Abstract
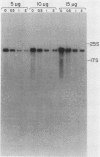
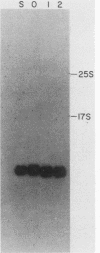
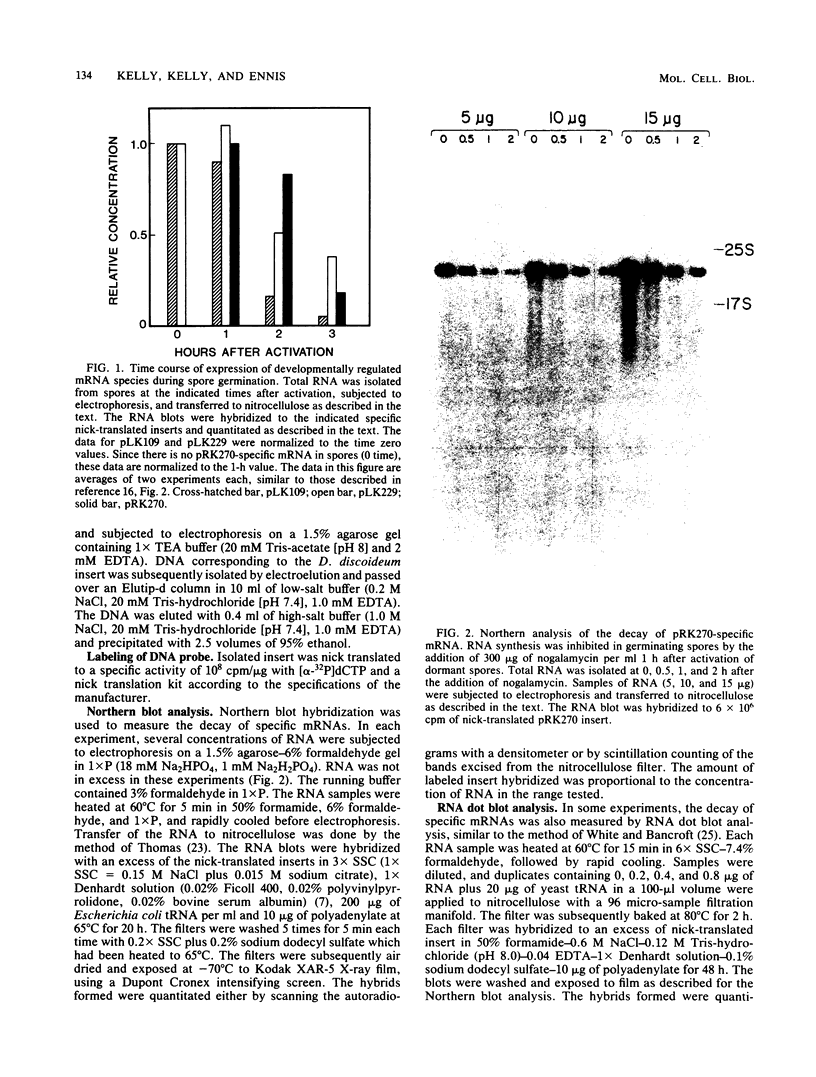
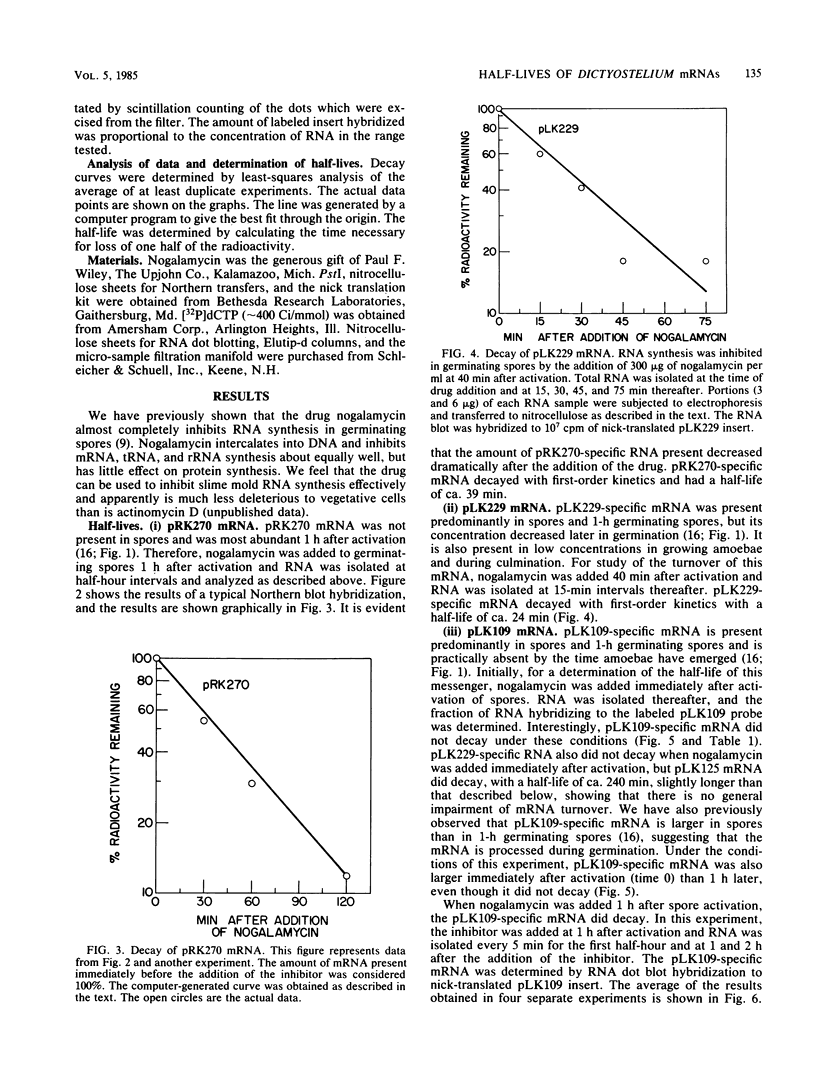
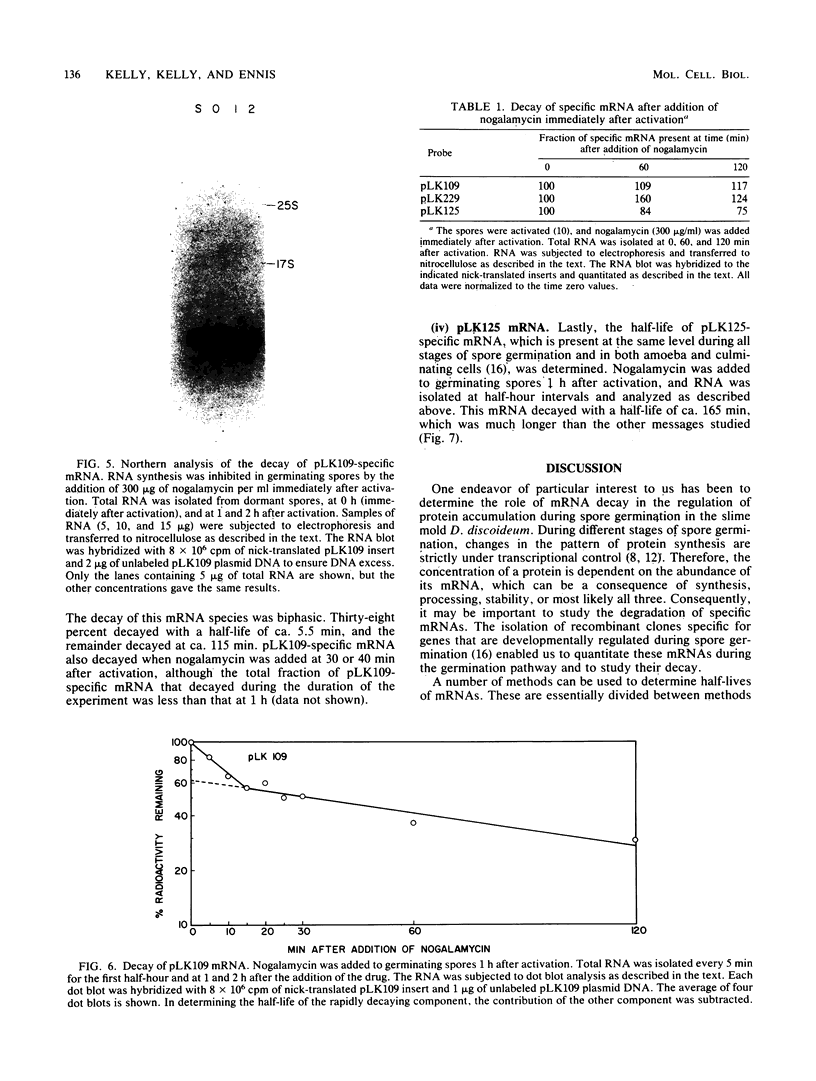
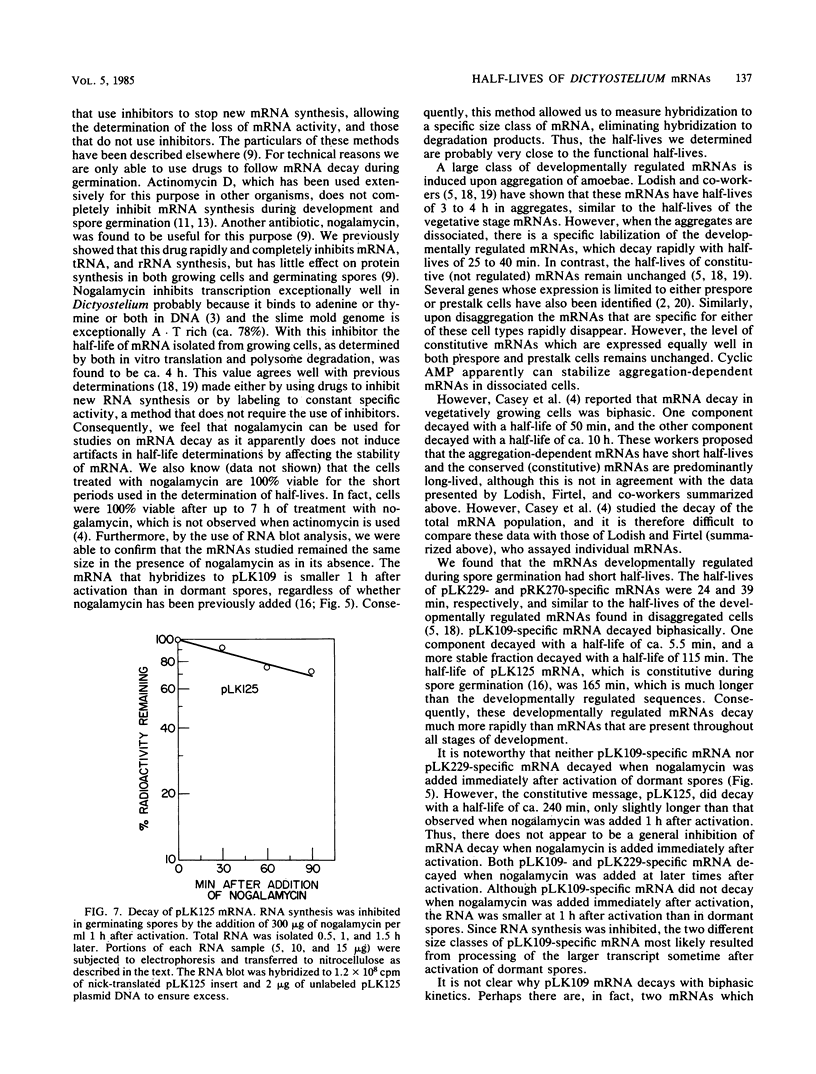
mRNA decay was studied during spore germination in Dictyoselium discoideum by the use of three previously isolated cDNA clones, pLK109, pLK229, and pRK270, which are specific for mRNAs developmentally regulated during spore germination. The half-life of a constitutive mRNA, pLK125, which is present throughout germination, growth, and development, as also determined. Nogalamycin, a DNA-intercalating compound, was used to inhibit RNA synthesis. Total RNA was isolated at intervals after addition of the drug, and the decay of mRNAs specific for the cDNA clones was determined by both Northern blot and RNA dot hybridization. If nogalamycin was added immediately after activation of dormant spores, neither pLK229 nor pLK109 mRNA decayed, but pLK125 mRNA did decay. Although pLK109 mRNA did not decay under these conditions, the RNA was smaller 1 h after activation than in dormant spores, indicating that it was processed normally. At 1 h after activation, pLK229-, pLK125-specific mRNAs decayed exponentially, with half-lives of 24, 39, and 165 min, respectively. Under the same conditions, decay of pLK109-specific mRNA was biphasic. Thirty-eight percent of the mRNA decayed with a half-life of 5.5 min, and the remainder decayed with a half-life of 115 min. It seems likely that nogalamycin inhibits the synthesis of an unstable component of the mRNA degradative pathway which is needed continuously for the decay of pLK109 mRNA. By extrapolating the curve representing the rapidly decaying component, a half-life of 18 min was calculated for pLK109-specific mRNA. The mRNAs developmentally regulated during spore germination have half-lives shorter than that of the constitutive messenger and shorter than the average half-life of 3 to 4 h previously determined for total Dicyostelium polyadenylated mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Casey L., Palatnik C. M., Jacobson A. Messenger RNA half-life in Dictyostelium discoideum. Dev Biol. 1983 Jan;95(1):239–243. doi: 10.1016/0012-1606(83)90023-4. [DOI] [PubMed] [Google Scholar]
- Chung S., Landfear S. M., Blumberg D. D., Cohen N. S., Lodish H. F. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. doi: 10.1016/0092-8674(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1966 Sep;56(3):880–887. doi: 10.1073/pnas.56.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dowbenko D. J., Ennis H. L. Regulation of protein synthesis during spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1791–1795. doi: 10.1073/pnas.77.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Nogalamycin inhibits ribonucleic acid synthesis in growing and developing cells of the slime mold Dictyostelium discoideum. Antimicrob Agents Chemother. 1981 Apr;19(4):657–665. doi: 10.1128/aac.19.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L., Sussman M. Mutants of Dictyostelium discoideum defective in spore germination. J Bacteriol. 1975 Oct;124(1):62–64. doi: 10.1128/jb.124.1.62-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Baxter L., Lodish H. F. Actinomycin D and the regulation of enzyme biosynthesis during development of Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):315–327. doi: 10.1016/0022-2836(73)90008-9. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Developmental changes in RNA and protein synthesis during germination of Dictyostelium discoideum spores. Dev Biol. 1978 Nov;67(1):189–201. doi: 10.1016/0012-1606(78)90308-1. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Protein and RNA synthesis during spore germination in the cellular slime mold Dictyostelium discoideum. Biochem Biophys Res Commun. 1977 Jul 11;77(1):282–289. doi: 10.1016/s0006-291x(77)80194-0. [DOI] [PubMed] [Google Scholar]
- Gross K. W., Jacobs-Lorena M., Baglioni C., Gross P. R. Cell-free translation of maternal messenger RNA from sea urchin eggs. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2614–2618. doi: 10.1073/pnas.70.9.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L. J., Kelly R., Ennis H. L. Characterization of cDNA clones specific for sequences developmentally regulated during Dictyostelium discoideum spore germination. Mol Cell Biol. 1983 Nov;3(11):1943–1948. doi: 10.1128/mcb.3.11.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Margolskee J. P., Lodish H. F. Half-lives of messenger RNA species during growth and differentiation of Dictyostelium discoideum. Dev Biol. 1980 Jan;74(1):37–49. doi: 10.1016/0012-1606(80)90051-2. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zusman D. R. Evidence for long-lived mRNA during fruiting body formation in myxococcus xanthus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1467–1471. doi: 10.1073/pnas.80.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Developmental phenomena in microorganisms and in higher forms. Annu Rev Microbiol. 1965;19:59–78. doi: 10.1146/annurev.mi.19.100165.000423. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]