Abstract
Nitric oxide modulates pain development. However, there is no evidence on the effect of nitroxyl (HNO/NO−) in nociception. Therefore, we addressed whether nitroxyl inhibits inflammatory hyperalgesia and its mechanism using the nitroxyl donor Angeli’s salt (AS; Na2N2O3). Mechanical hyperalgesia was evaluated using a modified Randall and Selitto method in rats, cytokine production by ELISA and nitroxyl was determined by confocal microscopy in DAF (a cell permeable reagent that is converted into a fluorescent molecule by nitrogen oxides)-treated dorsal root ganglia neurons in culture. Local pre-treatment with AS (17– 450 µg/paw, 30 min) inhibited the carrageenin-induced mechanical hyperalgesia in a dose- and time-dependent manner with maximum inhibition of 97%. AS also inhibited carrageenin-induced cytokine production. AS inhibited the hyperalgesia induced by other inflammatory stimuli including lipopolysaccharide, tumor necrosis factor-α, interleukin-1β and prostaglandin E2. Furthermore, the analgesic effect of AS was prevented by treatment with ODQ (a soluble guanylate cyclase inhibitor), KT5823 (a protein kinase G [PKG] inhibitor) or glybenclamide (an ATP-sensitive K+ channel blocker), but not with naloxone (an opioid receptor antagonist). AS induced concentration-dependent increase in fluorescence intensity of DAF-treated neurons in a L-cysteine (nitroxyl scavenger) sensitive manner. L-cysteine did not affect the NO+ donor S-Nitroso-N-acetyl-DL- penicillamine (SNAP)-induced anti-hyperalgesia or fluorescence of DAF-treated neurons. This is the first study to demonstrate that nitroxyl inhibits inflammatory hyperalgesia by reducing cytokine production and activating the cGMP/PKG/ATP-sensitive K+ channel signaling pathway in vivo.
Keywords: Nitroxyl, Angeli’s salt, nociception, pain, cytokine, nitric oxide
1.1. Introduction
Sensitization of primary sensory neurons is an essential process of inflammatory pain. In humans, this nociceptor sensitization usually leads to clinical conditions known as hyperalgesia (an increased response to a stimulus that is normally painful) or allodynia (pain due to a stimulus that does not normally provoke pain) (Verri et al., 2006). The mechanisms involved in the sensitization of primary sensory neurons may be divided into two processes. The first process includes non-neuronal events in which resident and recruited immune cells produce a sequence of hyperalgesic inflammatory mediators initiated by tumor necrosis factor α (TNFα). This triggers the release of interleukin-1β (IL-1β) and chemokines that in turn stimulate release of directly acting hyperalgesic mediators (Verri et al., 2006; Valerio et al., 2009). The most well known directly acting hyperalgesic mediators are prostaglandins, which activate their specific receptors present on the nociceptive neuron membrane. The second process includes neuronal events involving activation of receptors on primary nociceptive neurons that trigger intracellular signaling pathways, such as cAMP, protein kinase A, and protein kinase C (Aley and Levine, 1999; Khasar et al., 1999). These signaling pathways result in subsequent phosphorylation of voltage-dependent sodium channels (Gold et al., 1998) and inhibition of voltage-dependent potassium channels (Evans et al., 1999). Consequently, the nociceptor threshold is lowered and ultimately leads to an enhancement of neuron excitability.
Experimentally, the peripheral pharmacological control of inflammatory pain is based on two main strategies. The first is the use of drugs that prevent nociceptor sensitization, such as nonsteroidal anti-inflammatory drugs that inhibit prostaglandin synthesis (Ferreira, 1972) and, therefore, prevent the development of hyperalgesia. The second strategy is direct blockade of ongoing nociceptor sensitization, which can be achieved by use of peripheral morphine (opioids), dipyrone, or diclofenac (Lorenzetti and Ferreira, 1985; Ferreira et al., 1991; Cunha et al., 2010). In fact, these drugs reverse the already established hyperalgesia induced by prostaglandin E2 (PGE2) in rat and mice hind paws. In addition, several studies support the hypothesis that their antinociceptive activities are due to activation of the L-arginine/NO/cGMP/protein kinase G (PKG)/ATP-sensitive K+ channel pathway (Ferreira et al., 1991; Cunha et al., 2010).
NO is produced from the conversion of L-arginine to L-citruline by NO synthase isoforms and is involved in the regulation of several pathophysiological functions, such as vascular homeostasis, platelet aggregation, fibrinolysis, inflammation and pain (Furchgott and Zawadski, 1980; Furlong et al., 1987; Benjamim et al., 2000; Cunha et al., 2010; Paolocci et al., 2007). Focusing on pain, NO has dual roles in that it can either induce or reduce pain depending on its concentration and locale of production or delivery (Cury et al., 2011). Concerning the antinociceptive effect of NO, evidence shows that in the periphery NO donors or substances that increase neuronal concentrations of NO directly block inflammatory hyperalgesia (Cury et al., 2011). Within nociceptive neurons, NO binds with high affinity to the ferrous heme prosthetic group of soluble guanylate cyclase (sGC), resulting in pronounced stimulation of cGMP production (Griffiths et al., 2003). In turn, cGMP activates cGMP-dependent protein kinase (PKG), which seems to be responsible for the phosphorylation of ATP-sensitive K+ channels causing an increase in current (Cunha et al., 2010). This intracellular signaling pathway causes hyperpolarization of nociceptive neurons counteracting their enhanced excitability during the inflammatory process. This is an important peripheral mechanism of NO, which is activated, for instance, by morphine (Cunha et al., 2010).
NO• (uncharged form of NO) and oxidized nitrogen oxide congeners including peroxynitrite (ONOO−), nitrite (NO2−), nitrate (NO3−), nitrogen dioxide (NO2) and dinitrogen trioxide (N2O3) have attracted considerable attention in physiological and pathophysiological settings. By contrast, nitroxyl (HNO at physiological pH), the one-electron reduced and protonated sibling of NO, has been relatively overlooked (Miranda et al., 2003; Irvine et al., 2008; Wink et al., 1998). Attention to nitroxyl has increased due to its pharmacological effects, mainly in the cardiovascular system. Importantly, nitroxyl has been postulated to have a role as an endothelium-derived relaxing in mesenteric arteries and to function as a hyperpolarizing agent in resistance arteries (Andrews et al., 2009). Similarly to NO, nitroxyl also activates soluble guanylate cyclase (Wanstall et al., 2001; Irvine et al., 2003; Favaloro and Kemp-Harper, 2007; Irvine et al., 2007).
Taking into account the above information, we investigated the impact of the nitroxyl donor Angeli’s salt on inflammatory hyperalgesia induced by a variety of stimuli, and the underlying analgesic mechanism of observed effect.
1.2. Materials and Methods
1.2.1. Animals
A total of 520 male Wistar rats (180–220 g) were housed in temperature-controlled rooms (22–25°C), with access to water and food ad libitum. Experiments were conducted in accordance with the guidelines of International Association for Study of Pain and with the approval of the Ethics Committee of the Faculty of Medicine of Ribeirao Preto, University of Sao Paulo. All effort was made to minimize suffering.
1.2.2. Mechanical Hyperalgesia test
Mechanical hyperalgesia was analyzed as previously described (Ferreira et al., 1978). In this method, a constant pressure of 20 mmHg is applied (via a syringe piston moved by compressed air) to a 15 mm2 area on the dorsal surface of the hindpaw, and discontinued when the rat presented a typical “freezing reaction”. This reaction is comprised of brief apnoea, concomitant with retraction of the head and forepaws and reduction in the escape movements that animals normally make to free themselves from the position imposed by the experimental situation. Usually, the apnoea is associated with successive waves of muscular tremor. For each animal, the latency to the onset of the “freezing reaction” is measured before administration (zero time) and at different times after administration of the hyperalgesic agents. The intensity of mechanical hypersensitivity is quantified as the reduction in the reaction time, calculated by subtracting the value of the second measurement from the first (Ferreira et al., 1978). Reaction time was 31.5 ± 0.1 s (mean ± SEM; n = 36) before injection of the hyperalgesic agents.
1.2.3. TNFα and IL-1β production
Samples of cutaneous plantar tissue were collected 2 h after carrageenin injection and processed for ELISA assay to determine the levels of TNFα and IL-1β (Verri et al., 2010). Results are expressed as pg/100 mg of tissue.
1.2.4. Dorsal root ganglia (DRG) culture
Primary neuron cultures were prepared from the dorsal root ganglia (DRG) of rats as previously described (Cunha et al., 2010). Aliquots of cell suspensions were plated on Matrigel-coated coverslips (25 mm diameter) in 35 mm diameter Petri dishes at a final density of 1 × 106 cells per dish. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere for 24 h (Cunha et al., 2010). These cell cultures were analyzed by confocal microscopy as described below.
1.2.5. DAF-2 Fluorescence Measurements
After incubation of the affixed neurons with 10 µM DAF-2DA in Hanks/Hepes buffer (1 M HEPES on 1 L of Hanks, pH 7.4) for 30 min, optical microscopy was used to assess cell viability. During the experiments, cultures were maintained in the steel chamber at 37°C. The cells were mounted onto the stage of the scanning microscope (Leica TCS SP5 water lens). Digital images of DAF-2DA fluorescence were obtained after excitation at 488 nm (argon laser) using a 515 nm long-pass emission filter. After an initial period of baseline fluorescence collection (0–120 s), the cells were treated with AS (0.1–1 mM), SNAP (0.1–1 mM) (Cunha et al., 1999), L-cysteine (3 mM) (Andrews et al., 2009) or L-cysteine for 3 min before treatment with AS (1 mM) or SNAP (1 mM). The mean fluorescence intensity was determined from a linear measurement of fluorescence of 12–15 cells in randomly chosen fields of each slide, with a total of 3 slides per group.
1.2.6. Image Analysis
For confocal imaging, laser attenuation, pinhole diameter, photomultiplier sensitivity, and offset were kept constant for each set of experiments. Images were captured between 0–1200 s at 30 s intervals, and the changes in fluorescence intensities within regions of interests (circles drawn over cells) were quantitatively analyzed using the Leica SP5 software.
1.2.7. Drugs and stimuli
The following materials were obtained from the sources indicated. PGE2, glybenclamide, KT5823, naloxone, L-cysteine, and SNAP (S-Nitroso-N-acetyl-DL-penicillamine) were obtained from Sigma Aldrich (St Louis, MO, USA), Dulbecco’s modified Eagle medium (DMEM), Hanks, HEPES and the dye 4,5-diaminofluorescein diacetate (DAF-2DA) were obtained from Molecular Probes (St. Louis, MO, USA). Carragenin was obtained from FMC Corporation (Philadelphia, PA, USA). ODQ was purchased from Calbiochem (San Diego, CA, USA). Recombinant rat TNF-α and human IL-1β were acquired from the National Institute for Biological Standards and Control (South Mimms, Hertfordshire, UK). Angeli’s salt (Na2N2O3, sodium trioxodinitrate; AS) was synthesized and utilized as previously described (Smith and Hein, 1960). The stability of stock solutions prepared in 10 mM NaOH and stored at −20°C, was determined from the extinction coefficients at 250 nm (ε of 8000 M−1 cm−1 for Angeli’s salt) (Maragos et al., 1991). Test compounds were diluted in sterile saline, except for Angeli’s salt, which was prepared at 7 mg/ml of 10 mM NaOH, PGE2 at 500 µg/ml of ethanol, ODQ at 8 µg/50µl of DMSO 2% in saline, KT5823 at 1,5 µg/50 µl of DMSO 2% in saline or glybenclamide at 160 µg/50 µl of Tween 80 5% in saline. Angeli’s salt and PGE2 solutions were re-diluted in sterile saline for in vivo administration.
1.2.8. Experimental procedures
Rats were treated with Angeli’s salt (referred to as AS; 17–450 µg/paw, 15 min, diluted in 0.24–6.45 µl of 10 mM NaOH plus saline to complete 50 µL) before stimulus with carrageenin (100 µg/paw), and hyperalgesia was evaluated 3 and 5 h after inflammatory stimulus administration. The dose of 150 µg/paw of AS was chosen for subsequent experiments in which the inflammatory stimuli were LPS (500 ng/paw), TNFα (1 ng/paw), IL-1β (0.5 pg/paw) and PGE2 (100 ng/paw), and mechanical hyperalgesia was evaluated at the indicated time points. In another sets of experiments designed to determine the mechanism of action of AS, rats were treated with naloxone (1 µg/paw), ODQ (8 µg/paw), KT5823 (1.5 µg/paw), glybenclamide (160 µg/paw) or L-cysteine (16.7, 50 and 150 µg/paw) 30 min before AS (150 µg/paw) or SNAP (200 µg/paw) treatment, and the inflammatory stimulus, carrageenin (100 µg/paw), was injected 15 min after AS or vehicle administration. Mechanical hyperalgesia was evaluated 3 and 5 h after carrageenin injection. In the last series of experiments, dorsal root ganglia neurons cultures were treated with 0.1–1 mM of AS or SNAP (Cunha et al., 1999), L-cysteine (3 mM) (Andrews et al., 2009) or L-cysteine for 3 min before the treatment with the same concentration of AS or SNAP (1 mM) followed by confocal analysis in neurons.
1.2.9. Statistical analyses
The results are representative of two independent experiments and are presented as the mean ± SEM (n = 5 per group in each individual experiment). One-way ANOVA followed by Tukey’s t-test was performed to evaluate the differences between responses. Statistical differences were considered to be significant at P < 0.05.
1.3. Results
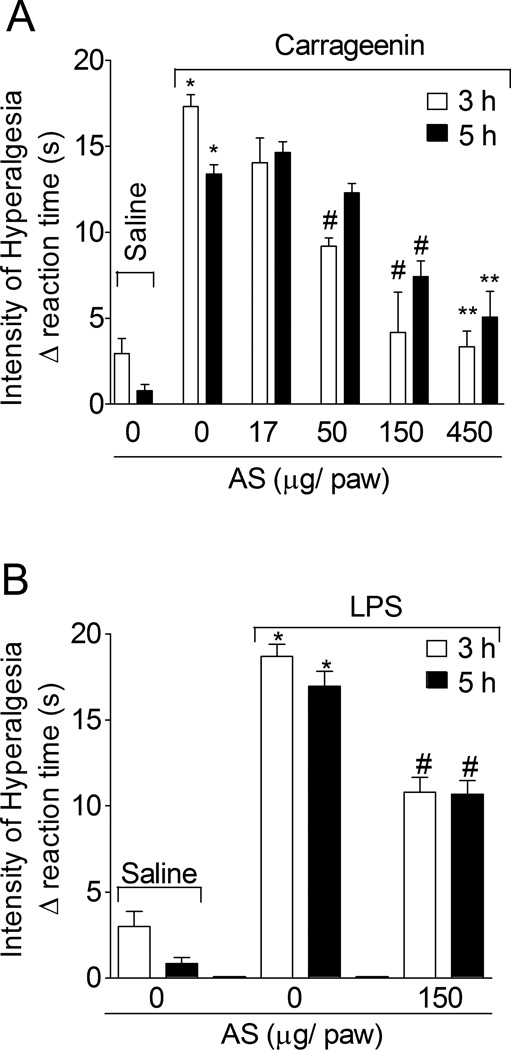
1.3.1. The nitroxyl donor, Angeli’s salt (AS), inhibits carrageenin- and lipopolysaccharide (LPS)-induced mechanical hyperalgesia
Rats were treated with AS (17–450 µg/paw, 15 min) or vehicle (6.45 µl of 10 mM NaOH plus saline to complete 50 µL) before carrageenin (100 µg/paw) stimulus, and the intensity of mechanical hyperalgesia was evaluated after 3 and 5 h (Fig. 1A). AS doses of 50, 150 and 450 µg/pawat 3 h and doses of 150 and 450 µg/pawat 5 h significantly inhibited carrageenin-induced mechanical hyperalgesia. A dose dependence was observed although the differences between 150 and 450 µg/pawwere not significant (Fig. 1A). Therefore, a dose of 150 µg/pawwas selected for subsequent experiments. Rats were treated with AS (150 µg/paw, 15 min) or vehicle (2.15 µl of 10 mM NaOH plus saline to dilute to 50 µL) before LPS (500 ng/paw) injection, and mechanical hyperalgesia was determined at 3 and 5 h (Fig. 1B). AS inhibited LPS-induced mechanical hyperalgesia at both time points.
Fig. 1. Angeli’s salt inhibits carrageenin- and LPS-induced mechanical hyperalgesia.
Panel A - Rats were treated with AS (17–450 µg/paw, 15 min) or vehicle (6.45 µl of NaOH 10 mM plus saline to dilute to 50 µL) before carrageenin (100 µg/paw) stimulus. Panel B - Rats were treated with Angeli’s salt (150 µg/paw, 15 min) or vehicle (2.15 µl of NaOH 10 mM plus saline to dilute to 50 µL) before lipopolysaccharide (LPS) (500 ng/paw) stimulus. Mechanical hyperalgesia was evaluated 3 and 5 h after stimulus. Vehicles were indicated as zero. n = 6 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (indicated as zero), #P<0.05 compared with positive + vehicle control group. **P<0.05 compared with AS dose of 50 µg•paw−1. One-way ANOVA followed by Tukey’s t test.
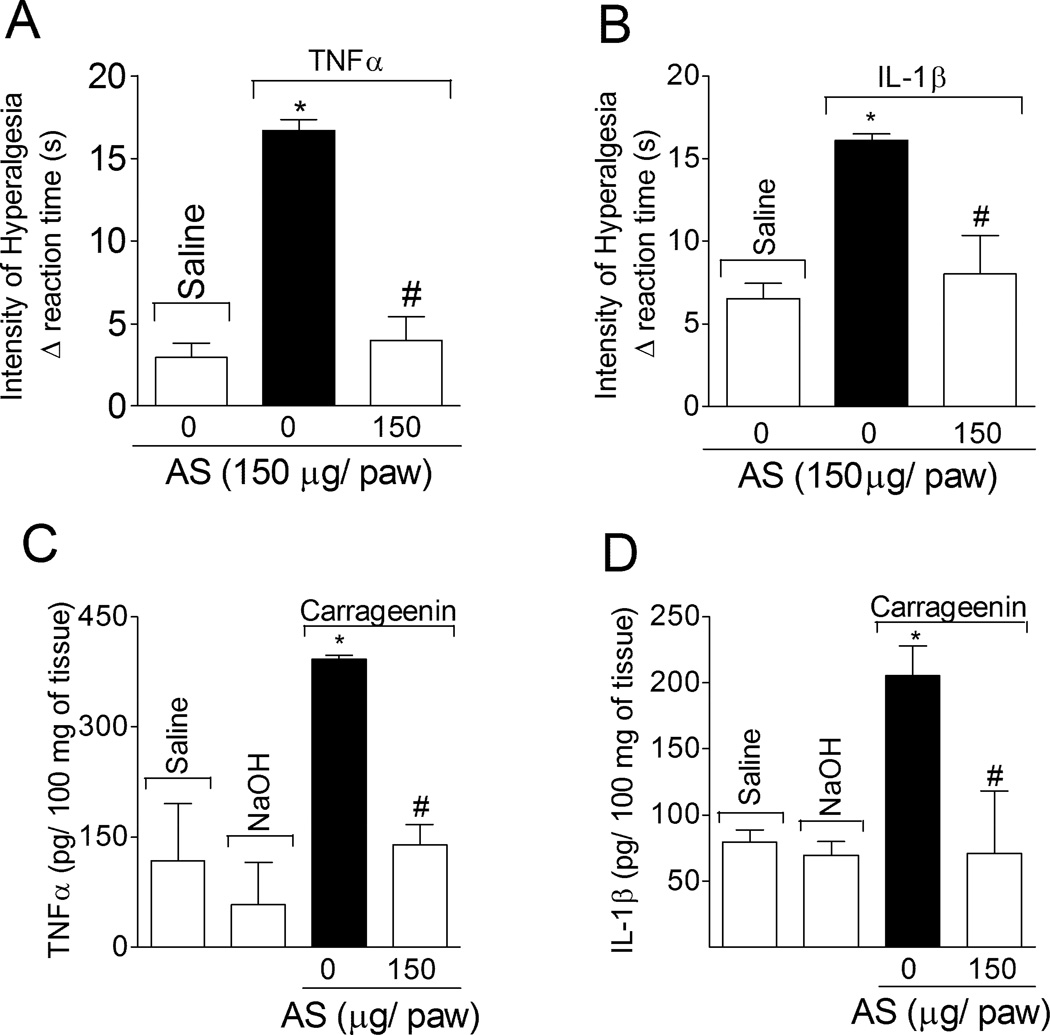
1.3.2. AS inhibits cytokine (TNFα and IL-1β)-induced hyperalgesia and carrageenin-induced cytokine production
Rats were treated with AS or vehicle (as in Fig. 1B) before TNFα (1 ng/paw; Fig. 2A) or IL-1β (0.5 pg/paw; Fig. 2B) stimulus, and mechanical hyperalgesia was evaluated after 3 h. AS inhibited TNFα- and IL-1β-induced hyperalgesia (Fig. 2A and 2B, respectively). In another set of experiments, rats were treated with AS or vehicle (as in Fig. 1B) before carrageenin (100 µg/paw) stimulus, and paw skin samples were collected 2 h after for cytokine level determination by ELISA. Local treatment with AS reduced carrageenin-induced TNFα (Fig. 2C) and IL-1β (Fig. 2D) production.
Fig. 2. AS inhibits cytokine-induced hyperalgesia and carrageenin-induced cytokine production.
Rats were treated with AS (150 µg/paw, 15 min) or vehicle (as in Fig. 1B) before stimulus with TNFα (1 ng/paw; Panel A) or IL-1β (0.5 pg/paw; Panel B). Mechanical hyperalgesia was evaluated 3 h after TNFα or IL-1β stimulus. The samples of subcutaneous plantar tissue were collected 2 h after carrageenin stimulus for TNFα (Panel C) and IL-1 β (Panel D) levels determination by ELISA. Vehicles were indicated as zero. n = 5 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (indicated as zero), #P<0.05 compared with positive + vehicle control group. One-way ANOVA followed by Tukey’s t test.
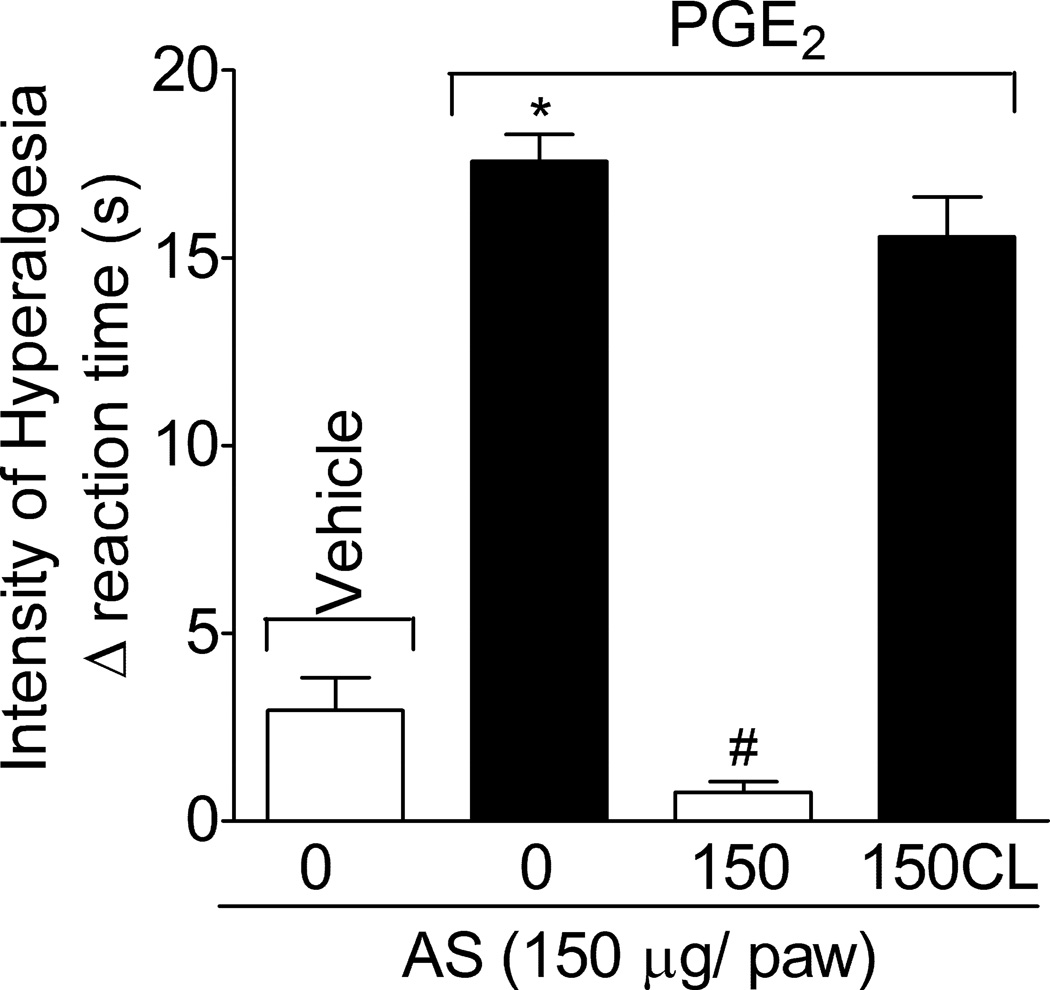
1.3.3. AS inhibits PGE2-induced mechanical hyperalgesia: local effect
Rats were treated with AS or vehicle (as in Fig. 1B) in the ipsilateral paw before PGE2 administration (100 ng/paw; Fig. 3), and the intensity of mechanical hyperalgesia was evaluated after 3 h, which is the peak of PGE2-induced mechanical hyperalgesia. AS significantly inhibited PGE2-induced hyperalgesia. AS treatment was also performed in the contra-lateral paw (150CL) to PGE2 stimulus (same dose, Fig. 3). The 150CL treatment with AS did not affect PGE2-induced hyperalgesia, indicating that the AS has local effects at the dose used.
Fig. 3. AS inhibits PGE2-induced hyperalgesia, local effect.
Rats were treated with AS or vehicle (as in Fig. 1B) in the ipsilateral or contra-lateral (CL) paw to PGE2 (100 ng•paw−1) stimulus. Mechanical hyperalgesia was evaluated 3 h after stimulus. Vehicles were indicated as zero. n = 6 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (indicated as zero), #P<0.05 compared with positive + vehicle control group. One-way ANOVA followed by Tukey’s t test.
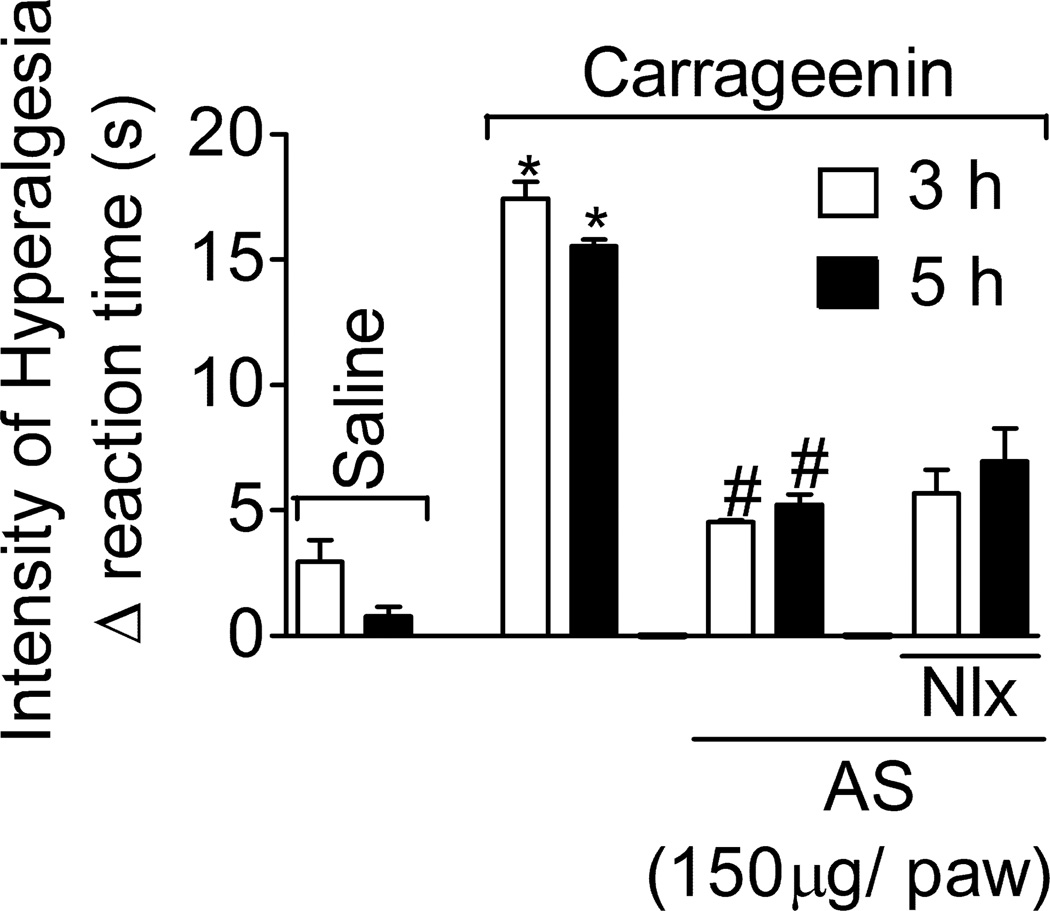
1.3.4. Naloxone does not affect AS inhibition of carrageenin-induced hyperalgesia
Rats were treated with naloxone (Nlx, opioid receptor antagonist, 1 mg/kg, i.p., 45 min) and AS (as in Fig. 1B) or vehicles before carrageenin (100 µg/paw) stimulus. Naloxone did not affect AS inhibition of carrageenin-induced mechanical hyperalgesia at 3 and 5 h (Fig. 4). Naloxone did not affect the nociceptive threshold per se (data not shown).
Fig. 4. Naloxone treatment does not affect AS inhibition of carrageenin-induced hyperalgesia.
Rats were treated with naloxone (Nlx, 1 mg/kg, i.p., diluted in saline) 30 min before AS or vehicle (as in Fig. 1B) treatment, and after an additional 15 min rats received the carrageenin (100 µg/paw) stimulus. Mechanical hyperalgesia was evaluated 3 and 5 h after stimulus. Vehicles were indicated as zero. n = 6 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (vehicle not indicated), #P<0.05 compared with positive + vehicle control group (vehicle not indicated). One-way ANOVA followed by Tukey’s t test.
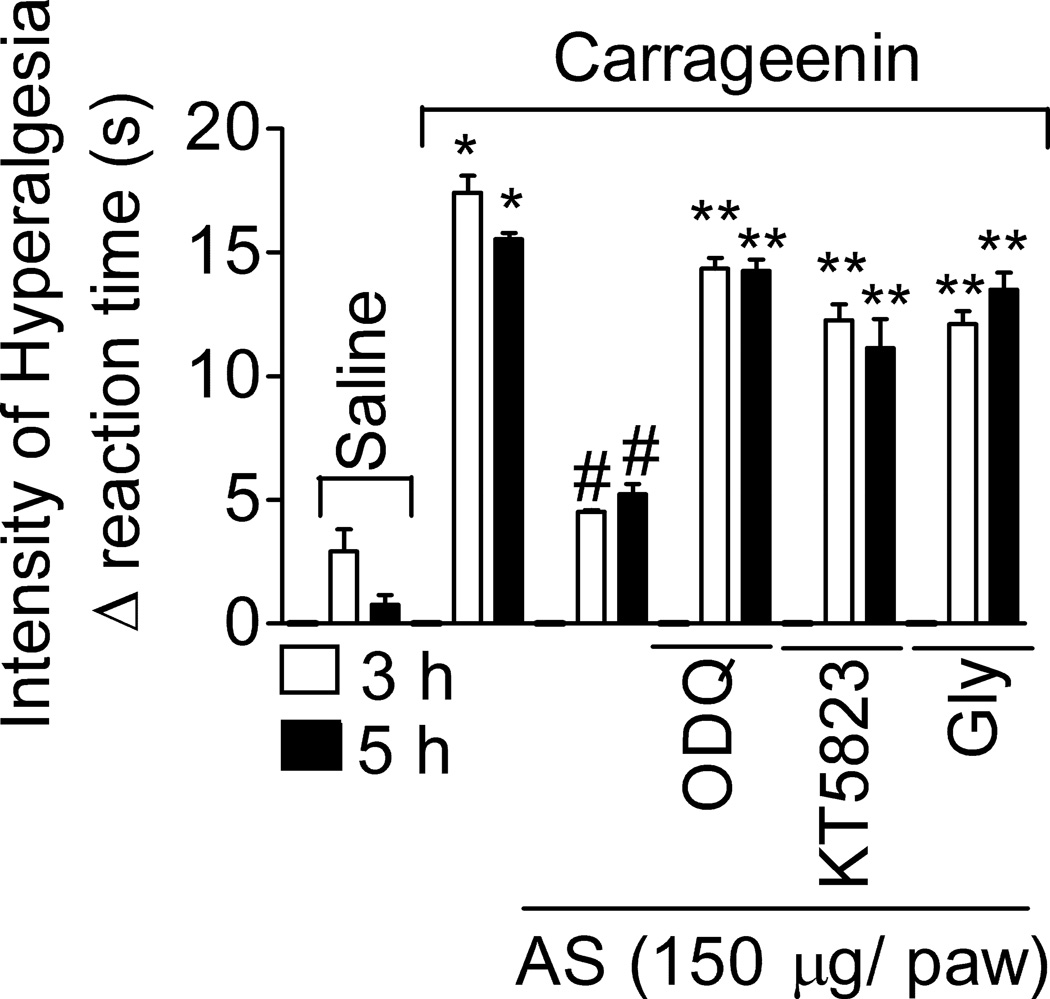
1.3.5. AS reduces mechanical inflammatory hyperalgesia by activating the GMPc/PKG/ATP-sensitive K+ channel signaling pathway
Rats were treated with ODQ (a soluble guanylate cyclase inhibitor, 8 µg/paw, diluted in 50 µl of DMSO 2% in saline), KT5823 (an inhibitor of PKG, 1.5 µg/paw, diluted in 50 µl of DMSO 2% in saline), glybenclamide (Gly; an ATP-sensitive K+ channels blocker, 160 µg/paw, diluted in 50 µl of Tween 80 5% in saline) or vehicle (Fig. 5) for 45 min, and AS (150 µg/paw) or vehicle for 15 min, before carrageenin administration (100 µg•paw−1). AS inhibition of carrageenin-induced hyperalgesia was prevented by ODQ, KT5823 and glybenclamide treatments at 3 and 5 h (Fig. 5). Control treatments with ODQ, KT5823 or glybenclamide in rats that received saline i.pl. did not alter the nociceptive threshold of rats (data not shown).
Fig. 5. AS inhibition of carrageenin-induced mechanical hyperalgesia depends on the cGMP/PKG/ATP-sensitive K+ channel pathway.
Rats were treated with ODQ (8 µg/paw, diluted in DMSO2% in saline), KT5823 (1,5 µg/paw, diluted in DMSO2% in saline) or glybenclamide (Gly; 160 µg/paw, diluted in Tween 80 5% in saline) 30 min before AS or vehicle (as in Fig. 1B) treatment, and after an additional 15 min rats received the carrageenin (100 µg/paw) stimulus. Mechanical hyperalgesia was evaluated 3 and 5 h after stimulus. Vehicles were indicated as zero. n = 6 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (vehicle not indicated), #P<0.05 compared with positive + vehicle (vehicle not indicated) control group. **P<0.05 compared with AS treatment. One-way ANOVA followed by Tukey’s t test.
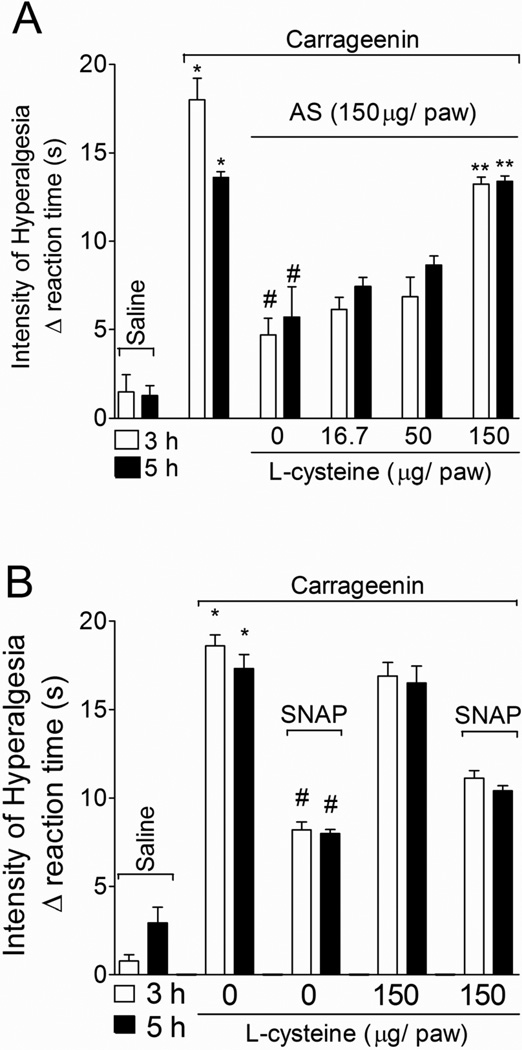
1.3.6. L-cysteine prevents AS, but not SNAP inhibition of carrageenin-induced hyperalgesia
Rats were treated with L-cysteine (a nitroxyl scavenger, 16.7–150 µg/paw, 30 min; Pino and Feelisch, 1994) and AS (150 µg/paw, 15 min) or vehicle before carrageenin (100 µg/paw) stimulus. Mechanical hyperalgesia was evaluated 3 and 5 h after carrageenin administration (Fig. 6A). L-cysteine treatment prevented AS-mediated inhibition of carrageenin-induced mechanical hyperalgesia in a dose-dependent manner (Fig. 6A). In another set of experiments, rats were treated with L-cysteine (150 µg/paw) or vehicle 30 min before SNAP (an NO+ donor, 200 µg/paw; Jin et al., 2011) administration, and after an additional 30 min, rats received carrageenin (100 µg/paw) stimulus (Fig. 6B). SNAP inhibited carrageenin-induced mechanical hyperalgesia at 3 and 5 h (Fig. 6B), but was not sensitive to L-cysteine treatment at the dose of 150 µg/paw. Therefore, the scavenger activity of L-cysteine was selective to NO− without affecting NO• in vivo.
Fig. 6. L-cysteine prevents AS, but not SNAP inhibition of carrageenin-induced mechanical hyperalgesia.
A - Rats were pre treated with L-cysteine (16.7, 50 and 150 µg/paw, 30 min) before AS or vehicles (as in Fig. 1B), and after additional 15 min rats received carrageenin (100 µg/paw) stimulus. B - Rats were pre treated with SNAP (200 µg/paw, 30 min) before L-cysteine or vehicles (as above), and after additional 15 min rats received carrageenin (100 µg/paw) stimulus. Mechanical hyperalgesia was evaluated 3 and 5 h after stimulus. Vehicles were indicated as zero. n = 6 rats per group per experiment, representative of two separated experiments. *P<0.05 compared with the negative control – saline + vehicle (vehicle not indicated), #P<0.05 compared with positive + vehicle (vehicle not indicated) control group. **P<0.05 compared with AS treatment and the lower dose of L-cysteine. One-way ANOVA followed by Tukey’s t test.
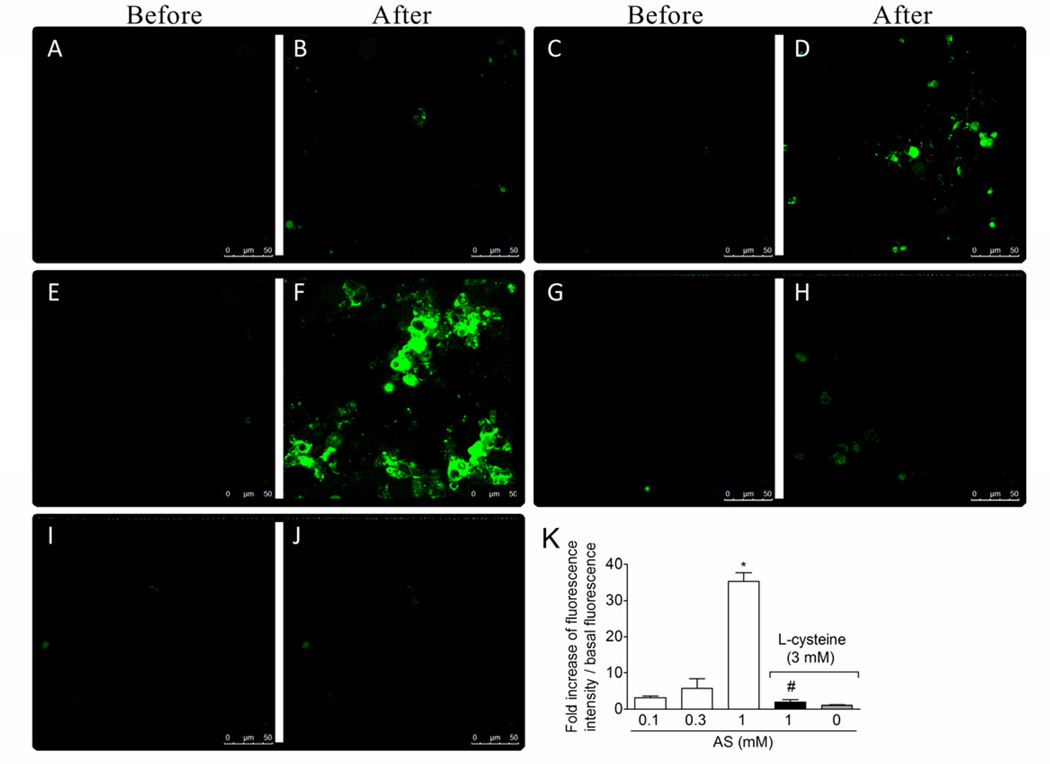
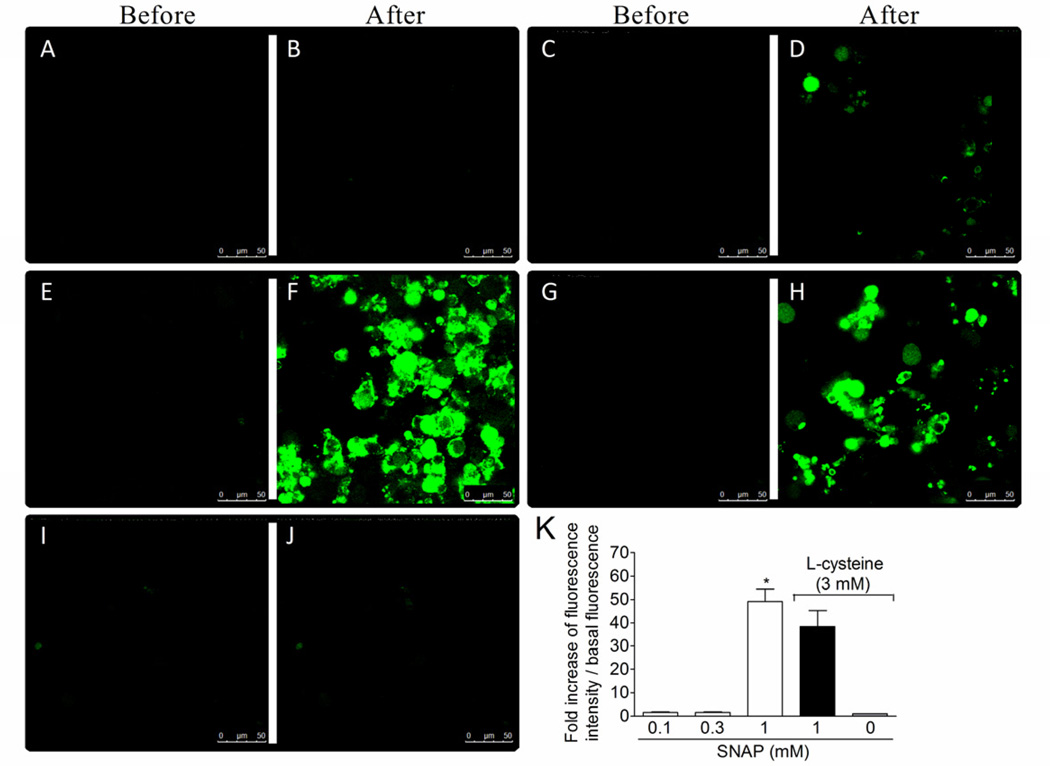
1.3.7. Effect of the nitroxyl scavenger L-cysteine in AS and SNAP donation of nitric oxide in dorsal root ganglia (DRG) neurons
DAF-2DA (4,5-diaminofluorescein diacetate) is a cell permeable reagent that undergoes hydrolysis of the diacetate groups by cytosolic esterases resulting in sequestration of DAF-2 inside the cell. DAF-2 reacts with the autoxidation product of both NO (Kojima et al., 1998) and nitroxyl (Espey et al., 2002). In the later case, this process is independent of NO• and ONOO− (Espey et al., 2002). Nitrogen oxides converts the non-fluorescent dye, DAF-2, to its fluorescent triaole derivative, DAF-2T. DRG neurons were treated with DAF-2DA and washed. Basal fluorescence (before) was determined as shown in representative images (Fig. 7A, 7C, 7E, 7G, and 7I) followed by novel measures (after) of fluorescence during 1200 s at 30 s intervals after adding AS (0 –1 mM) to the medium (Fig. 7A–7F). There was a concentration-dependent increase of fluorescence with significant increase with 1 mM AS (Fig. 7B, 7D and 7F). L-cysteine (3 mM, Andrews et al., 2009) treatment 3 min before AS (1 mM) reduced the fluorescence to control levels as shown in the representative image (Fig. 7H) and fluorescence analysis (Fig. 7K). Using the same protocol of Fig. 7, but with SNAP as a donor of NO+ (Jin et al., 2011) it was observed that SNAP induced significant increase of fluorescence at 1 mM (Fig. 8F) compared to the lower concentrations of SNAP (Fig. 8A–D) and background fluorescence (Fig. 8E). However, L-cysteine did not affect SNAP-induced increase of fluorescence (Fig. 8G–H). L-cysteine alone did not alter the intracellular basal levels of nitrogen oxides (Fig. 7I–J and 8I–J). Therefore, DAF-2DA detects NO with different charges such as NO+ and HNO/NO−, and AS induces the delivery of HNO/NO− in neurons corroborating its possible neuronal effect.
Fig. 7. AS increases nitroxyl levels in neurons.
The basal fluorescence of DAF-2DA treated neurons was captured (0 – 30 s) as shown in the representative images in panels A, C, E, G and I. DRG neurons were treated with L-cystein (nitroxyl scavenger, 3 mM) or medium 3 min before AS treatment (01.-1 mM, panels B, D, F, H and K). Fluorescent images were captured during 1200 s at 30 s intervals. Fluorescence values were collected by drawing circles boxes over the cells in each field, which ranged 12 to 15 cells per treatment. Panels A–J show representative images and panel K the image analysis. Vehicles were indicated as zero. The total number of investigated cells ranged from 12 to 15 per slide with a total of 3 slides per group. *P<0.05 compared with the 0.1 and 0.3 mM concentrations of AS, #P<0.05 compared with AS 1 mM group. One-way ANOVA followed by Tukey’s t test.
Fig. 8. L-cysteine does not affect SNAP-induced increase of nitric oxide in neurons.
The basal fluorescence of DAF-2DA treated neurons was captured (0 – 30 s) as shown in the representative images in panels A, C, E, G and I (before). DRG neurons were treated with L-cystein (nitroxyl scavenger, 3 mM) or medium 3 min before SNAP treatment (01.-1 mM, panels B, D, F, H and K). Fluorescent images were captured during 1200 s at 30 s intervals (after). Vehicles were indicated as zero. Fluorescence values were collected by drawing circles boxes over the cells in each field, which ranged from 12 to 15 cells per slide with a total of 3 slides per group. *P<0.05 compared with the 0.1 and 0.3 mM concentrations of SNAP. One-way ANOVA followed by Tukey’s t test.
1.4. Discussion and conclusions
The biological roles of nitroxyl have not been explored to a similar extent as those of NO. Herein, we demonstrated for the first time that the nitroxyl donor Angeli’s salt reduced mechanical hyperalgesia induced by a variety of stimuli such as carrageenin, LPS, cytokines and PGE2 by inhibiting carrageenin-induced cytokine production and triggering a peripheral cGMP/PKG/ATP-sensitive K+ channel antinociceptive signaling pathway in rats.
Generally, either NO or oxidized nitrogen oxides are assumed to mediate biological functions including those of endothelium relaxation, inhibition of platelet aggregation, inflammation and infection, and modulation of pain neurotransmission (Furchgott and Zawadski, 1980; Furlong et al., 1987; Benjamim et al., 2000; Cunha et al., 2010). However, there is also evidence that HNO/NO− may be formed endogenously (Pufahl et al., 1995; Schmidt et al., 1996; Hobbs et al, 1997; Rusche et al., 1998; Adak et al., 2000) and can induce, for instance, vasorelaxation (Wanstall et al., 2001; Irvine et al., 2003).
Indeed, endogenous production of HNO/NO− may occur via NO synthase itself (Hobbs et al., 1994; Schmidt et al., 1996; Rusche et al., 1998), particularly in the absence of the cofactor tetrahydrobiopterin or after oxidation of the intermediate Nω-hydroxy-L-arginine (Fukuto et al., 1992; Pufahl et al., 1995). Oxidation of hydroxylamine has also been shown to produce HNO/NO− (Donzelli et al., 2008). Furthermore, nitroxyl may be produced from the reduction of NO by mitochondrial cytochrome c (Sharpe and Cooper, 1998), xanthine oxidase (Saleem and Ohshima, 2004) or hemoglobin (Gow and Stamler, 1998). Similarly, the reaction of S-nitrosothiols with thiols can yield HNO at physiological pH (Arnelle and Stamler, 1995; Wong et al., 1998). However, whether HNO/NO− is in fact produced endogenously remains to be determined.
The most convenient, well-studied and utilized HNO/NO− donor is Angeli’s salt (Bonner and Ravid, 1975). This donor has been pivotal in the discovery of the pharmacology of HNO (Miranda et al., 2005). Local treatment with AS reduced the hyperalgesia induced by carrageenin, LPS, TNFα, IL-1β and PGE2. Thus, this anti-hyperalgesic effect of the HNO/NO− donor is replicable upon a variety of stimuli. AS also inhibited the carrageenin-induced TNFα and IL-1β production, which are cytokines derived from local non-neuronal cells in the paw tissue (Verri et al., 2006). This indicates that AS might present non-neuronal effects.
In addition, considering that PGE2 is a directly sensitizing mediator, the inhibition of PGE2-induced hyperalgesia by AS indicates that HNO also presents a neuronal effect similar to the assumed role of NO. Corroborating a neuronal effect, AS induced an increase of fluorescence in DAF-2DT treated neurons, which was reduced by the HNO/NO− scavenger L-cysteine. In turn, L-cysteine did not affect the fluorescence induced by SNAP confirming that HNO/NO− effects were being observed with AS. These in vitro results corroborate the in vivo data demonstrating that L-cysteine inhibits AS, but not SNAP-induced anti-hyperalgesia. The down-modulation of neuronal sensitization by NO is an important mechanism activated by clinically and experimentally available drugs and mediators such as morphine, atorvastatin, kaurenoic acid and PGJ2 (Ferreira et al., 1991; Santodomingo-Garzon et al., 2006; Napimoga et al., 2008; Cunha et al., 2010; Mizokami et al., 2012). However, it remains to be determined whether these drugs increase the neuronal levels of HNO/NO−, NO or other more oxidized nitrogen oxides.
HNO/NO− is capable of activating sGC to produce cGMP similarly to NO (Wanstall et al., 2001; Irvine et al., 2003; Favaloro and Kemp-Harper, 2007; Irvine et al., 2007; Irvine et al., 2008; Fukuto et al., 1992), and there are several possible explanations for this activity. First, oxidation of HNO/NO− to NO, which in turn interacts with Fe2+ in the heme group of sGC, would lead to activation of sGC. In fact HNO has been shown to be oxidized in cultured endothelial cells in the presence of superoxide dismutase (Zeller et al., 2009). However, AS decomposes to only 1–2% of NO• comparing to 98–99% of HNO/NO− (Zeller et al., 2009). Moreover, there is reduction of superoxide dismutase activity in carrageenin-induced paw inflammation (Wang et al., 2004) suggesting an inadequate environment for AS decomposition to NO•. The second explanation (NO•-independent mechanism) suggests that HNO/NO− could be directly activating the oxidized form of sGC containing Fe3+ which is NO•-insensitive (Irvine et al., 2008). The second explanation takes into account that HNO/NO− and NO• present different activities in ex-vivo pharmacological and functional studies in isolated organ bath. For instance, scavenging NO• with hydroxocobalamin or HNO/NO− with L-cysteine and 4-aminopyridine decreases the sensitivity to acethylcoline (Ach)-induced vasorelaxation (Andrews et al, 2009). In rat mesenteric arteries, blocking the effects of endothelium-derived hyperpolarizing factor (EDHF) with charybdotoxin and apamin decreases ACh-mediated relaxation unmasking a nitrogen oxide-dependent component, mediated equally by HNO and NO since hydroxocobalamin and L-cysteine in combination abolished vasorelaxation to ACh. Furthermore, ACh-evoked hyperpolarizations, resistant to EDHF inhibition, are virtually abolished by 4-aminopyridine, indicating an HNO role (Andrews et al., 2009). Further supporting that the effects of HNO are not explained solely by simple conversion to NO, HNO is known for activating voltage-dependent K+ (Kv) channels (Irvine et al., 2003) in the same vascular bed while NO activates calcium-activated K+ channels (KCa) (Mistry and Garland, 1998; Sampson et al., 2001).
Importantly, we report that the HNO donor AS inhibited mechanical hyperalgesia activating the cGMP/PKG/ATP-sensitive K+ channel pathway using similar tools (ODQ, KT5823 and glybenclamide) as in previous studies demonstrating the anti-hyperalgesic effect and mechanism of NO (Ferreira et al., 1991; Tonussi et al., 1994; Sachs et al., 2004; Cunha et al., 2010). Thus, it remains to be determined whether NO and/or HNO are/is the molecule(s) responsible for the peripheral analgesic effects of morphine, diclofenac, dipyrone, atorvastatin and PGJ2 (Lorenzetti and Ferreira, 1985; Ferreira et al., 1991; Tonussi and Ferreira, 1992; Santodomingo-Garzon et al., 2006; Cunha et al., 2010). Considering the relevance of morphine as an analgesic drug, and depending on the relative roles of NO and/or HNO/NO− in its peripheral antihyperalgesic mechanism, it might be worthy developing novel HNO/NO− or mixed HNO/NO−/NO donors to bypass the opioid receptor, and avoid its desensitization that occurs following prolonged treatment with non-internalizable opioid agonists such as morphine (Koch and Höllt, 2008), and therefore, maintaining controlled analgesia with reduced side effects. In this sense, the analgesic effect of AS was not altered by the opioid receptor antagonist naloxone, confirming that HNO/NO− induces analgesia in an opioid receptor and endogenous opioid release independent fashion.
To conclude, we demonstrated that the nitroxyl donor Angeli’s salt inhibits mechanical hyperalgesia by inhibiting cytokine production and triggering the antinociceptive cGMP/PKG/ATP-sensitive K+ channel signaling pathway in vivo.
Highlights.
-
-
Angeli’s salt is a donor of HNO/NO−
-
-
This is the first study showing the analgesic effect of HNO/NO− using Angeli’s salt
-
-
Angeli’s salt inhibited the mechanical hyperalgesia induced by varied stimuli
-
-
Its mechanisms include peripheral inhibition of cytokine production
-
-
And activation of the the cGMP/PKG/ATP-sensitive K+ channel signaling pathway
Acknowledgments
The authors gratefully acknowledge the technical assistance Sergio R. Rosa, Giuliana B. Francisco, Larissa S. Ferrari, Renata M. Martinez, Miriam S.N. Hohmann, and Talita P. Domiciano. This work was supported by Brazilian grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Fundação Araucária, FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and Decit/SCTIE/MS (Departamento de Ciência e Tecnologia da Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Ministério da Saúde) intermediated by CNPq and support of Fundação Araucária, Brazil. Support by the National Institutes of Health (R01-GM076247) to KMM is also acknowledged.
Abbreviations
- AS
Angeli’s salt
- HNO/NO−
Nitroxyl
- PGE2
Prostaglandin E2
- TNFα
Tumor necrosis factor alpha
- LPS
lipopolysaccharide
- IL-1β
Interleukin-1 beta
- sGC
soluble guanylate cyclase
- PKG
protein kinase G
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Adak S, Wang Q, Stuehr DJ. Arginine conversion of nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. J. Biol. Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J. Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnelle DR, Stamler JS. NO+, NO•, NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J. Infect. Dis. 2000;182:214–223. doi: 10.1086/315682. [DOI] [PubMed] [Google Scholar]
- Bonner FT, Ravid B. Thermal decomposition of oxyhyponitrite (sodium trioxodinitrate(II) in aqueous solution. Inorg. Chem. 1975;14:558–563. [Google Scholar]
- Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Jr, Funez MI, Dias QM, Schivo IR, Domingues AC, Sachs D, Chiavegatto S, Teixeira MM, Hothersall JS, Cruz JS, Cunha FQ, Ferreira SH. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. 2010;107:4442–4447. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha FQ, Teixeira MM, Ferreira SH. Pharmacological modulation of secondary mediator systems--cyclic AMP and cyclic GMP--on inflammatory hyperalgesia. Br. J. Pharmacol. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011;25:243–254. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Donzelli S, Espey MG, Flores-Santana W, Switzer CH, Yeh GC, Huang J, Stuehr DJ, King SB, Miranda KM, Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic. Biol. Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Miranda KM, Thomas DD, Wink DA. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic. Biol. Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J. Physiol. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc. Res. 2007;73:587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat. New. Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991;201:121–122. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Correa FMA. Central and peripheral antialgesic action of aspirin-like drugs. Eur. J. Pharmacol. 1978;53:39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- Fukuto JM, Wallace GC, Hszieh R, Chaudhuri G. Chemical oxidation of N-hydroxyguanidine compounds. Release of nitric oxide, nitroxyl and possible relationship to the mechanism of biological nitric oxide generation. Biochem. Pharmacol. 1992b;43:607–613. doi: 10.1016/0006-2952(92)90584-6. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furlong B, Henderson AH, Lewis MJ, Smith JA. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br. J. Pharmacol. 1987;90:687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J. Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- Griffiths C, Wykes V, Bellamy TC, Garthwaite J. A new and simple method for delivering clamped nitric oxide concentrations in the physiological range: application to activation of guanylyl cyclase-coupled nitric oxide receptors. Mol. Pharmacol. 2003;64:1349–1356. doi: 10.1124/mol.64.6.1349. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends. Pharmacol. Sci. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Fukuto JM, Ignarro LJ. Formation of free nitric oxide from l-arginine by nitric oxide synthase: direct enhancement of generation by superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Kemp-Harper BK. NO− activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49:885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends. Pharmacol. Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Jin XG, Chen SR, Cao XH, Li L, Pan HL. Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J. Biol. Chem. 2011;286:33190–33202. doi: 10.1074/jbc.M111.270967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Höllt V. Role of receptor internalization in opioid tolerance and dependence. Pharmacol. Ther. 2008;117:199–206. doi: 10.1016/j.pharmthera.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 1998;70:2446–2454. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Lorenzetti BB, Ferreira SH. Mode of analgesic action of dipyrone: direct antagonism of inflammatory hyperalgesia. Eur. J. Pharmacol. 1985;114:375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KM, Nagasawa HT, Toscano JP. Donors of HNO. Curr. Top. Med. Chem. 2005;5:649–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami SS, Arakawa NS, Ambrosio SR, Zarpelon AC, Casagrande R, Cunha TM, Ferreira SH, Verri WA., Jr Kaurenoic Acid from Sphagneticola trilobata Inhibits Inflammatory Pain: Effect on Cytokine Production GMP–Protein Kinase G–ATP-Sensitive Potassium Channel Signaling Pathway and Activation of the NO–Cyclic. J. Nat. Prod. 2012;75:896–904. doi: 10.1021/np200989t. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, Verri WA, Jr, Cunha FQ, Ferreira SH. 15d-prostaglandin J2 inhibits inflammatory hyperalgesia: involvement of peripheral opioid receptor. J. Pharmacol. Exp. Ther. 2008;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. The pharmacology of nitroxyl (HNO) and its therapeutic potential: Not just the janus face of NO. Pharmacology & Therapeutics. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino RZ, Feelisch M. Bioassay discrimination between nitric oxide (NO.) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- Pufahl RA, Wishnok JS, Marletta MA. Hydrogen peroxide supported oxidation of NG-hydroxyl-L-arginine by nitric oxide synthase. Biochemistry. 1995;34:1930–1941. doi: 10.1021/bi00006a014. [DOI] [PubMed] [Google Scholar]
- Rusche KM, Spiering MM, Marletta MA. Reactions catalyzed by tetrahydrobiopterin-free nitric oxide synthase. Biochemistry. 1998;37:15503–15512. doi: 10.1021/bi9813936. [DOI] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hyperalgesia: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc. Natl. Acad. Sci. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Ohshima H. Xanthine oxidase converts nitric oxide to nitroxyl that inactivates the enzyme. Biochem. Biophys. Res. Commun. 2004;315:455–462. doi: 10.1016/j.bbrc.2004.01.081. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Plane F, Garland CJ. Involvement of cyclic GMP and potassium channels in relaxation evoked by the nitric oxide donor, diethylamine NONOate, in the rat small isolated mesenteric artery. Naunyn. Schmiedebergs. Arch. Pharmacol. 2001;364:220–225. doi: 10.1007/s002100100453. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Garzón T, Cunha TM, Verri WA, Jr, Valério DA, Parada CA, Poole S, Ferreira SH, Cunha FQ. Atorvastatin inhibits inflammatory hyperalgesia. Br. J. Pharmacol. 2006;149:14–22. doi: 10.1038/sj.bjp.0706836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HHW, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. NO• from NO synthase. Proc. Natl. Acad. Sci. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MA, Cooper CE. Reactions of nitric oxide with mitochondrial cytochrome c: a novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 1998;332:9–19. doi: 10.1042/bj3320009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PAS, Hein GE. The Alleged Role of Nitroxyl in Certain Reactions of Aldehydes and Alkyl Halides. J. Am. Chem. Soc. 1960;82:5731–5746. [Google Scholar]
- Tonussi CR, Ferreira SH. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain. 1992;48:421–427. doi: 10.1016/0304-3959(92)90095-S. [DOI] [PubMed] [Google Scholar]
- Tonussi CR, Ferreira SH. Mechanism of diclofenac analgesia: direct blockade of inflammatory sensitization. Eur. J. Pharmacol. 1994;251:173–179. doi: 10.1016/0014-2999(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Valério DA, Ferreira FI, Cunha TM, Alves-Filho JC, Lima FO, De Oliveira JR, Ferreira SH, Cunha FQ, Queiroz RH, Verri WA., Jr Fructose-1,6-bisphosphate reduces inflammatory pain-like behaviour in mice: role of adenosine acting on A1 receptors. Br. J. Pharmacol. 2009;158:558–568. doi: 10.1111/j.1476-5381.2009.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hyperalgesic role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, Oliveira FR, Teixeira MM, Silva JS, McInnes IB, Ferreira SH, Louzada-Junior P, Liew FY, Cunha FQ. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 2010;69:1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR. Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br. J. Pharmacol. 2001;134:463–472. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Feelisch M, Fukuto J, Chistodoulou D, Jourd'heuil D, Grisham MB, Vodovotz Y, Cook JA, Krishna M, DeGraff WG, Kim S, Gamson J, Mitchell JB. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 1998;351:66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- Wong PSY, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, et al. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- Zeller A, Wenzl MV, Beretta M, Stessel H, Russwurm M, Koesling D, Schmidt K, Mayer B. Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli's salt. Mol. Pharmacol. 2009;76:1115–1122. doi: 10.1124/mol.109.059915. [DOI] [PubMed] [Google Scholar]