Abstract
Background
The pathogenesis of several common gastric motility diseases and functional gastrointestinal disorders remains essentially unexplained. Gastric wall biopsies that include the muscularis propria to evaluate the enteric nervous system, interstitial cells of Cajal and immune cells can provide important insights to our understanding of the etiology of these disorders.
Objectives
To determine 1. Technical feasibility, reproducibility and safety of performing a full thickness gastric biopsy (FTGB) using a submucosal endoscopy with mucosal flap (SEMF) technique; 2. Technical feasibility, reproducibility and safety of tissue closure using an endoscopic suturing device; 3. Ability to identify myenteric ganglia in resected specimens; 4. Long-term safety.
Design
Single center, pre-clinical survival study.
Setting
Animal research laboratory – Developmental Endoscopy Unit.
Subjects
Twelve domestic pigs.
Interventions
Animals underwent a SEMF procedure with gastric muscularis propria resection. The resultant offset mucosal entry site was closed using an endoscopic suturing device. Animals were survived for 2 weeks.
Main Outcome Measurements
1. Technical feasibility, reproducibility and safety of the procedure 2. Clinical course of the animals 3. Histological and immunochemical evaluation of the resected specimen to determine if myenteric ganglia were present in the sample.
Results
FTGB was achieved using the SEMF technique in all 12 animals. The offset mucosal entry site was successfully closed using the suturing device in all animals. Mean resected tissue size was 11 mm. Mean total procedure time was 61 mins with 2–4 interrupted sutures placed per animal. Histology showed musclaris propria and serosa confirming full thickness resections in all animals. Myenteric ganglia were visualized in 11/12 animals. The clinical course was uneventful. Repeat endoscopy and necropsy at 2 weeks showed absence of ulceration at either the mucosal entry sites or overlying the more distal muscularis propria resection sites. There was complete healing of the serosa in all animals with minimal single band adhesions in 5/12 animals. Retained sutures were present in 10/12 animals.
Limitations
Animal experiment.
Conclusion
FTGB using the SEMF technique and endoscopic suturing device is technically feasible, reproducible and safe. The larger tissue specimens will allow for improved analysis of multiple cell types.
INTRODUCTION
The etiology of gastrointestinal neuromuscular diseases (GIND), including functional gastrointestinal disorders remains largely unknown. There is recent evidence to support underlying neuromuscular pathologic changes that are heterogeneous and include loss of interstitial cells of Cajal (ICC) and enteric nerves, and presence of inflammatory infiltrates1,2,3,4,5. For example, surgically obtained full thickness gastric biopsy (FTGB) samples from patients with gastroparesis show a decrease in ICC in 50% of patients, an immune infiltrate in 45% and a decrease in nerve fibers6. The presence of an immune infiltrate correlated with nausea and vomiting7. Non-surgically obtained FTGB that include the muscularis propria to evaluate the enteric nervous system, ICCs, immune cells and other related cells are essential to further our understanding of the pathophysiology of these disorders and intervene earlier in the disease process. Mucosal-based biopsies are insufficient as they do not allow evaluation of the deep muscle layers as well as the myenteric plexus present between the inner circular and outer longitudinal muscle layers. Our earlier work with experimental endoscopic techniques was limited by a combination of poor safety data and inadequate tissue sampling8,9. Endoscopic acquisition of FTGB that is safe, effective and minimally invasive would contribute to accurate diagnosis and identification of patients who would benefit from targeted therapy.
The aims of this study were to determine 1. Technical feasibility, reproducibility and safety of performing a FTGB using a submucosal endoscopy with mucosal flap (SEMF) technique; 2. Reliable tissue closure using endoscopic suturing; 3. Ability to identify myenteric ganglia in resected specimens; 4. Long-term safety.
MATERIALS AND METHODS
Experimental Design
This pre-clinical survival study in a pig model was approved by the Institutional Animal Care and Use Committee. Twelve pigs were studied. Each animal underwent a SEMF procedure with FTGB followed by closure of the offset mucosal entry point using an endoscopic suturing device. Animals were survived for two weeks at which time a repeat endoscopy was performed followed by necropsy. The main study outcome measurements were clinical course of animals, technical feasibility, reproducibility and short and long term (2 weeks) safety of the procedure. Data was recorded on the procedure, clinical course and follow-up endoscopy with necropsy. Data analysis was descriptive for this feasibility study.
Animals
Fourteen domestic 35 kg pigs were approved for the study with the intent of using 12. Endoscopy was performed in each animal under general anesthesia. Animals were allowed a liquid diet 48 hours before the procedure and water only ad libidum 24 hours pre-procedure. Antibiotics were administered for 5 days post-procedure (ceftiofur 5 mg/kg IM daily and metronidazole 1g bid PO). Analgesia (buprenorphine hydrochloride 0.03 mg/kg IM) was given immediately post-procedure. Animals were placed on a liquid diet for one day post- procedure, softened food on the second day, and by the third day animals resumed regular feed if tolerated. Each animal received oral proton pump inhibitors (nexium 40mg bid PO) for 7 days post procedure. After a two week survival period, repeat endoscopy was performed. Animals were chemically euthanized (pentobarbital 100mg/ kg IV) immediately after endoscopy and this was followed by necropsy.
Procedure
Step 1 Submucosal endoscopy with mucosal flap (SEMF)
The intent was to create a submucosal tunnel within which a full thickness biopsy that included the muscularis propria would be obtained. The resection site was offset from the mucosal entry point to the submucosal tunnel by approximately 4–5 cm. The overlying mucosal flap created by tunneling through the submucosa was used as a sealant flap protecting the peritoneum from contamination with gastric contents.
A large submucosal fluid cushion (SFC) was initially formed using saline (~ 40mls) injected via a standard needle injection catheter (23 gauge Injector Force; Olympus America, Center Valley, PA). A small incision (<5 mm) was made on the proximal aspect of the SFC using a needle knife, which served as the mucosal entry point. A tunneling balloon – 18mm diameter (Apollo Endosurgery Inc, Austin, TX) was inserted into the SFC and as the balloon was inflated - Figures 1A and 1B, the unique progressive unfurling of this dilation balloon created a submucosal tunnel revealing muscularis propria. The length of the submucosal tunnel was variable and depended on the length of the balloon used (5–8cm) and degree of balloon inflation.
FIGURE 1.
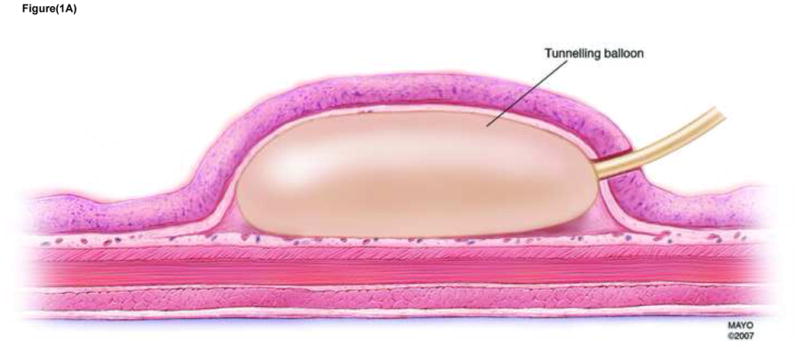
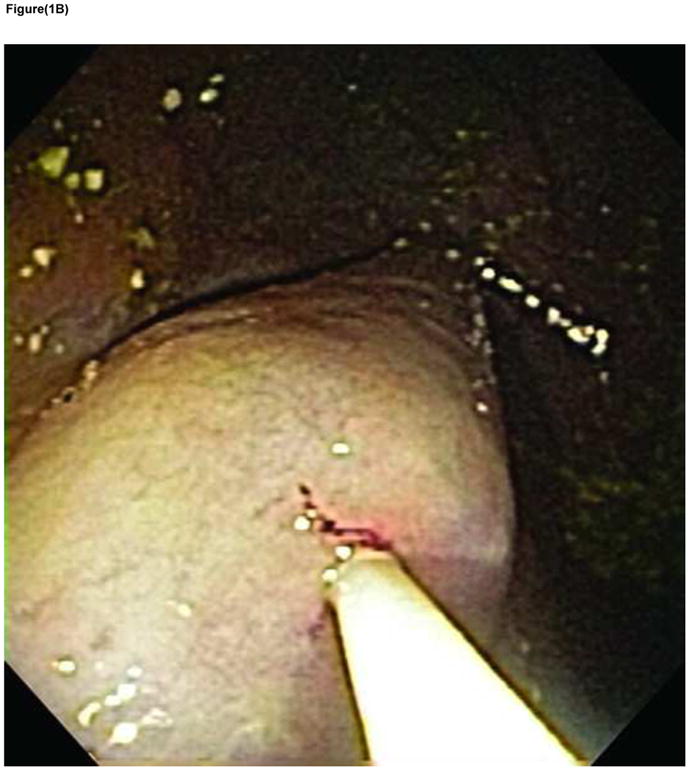
FIGURE 1A Dissection of submucosal space with tunneling balloon
FIGURE 1B Endoscopic view of tunneling balloon dilated within the submucosal space
After the submucosal tunnel was created, a double channel endoscope (2T 160, Olympus America, Center Valley, PA) with an endoscopic mucosal resection (EMR) type cap attached was advanced through the submucosal tunnel. The EMR clear cap maintained tunnel patency and allowed for improved visualization. An endoscopic Doppler probe (VTI Vascular Technology, Nashua, NH) was advanced through the endoscope working channel and placed within this submucosal space to identify any underlying blood vessels. Then a spiral tissue helix (Apollo Endosurgery Inc, Austin, TX) or rat tooth grasping forceps (Olympus America, Center Valley, PA) was used to tent the muscularis propria towards the endoscope and into the cap. Using electrocautery the muscularis propria was resected using a spiral snare (Olympus America, Center Valley, PA) or hexagonal snare (Traxtion US Endoscopy, Mentor, OH). Tissue was retrieved and submitted for analysis.
Step 2 Endoscopic Suturing
The suturing device (Overstitch, Apollo Endosurgery Inc, Austin, TX) was attached to the endoscope and advanced into the stomach through an overtube (US Endoscopy, Mentor, OH) placed within the esophagus. The Overstitch suturing device simulates free-hand suturing and allows for controlled suture placement. The offset mucosal entry point was closed by interrupted polypropelene 3.0 sutures. Closure was considered adequate if the entry site was visibly closed without gaps and there was sustained distension of the gastric lumen with air insufflation suggesting no air leak.
Tissue Samples
The resected tissues were transported over ice to the laboratory in Ham F12 media (Invitrogen, Carlsbad, CA). Resected tissue was measured and sectioned. Hematoxylin and Eosin staining was used to determine which muscle layers were included in the resected specimen, and an antibody to PGP9.5 (Protein Gene Product 9.5) was used as a general neuronal marker, to determine if myenteric neurons were present in the sample8,9.
RESULTS
In order to study 12 animals, 14 pigs were enrolled. Two were excluded early in the study after one death from anesthesia related complications and the other had a superficial mucosal tear over the tunnel. In the former, necropsy was performed and no abnormality detected within the peritoneal cavity with an unremarkable post-biopsy site. In the latter, a muscularis propria resection was not performed but the animal recovered well. In this setting the procedure could be hypothetically repeated after mucosal healing in 4–6 weeks.
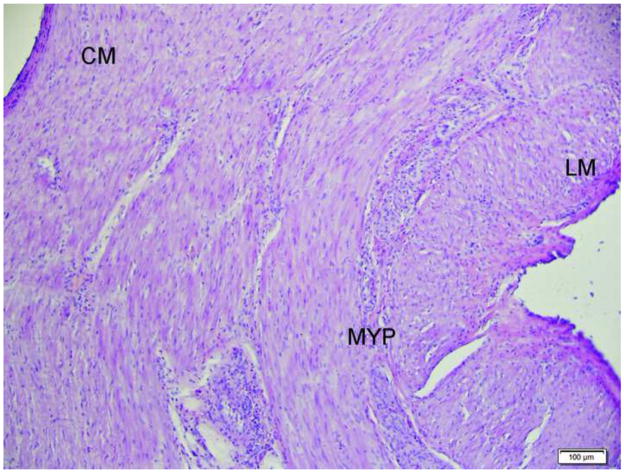
FTGB was achieved using the SEMF technique in all 12 animals. The peritoneal cavity was visualized in each animal providing endoscopic confirmation of a full thickness resection - Figure 2. The offset mucosal entry site was successfully closed in all animals using the endoscopic suturing device - Figure 3 No immediate procedure related complications occurred. Histology showed muscularis propria and serosa confirming full thickness resections in all animals - Figure 4. Multiple myenteric ganglia were visualized in 11/12 animals using PGP9.5 antibodies - Figure 5. In one animal the snare slipped during resection resulting in a smaller sample which was full thickness but without identifiable myenteric ganglia. Mean total procedure time from submucosal injection to completion of suturing was 61 mins (range 40–95mins). In the latter 6 animals, the resected tissues were measured before fixation with a recorded mean long axis length of 11 mm (range 7 to 13mm) – Figure 6. Resections were performed from either the anterior or posterior gastric body. Two to 4 interrupted sutures were placed per animal. Procedure feasibility and safety did not differ with the use of rat tooth grasping forceps (n=6) versus spiral tissue helix (n=6), and spiral snare (n=6) versus hexagonal snare (n=6). The clinical course was uneventful in all animals. Repeat endoscopy at 2 weeks showed stellate scarring at the mucosal entry sites, and absence of mucosal ulceration at either the entry sites or overlying the more distal muscularis propria resection sites - Figure 7 Necropsy after endoscopy showed complete healing of the serosa in all animals with minimal single band adhesions in 5/12 animals - Figure 8. Retained sutures were present in 10/12 animals.
FIGURE 2.
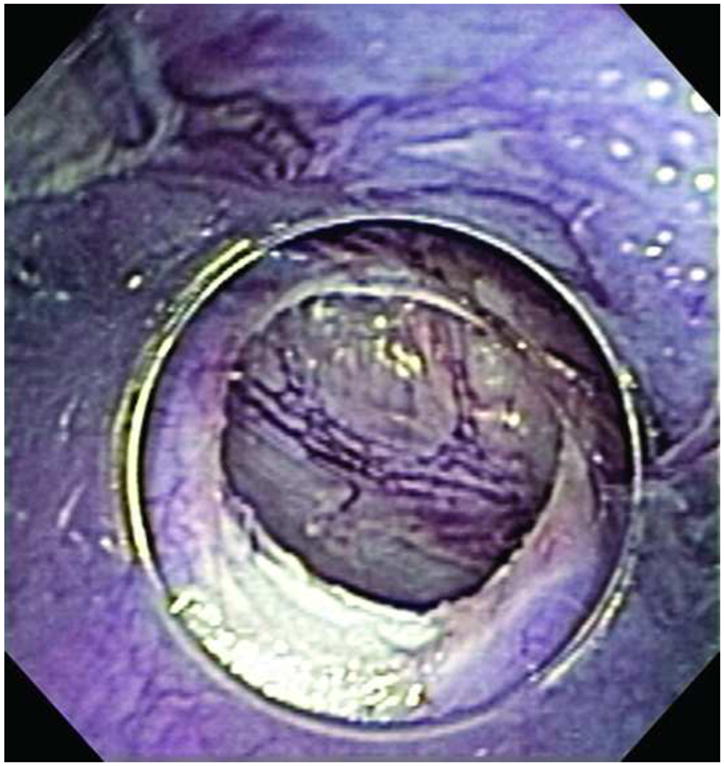
Visualization of the peritoneum after FTGB
FIGURE 3.
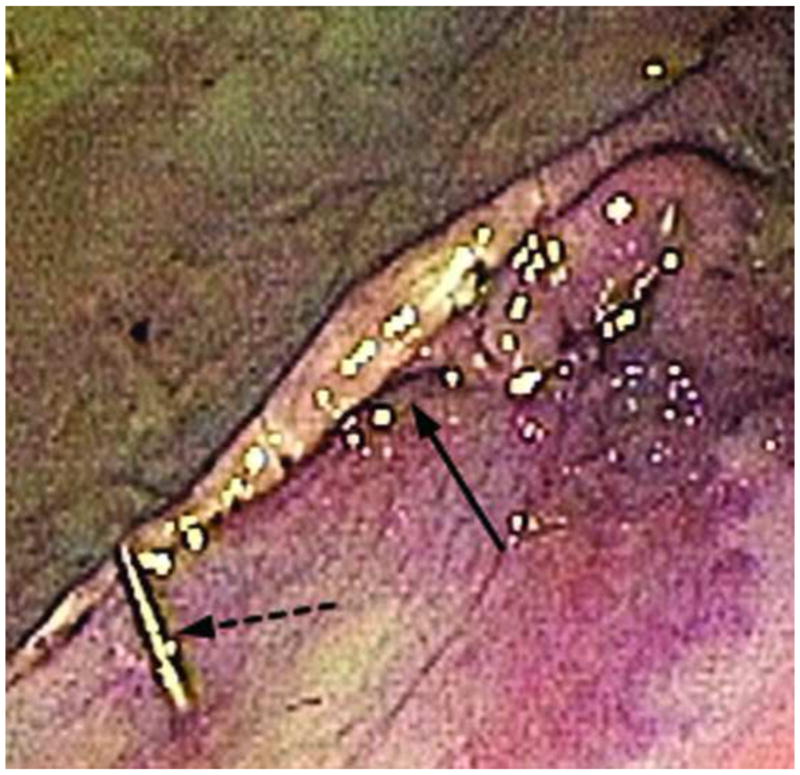
Closure ofoffset mucosal entry point (solid arrow) after endoscopic suturing with suture tag visible (broken arrow)
FIGURE 4.
H & E stained section showing the presence of circular muscle (CM), myenteric plexus (MYP) and longitudinal muscle (LM) in the sample. Scale bar = 100 μm
FIGURE 5.
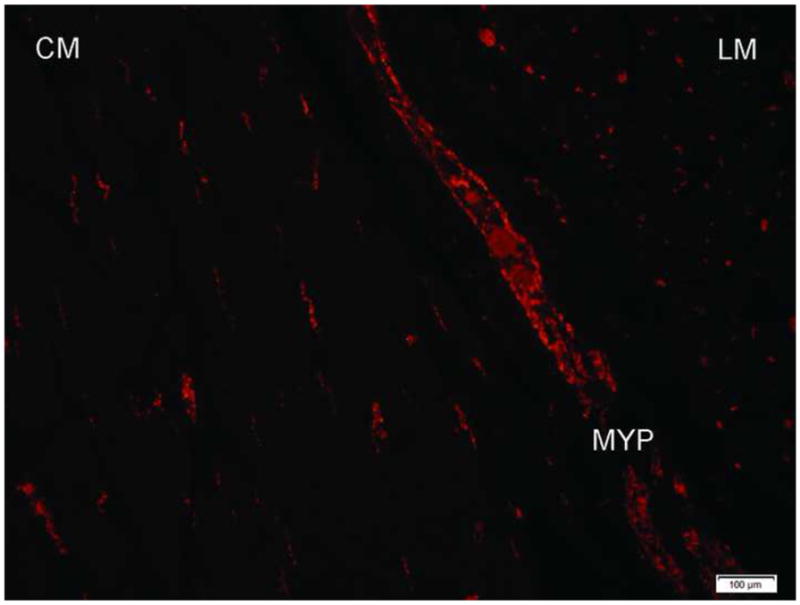
Resected tissue showing anti-PGP9.5 immunopositive myenteric ganglia. Circular muscle (CM), myenteric plexus (MYP), longitudinal muscle (LM). Scale bar = 100 μm
FIGURE 6.
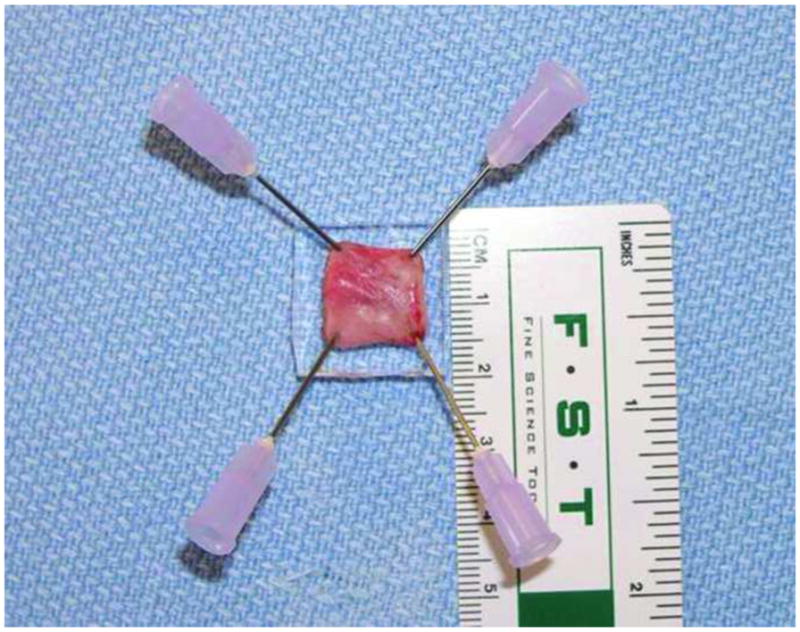
Resected tissue
FIGURE 7.
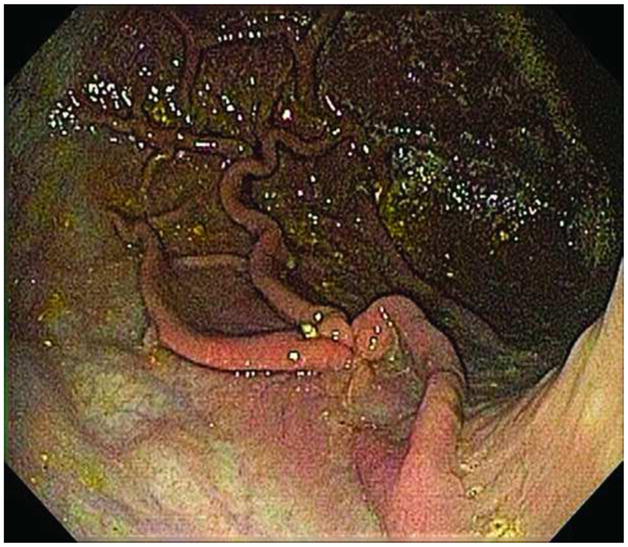
Stellate scar at mucosal entry site seen at endoscopy
FIGURE 8.
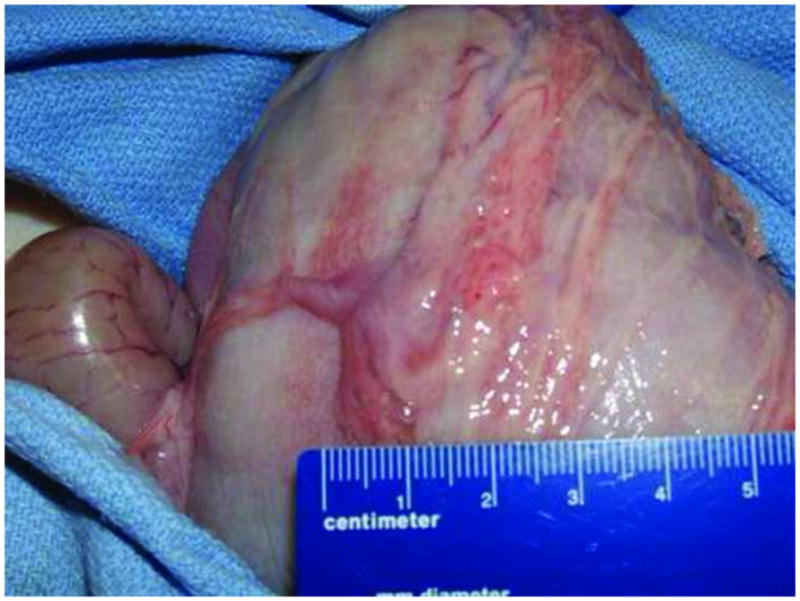
Serosa with Single Band Adhesion
DISCUSSION
This preclinical survival study evaluated the technical feasibility, reproducibility and safety of a FTGB using the SEMF technique and endoscopic suturing. Using this novel technique a full thickness biopsy of the entire muscularis propria that included oblique, circular and longitudinal muscle layers could be technically achieved with sufficient tissue obtained from the intermuscular layer to identify multiple myenteric ganglia using PGP9.5 antibodies. This is important as myenteric ganglia do not form a continuous layer and therefore the sample needs to be sufficiently large to capture several ganglia. The significant benefit of the SEMF technique is the presence of the overlying mucosal flap that serves as a safety valve to seal the gastric wall perforation. Effective closure of the mucosal entry point was achieved in all animals using the endoscopic suturing device. All 12 animals had an uncomplicated clinical course with complete healing of the mucosal and serosal aspects of the resection sites at follow-up endoscopy and necropsy. There was acquisition of ample tissue samples comparable to surgical specimens and in close accordance with the guidelines of the International working group on histological techniques10,11. In human trials, the target site will be the anterior gastric body, approximately 9cm proximal to the pylorus as recommended by the guidelines of the International working group10. We anticipate that the resection technique, in theory should be easier in a human study because of the improved endoscope position within the stomach compared to the ‘near-retroflexed’ position of the endoscope when working in the porcine stomach.
This procedure reflects an important directional shift in approaching invasive and complex endoscopic techniques. We have previously reported on the evaluation of different existing endoscopic approaches for acquisition of deep biopsies of the gastric muscle wall to include the intermuscular layer. However, all the studied techniques including the innovative ‘no hole’ double EMR were limited by the lack of adequate tissue and/or safety 8,9. The ‘no hole’ EMR technique involved an initial gastric EMR followed by creating a pseudopolyp of the exposed muscularis propria using endoloops and T-tag tissue anchors. The pseudopolyp was then resected. This study explored the concept of obtaining deep muscle wall biopsies using a unique approach of resection without perforation. The SEMF technique was pioneered by research in our Developmental Endoscopy Unit as a concept to use the submucosa as an intramural working space for endoscopic interventions into or beyond the gut wall 12,13,14,15. The offset mucosal entry into the submucosal space and exit into the peritoneal cavity minimizes peritoneal contamination and the overlying mucosa serves as a sealant flap valve. This unique SEMF approach has been successfully performed clinically to access the peritoneal cavity for NOTES (natural orifice translumenal endoscopic surgery) applications and for the performance of myotomy in achalasia.16,17
Percutaneous endoscopically assisted transenteric full thickness biopsy is a novel clinically applied method for assessing histopathological abnormalities in GIND patients. Initial experience showed abnormalities identified in 44% of patients such as possible degenerative leiomyopathy18,19. The limitation of this technique compared with the SEMF technique or that obtained by standard laparoscopy is the small sample size that is less than the size recommended by the International working group guidelines and that does potentially reduce diagnostic yield20. Another approach that has been published in a non-survival study evaluated colonic endoscopic full thickness biopsies using an EMR-based technique, future studies are needed to assess safety of this procedure21. Other options for evaluating myenteric ganglia are also being investigated. The use of an innovative submucosal probe-based confocal laser endomicroscopy that provides optical histologic imaging is currently being evaluated in pre-clinical studies with promising results22,23. Identifying a neuron-specific fluorescent stain for human use and addressing any potential toxicity or long term effects of these neuronal probes are underway. However it is likely that subtyping neurons, immune cells and ICC will continue to require tissue acquisition. In this context our study technique employing an invasive endoscopic approach allows for acquisition of sufficient tissue to facilitate quantitative and qualitative analysis of multiple cell types. The ready availability of such an endoscopic technique may lead to invaluable insights into the pathophysiology and potential novel targeted therapy of GINM disorders.
Take Home Message.
Full-thickness gastric biopsy using the submucosal endoscopy with mucosal flap technique with endoscopic suturing is feasible, reproducible, and safe. Ample tissue samples can be obtained using this technique to allow for analysis of multiple cell types including myenteric ganglia, and interstial cells of Cajal.
Such an endoscopic approach reflects an important directional shift in diagnostic procedures that may lead to invaluable insights into the pathophysiology and potential novel targeted therapy of gastrointestinal neuromuscular disorders.
Acknowledgments
Grant support: Funded by Weissman Fund and NIH grants DK68055 and DK57061.
Acronyms
- FTGB
Full thickness gastric biopsy
- SEMF
Submucosal endoscopy with mucosal flap
- GIND
Gastrointestinal neuromuscular diseases
- ICC
Interstial cells of Cajal
- SFC
Submucosal fluid cushion
- EMR
Endoscopic mucosal resection
- PGP9.5
Protein Gene Product 9.5
- NOTES
Natural orifice translumenal endoscopic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harberson J, Thomas RM, Harbison SP, Parkman HP. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55(2):359–70. doi: 10.1007/s10620-009-1071-2. [DOI] [PubMed] [Google Scholar]
- 2.Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130(3):759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59(12):1716–26. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–64. 2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20 (Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 6.Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, et al. NIDDK Gastroparesis Clinical Research Consortium. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–85. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The NIDDK Gastroparesis Clinical Research Consortium (GpCRC) Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, et al. Clinical-histological associations in gastroparesis: Results from the Gastroparesis Clinical Research Consortium. NeuroGastroenterology and Motility. doi: 10.1111/j.1365-2982.2012.01894.x. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan E, Gostout CJ, Lurken MS, Talley NJ, Locke GR, Szarka LA, et al. Evaluation of endoscopic approaches for deep gastric-muscle-wall biopsies: what works? Gastrointest Endosc. 2008;67(2):297–303. doi: 10.1016/j.gie.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Rajan E, Gostout CJ, Lurken MS, Talley NJ, Locke GR, Szarka LA, et al. Endoscopic “no hole” full-thickness biopsy of the stomach to detect myenteric ganglia. Gastrointest Endosc. 2008;68(2):301–7. doi: 10.1016/j.gie.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118(2):271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 11.Knowles CH, Veress B, Tornblom H, Wallace S, Paraskeva P, Darzi A, et al. Safety and diagnostic yield of laparoscopically assisted full-thickness bowel biospy. Neurogastroenterol Motil. 2008;20(7):774–9. doi: 10.1111/j.1365-2982.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 12.Sumiyama K, Gostout CJ. Clinical applications of submucosal endoscopy. Curr Opin Gastroenterol. 2011;27(5):412–7. doi: 10.1097/MOG.0b013e328349cf8e. [DOI] [PubMed] [Google Scholar]
- 13.Sumiyama K, Tajiri H, Gostout CJ. Submucosal endoscopy with mucosal flap safety valve (SEMF) technique: a safe access method into the peritoneal cavity and mediastinum. Minim Invasive Ther Allied Technol. 2008;17(6):365–9. doi: 10.1080/13645700802528512. [DOI] [PubMed] [Google Scholar]
- 14.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA. Chemically assisted endoscopic mechanical submucosal dissection by using mesna. Gastrointest Endosc. 2008;67(3):534–8. doi: 10.1016/j.gie.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Marler RJ. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65(4):688–94. doi: 10.1016/j.gie.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Lee CK, Lee SH, Chung IK, Lee TH, Lee SH, Kim HS, et al. Human diagnostic transgastric peritoneoscopy with the submucosal tunnel technique performed with the patient under conscious sedation (with video) Gastrointest Endosc. 2010;72(4):889–91. doi: 10.1016/j.gie.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos SN, Harris MD, Hida S, Brathwaite C, Demetriou C, Grendell J. Endoscopic submucosal myotomy for the treatment of achalasia (with video) Gastrointest Endosc. 2010;72(6):1309–11. doi: 10.1016/j.gie.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Andrews CN, Mintchev P, Neshev E, Fraser HF, Storr M, Bathe OF, et al. Percutaneous endoscopically assisted transenteric full-thickness gastric biopsy: initial experience in humans. Gastrointest Endosc. 2011;73(5):949–54. doi: 10.1016/j.gie.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Fraser H, Neshev E, Storr M, Urbanski SJ, Andrews CN. A novel method of full-thickness gastric biopsy via a percutaneous, endoscopically assisted, transenteric approach. Gastrointest Endosc. 2010;71(4):831–4. doi: 10.1016/j.gie.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, et al. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118(2):271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 21.Neunlist M, Coquenlorge S, Aubert P, Duchalais-Dassonneville E, des Varannes SB, Meurette G, et al. Colonic endoscopic full-thickness biopsies: from the neuropathological analysis of the myenteric plexus to the functional study of neuromuscular transmission. Gastrointest Endosc. 2011;73(5):1029–34. doi: 10.1016/j.gie.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Ohya TR, Sumiyama K, Takahashi-Fujigasaki J, Dobashi A, Saito S, Tajiri H. In vivo histologic imaging of the muscularis propria and myenteric neurons with probe-based confocal laser endomicroscopy in porcine models (with videos) Gastrointest Endosc. 2012;75(2):405–10. doi: 10.1016/j.gie.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Sumiyama K, Tajiri H, Kato F, Imura T, Ono K, Ikeda K, et al. Pilot study for in vivo cellular imaging of the muscularis propria and ex vivo molecular imaging of myenteric neurons (with video) Gastrointest Endosc. 2009;69(6):1129–34. doi: 10.1016/j.gie.2008.08.007. [DOI] [PubMed] [Google Scholar]