholar]
- 16.Simba DO, Msamanga GI. Use of cold-chain to assess vaccine exposure to adverse temperatures in rural Tanzania. East Afr Med J. 1994;71:445–6. [PubMed] [Google Scholar]
- 17.Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007;25:3980–6. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 18.Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine. 2007;25:1328–33. doi: 10.1016/j.vaccine.2006.09.092. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Temperature sensitivity of vaccines. 2006 http://www.who.int/vaccines-documents/DocsPDF06/847.pdf.
- 20.PATH. 2009–2012 Optimize strategy. 2009 www.who.int/immunization-delivery/systems_policy/Optimize_Strategy_2009-2012.pdf.
- 21.Dicko M. Supply chain integration. Cold chain and logistics management meeting. 2009 http://www.technet21.org/Technet_forum/Immunization_Newsletter2010/Dec_12_2010/5_Integration_Presentation_Technet_02Dec10.ppt.
- 22.Centers for Disease Control and Prevention. Vaccine management business improvement project. http://www.cdc.gov/vaccines/programs/vmbip/default.htm.
- 23.Centers for Disease Control and Prevention. The pink book: epidemiology and prevention of vaccine preventable diseases. (12) 2011 http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/vac-storage.pdf [chapter 5, Vaccine storage and handling]
- 24.LEK Consulting LLC. Liquid nitrogen (LN2) cold-chain for malaria vaccine distribution in Africa. 2008 http://www.who.int/vaccine_research/documents/Lee_LN_Infrastructure_in_Africa_MalVac_00_.pdf[cited in: Pinder et al., 2010, MALVAC 2009: Progress and challenges in Development of whole organism malaria vaccines for endemic countries, 3–4 June 2009, Dakar, Senegal. Vaccine 28: 4695-4702]
- 25.Hutton G, Tediosi F. The costs of introducing a malaria vaccine through the expanded program on immunization in Tanzania. Am J Trop Med Hyg. 2006;75(2 Suppl):119–30. doi: 10.4269/ajtmh.2006.75.119. [DOI] [PubMed] [Google Scholar]
- 26.Meussen ENT, Walker J, Peters A, Pastoret P-P, Jungersen G. Current status of veterinary vaccines. Clin Microbiol. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasler JF. The current status and future of commercial embryo transfer in cattle. Anim Reprod Sci. 2003;79:245–64. doi: 10.1016/s0378-4320(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 28.Hiemstra SJ, van der Lende T, Woelders H. The potential of cryopreservation and reproductive technologies for animal genetics resources conservation strategies. 2005 http://www.fao.org/biotech/docs/hiemstra.pdf.
- 29.Westermann J, Körner IJ, Kopp J, Zenke S, Dörken B, Pezzutto A. Cryopreservation of mature monocyte-derived human dendritic cells for vaccination: influence on phenotype and functional properties. Cancer Immunol Immunother. 2003;52:194–8. doi: 10.1007/s00262-002-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liseth K, Ersvaer E, Hervig T, Bruserud Ø. Combination of intensive chemotherapy and anticancer vaccines in the treatment of human malignancies: the hematological experience. J Biomed Biotech. 2010 doi: 10.1155/2010/692097. http://dx.doi.org/10.1155/2010/692097._Article_ID_692097. [DOI] [PMC free article] [PubMed]
- 31.Di Giulio G, Lynen G, Morzaria S, Oura C, Bishop R. Live immunization against East Coast fever – current status. Trends Parasitol. 2009;25:85–92. doi: 10.1016/j.pt.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Management Sciences for Health. Managing drug supply. USA: Kumarian Press; 1997. [Google Scholar]
- 33.World Health Organization. Vaccine volume calculator in cold chain and logistics tools. 2009 http://www.who.int/immunization_delivery/systems_policy/logistics/en/index4.html.
- 34.World Health Organization. Logistics planning tool in cold chain and logistics tools. 2009 http://www.who.int/immunization_delivery/systems_policy/logistics/en/index5.html.
- 35.World Health Organization. Vaccine management and logistics: vaccine forecast and needs estimation. 2010 http://www.who.int/immunization_delivery/systems_policy/logistics/en/index2.html.
- 36.World Health Organization. New Product Information Sheets (PIS) since 2000 edition. http://www.who.int/immunization_standards/vaccine_quality/new_sheets_intro/en/index.html.
- 37.Medical Stores Department. Medical Stores Department Tanzania; 2002–2010. Available from: http://www.msd.or.tz/ [cited 2009]
- 38.National Bureau of Statistics: United Republic of Tanzania, Tanzania. Statistics for development. http://www.tanzania.go.tz/nbsf.html.
- 39.Manzi F. Ifakara Research and Development Centre; Ifakara, Tanzania: Feb, 2011. [Google Scholar]
- 40.World Bank. World Development Indicators, 2.1 Population Dynamics. 2011 http://data.worldbank.org/country/tanzania.
- 41.World Health Organization. Monitoring vaccine wastage at country level. 2003 http://whqlibdoc.who.int/hq/2005/WHO_V&B_03.18.Rev.1_eng.pdf(WHO/V&B/03.18)
- 42.World Health Organization. Immunization service delivery and accelerated disease control: projected vaccine wastage. 2011 http://www.who.int/immunization_delivery/systems_policy/logistics_projected_wastage/en/index.html.
- 43.TANESCO. 2009 Available from: http://www.tanesco.co.tz/
- 44.Kagaruki K. Expanded Program on Immunizations Tanzania. 2009. Summary of District Transport Monthly Budgets 2002/2003. [Google Scholar]
- 45.Pinder M, Moorthy VS, Akanmori BD, Genton B, Brown GV. MALVAC 2009 Progress and challenges in development of whole organism malaria vaccines for endemic countries, 3–4 June 2009, Dakar, Senegal. Vaccine. 2010;28:4695–702. doi: 10.1016/j.vaccine.2010.04.091. [DOI] [PubMed] [Google Scholar]
- 46.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]