Abstract
Wnt/β-catenin signaling promotes neural differentiation by activation of the neuron-specific transcription factors, Neurogenin1 (Ngn1), NeuroD and Brn3a, in the nervous system. Since neurons in cranial sensory ganglia and dorsal root ganglia transiently express Ngn1, NeuroD and Brn3a during embryonic development, we hypothesized that Wnt proteins could instructively promote a sensory neuronal fate from mesencymal stem cells (MSCs) directed to differentiate into neurons. Consistent with our hypothesis, Wnt1 induced expression of sensory neuron markers including Ngn1, NeuroD and Brn3a, as well as glutamatergic markers in neurally-induced MSCs in vitro and promoted engraftment of transplanted MSCs in the inner ear bearing selective loss of sensory neurons in vivo. Given the consensus function of T cell leukemia 3 (Tlx3), as a glutamatergic selector gene, we postulated that the effects of canonical Wnt signaling on sensory neuron and glutamatergic marker gene expression in MSCs may be mediated by Tlx3. We first confirmed that Wnt1 indeed up-regulates Tlx3 expression, which can be suppressed by canonical Wnt inhibitors. Next, our chromatin immunoprecipitation assays revealed that T-cell factor 3/4 (TCF3/4), Wnt-activated DNA binding proteins, interact with a regulatory region of Tlx3 in MSCs after neural induction. Furthermore, we demonstrated that forced expression of Tlx3 in MSCs induced sensory and glutamatergic neuron markers after neural induction. Together, these results identify Tlx3 as a novel target for canonical Wnt signaling that confers somatic stem cells with a sensory neuron phenotype upon neural induction.
Keywords: Wnt, Tlx3, mesencymal stem cells, differentiation, glutamatergic neurons, transplantation
Introduction
Mesencymal stem cells (MSCs) possess several unique properties that make them a particularly attractive source of cells for cell-based therapy. MSCs have the potential to self-renew and differentiate into multiple cell types [1, 2], and have been used to replace damaged cells in the nervous system using animal models of neurological disorders or traumatic brain injury [3, 4]. Recent evidence suggests that MSCs can be used not only to replace damaged neurons, but also to promote endogenous neuronal cell repair or survival by releasing neurotrophic factors [5–7]. These results demonstrate multiple characteristics of MSCs that promote them as donor cells for cellular repair as well as, potential delivery vectors for therapeutic agents.
We previously demonstrated that Sonic hedgehog (Shh) and retinoic acid (RA) synergistically promote expression of sensory neuron markers, including GATA3, Sox10, GluR4 (Glutamate receptor4), and P2X3 (purinergic receptor P2X, ligand-gated ion channel 3) in MSCs directed to differentiate into neurons. However, Shh and RA failed to induce expression of the POU-domain transcriptionfactor Brn3a, which is expressed in the majority of peripheral sensory neurons during development [8–10]. Surprisingly, a conditioned medium prepared from embryonic day 10 (E10) mouse hindbrain/somite/otocyst induced expression of Brn3a, as well as, Ngn1 and NeuroD [11]. The effects of the conditioned medium appeared to be specific, since no change in the GATA3 or Sox10 expression level was detected. These results prompted us to postulate that a soluble protein(s), other than Shh or RA, in the conditioned medium could regulate Brn3a, Ngn1 and NeuroD in MSCs.
Several lines of evidence suggest that Wnt signaling plays pivotal roles in cell fate specification in the nervous systems. Wnt genes encode secreted glycoproteins that exert diverse functions during embryogenesis depending on the cellular and developmental contexts. Canonical Wnt signaling allows β-catenin to translocate to the nucleus, where it interacts with T-cell factor (TCF) family of DNA-binding proteins and regulate transcription [12–14]. Neural crest progenitor cells were shown to acquire Brn3a expression at the expense of Sox10 expression by Wnt/β-catenin signaling [15, 16]. Furthermore, Wnt signaling is essential for generation of otic progenitor cells, some of which give rise to auditory sensory neurons, at early stages of inner ear development [17, 18]. Wnt has also been shown to promote neuronal differentiation from embryonic, somatic and neural stem cells [19–21]. Based on these previous studies, we hypothesized that Wnts are the soluble proteins in the conditioned medium, which promote sensory neuronal fate specification from MSCs after neural induction.
Materials and Methods
Mesencymal Stem Cell Culture
MSCs were isolated from the femurs and tibias of 5–7 week old C57BL/6 wild-type mice (Jackson Lab, Bar Harbor, ME), maintained as described previously [11, 22]. These MSCs expressed common MSC markers, but lacked expression of hematopoietic cell markers [22]. Some of the cultured MSCs were plated on poly-D-lysine-coated culture dishes at 5 × 104 cells/cm2. To initiate neural differentiation, culture medium was replaced with neural induction medium containing DMEM, 10 ng/mL FGF2 (Peprotec, Rocky Hill, NJ), 2% B27 (Invitrogen, Carlsbad, CA), 5 μM Forskolin (Sigma, St. Louis, MO), 125 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma,) with 10 μM β-mercaptoethanol (β-ME), and one of the following reagents: (1) recombinant human Wnt1 (1–400 ng/mL, Peprotec), (2) recombinant human Wnt3a (1–400 ng/mL, R&D Systems, Minneapolis, MN), (3) no factor (control). The cells were incubated for an additional 3 or 7 days. In function-blocking experiments, recombinant mouse Dkk1 (1–100μg/mL, R&D Systems) or recombinant mouse sFRP2 (0.1–500 ng/mL, R&D Systems) were added to neural induction medium containing Wnt1 (100 ng/mL) prior to the start of anincubation period.
Chromatin Immunoprecipitation Assays
Chromatin immuneprecipitation was performed using the Chip-IT Express kit (Active Motif) according to the manufacture’s instructions. MSCs were incubated for 4 days in maintenance medium, followed by neural induction medium in the presence or absence of 100 ng/mL Wnt1. Cells were incubated for an additional 2 days, after which nuclear protein and genomic DNA were cross-linked by incubation with 1% formaldehyde. The nuclei were collected by dounce homogenization and centrifugation, and sonicated to shear chromatin to an average of 200–800 bp fragments. Immunoprecipitations were performed with anti-TCF3/4 antibody (Millipore, 6F12-3, Billerica, MA) or the corresponding pre-immune serum using Protein G magnetic beads. The isolated DNA was subjected to PCR analyses using primers flanking the TCF binding sites in the Tlx3 promoter and its 3′ non-coding region. A sample representing 0.5% of the total chromatin used for immunoprecipitation reactions was used as an input control. A primer pair for the CyclinD1 promoter [23] was used as a positive control, whereas a primer pair for region 1 (Fig. 5D) that does not contain the TCF binding motif in the Tlx3 regulatory region was used as a negative control. PCR products were resolved on 2% agarose gels and visualized using ethidium bromide staining. The identity of PCR products was confirmed by cloning DNA fragments into pCR vectors (Invitrogen) and sequence analysis.
Figure 5. Tlx3 is up-regulated directly by canonical Wnt signaling.
(A, B) qRT-PCR analysis for Tlx3 in MSCs at day 7 of neuron induction with Wnt1 and either Dkk1 (1, 10 or 100 ng/mL) or sFRP2 (1, 10 or 100 ng/mL). (C, D) Chromatin immunoprecipitation with anti-TCF3/4 antibody on the resulting genomic DNA was conducted on neurally-induced MSCs. PCR probes to the various promoters relative to the Tlx3 coding region are indicated in (C). The red vertical lines indicate the positions of a consensus TCF binding sequence. (D) The promoter region 3 exhibits binding with TCF3/4 in the presence of Wnt1, whereas other regions lack binding. Cyclin D1 promoter, a known TCF target, was used as a positive control. IgG represents the amount of DNA immunoprecipitated by normal mouse IgG (negative control). Input DNA represents a sample representing 0.5% of the total chromatin used for immunoprecipitation reactions (input control). (E) pLG3 luciferase reporter vector containing Tlx3 promoter (WT) and its mutant within the region 3 TCF binding site (Mt). (F) Relative luciferase activity in pLG3 control vector (Ctrl), Tlx3 promoter (WT) and mutant Tlx3 promoter (mt) expressing neurally- induced MSCs with Wnt1 for 3 days. *P<0.05, **P<0.001
Luciferase Reporter Assays
Reporter constructs were prepared using the pGL3 vector (Promega, Madison, WI) that contains a Firefly luciferase reporter gene. A PCR fragment containing the 2,849 bp sequence lying upstream of the Tlx3 start codon was cloned into the pGL3 vector. A mutation in the Tlx3 promoter was introduced using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). For the luciferase reporter assay, MSCs were co-transfected with the Tlx3 promoter-reporter plasmid (wild type or mutant construct) and the pGL4.74 [hRluc/TK] plasmid encoding Renilla luciferase (Promega). Transfected cells were grown in neural induction medium containing Wnt1 (400 ng/mL) for 3 days. Luciferase activity was measured using the dual luciferase assay system (Promega). Firefly luciferase activity was normalized relative to the activity of Renilla luciferase.
Surgery and Cell Implantation\
Mongolian gerbils (Charles River, Wilmington, MA) at the age of 4 month old were used in this study. The experimental protocol for this study was approved by the institutional animal care and use committee at the Indiana University School of Medicine. Surgery procedures have been previously described [24, 25]. Briefly, following anesthesia, an incision was made to expose the left posterior side of the skull. A piece of gelform (Pfizer, New York, NY) soaked with 5 μL ouabain was placed in the round window niche. The animals were allowed to recover for one month, during which progressive degeneration of spiral ganglion neurons takes place. Following the recovery period, the animals were anesthetized and a suspension of cultured MSCs (1×106 cells/μL) in 10 μL PBS (left ear) or 10 μL PBS (right ear, control) was injected into the modiolus through a 30-gauge needle that was inserted into the bony wall of the basal turn of the cochlea.
An Alzet mini-osmotic pump (Model 2004) was used to deliver neural induction medium and Wnt1. The flow rate for infusion at 37°C was 0.25 μL/hr. Under the sterile condition, the osmotic pump was loaded with neural induction medium that contained 5-times higher concentrations of agents than the one used for in vitro experiments. The osmotic pump was placed in a 37°C saline bath for 12 hrs, which allowed the pump to be fully functional immediately upon implantation. The infusion tip of the cannula was made by stripping the Teflon coating from 36-gauge platinum iridium wire (Cooner Wire Co., Chatsworth, CA). A 1.25 cm piece of this Teflon Tubing (0.13 mm ID; 0.18 mm OD) was inserted into the end of a 12 mm polyurethane tubing (0.64 mm ID; 1.10 mm OD) (Micro-Renathane, Braintree Scientific Inc., Braintree, MA) and secured with silicon rubber (Dow Corning, MDX 4–4210), leaving 6 mm as a fine infusion tip (supplemental Fig. 1). Seventy two hours after the implantation, the osmotic pump was changed to a new pump containing 500 ng/mL Wnt1 and 250 ng/mL BDNF under general anesthesia. The animals carried the second osmotic pump for the following 3 weeks, after which they were euthanized and their temporal bones were processed for immunohistochemistry.
Results
Sensory neuron markers and glutamate receptors in neurally-induced MSCs are up-regulated by Wnt1 in a dose-dependent manner
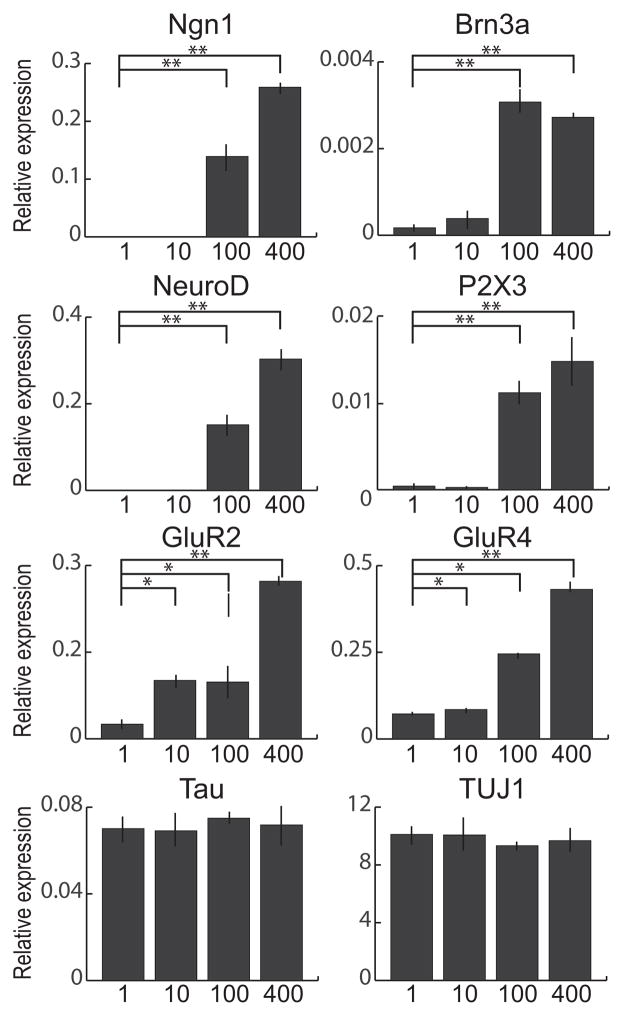
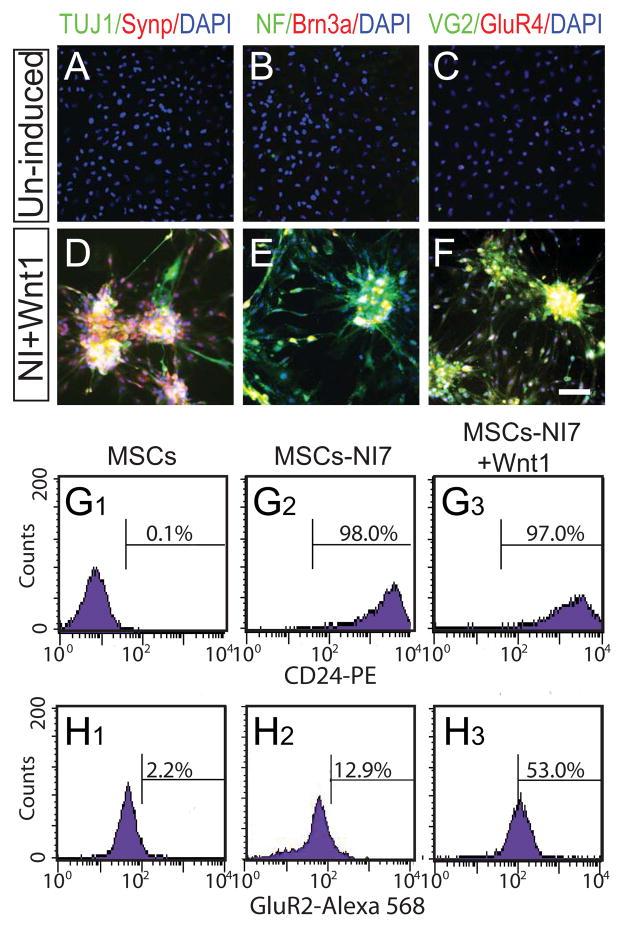
Since the Wnt receptors Frizzled (Fz) and low-density lipoprotein receptor-related protein (LPR) 5/6 as well as key Wnt signaling components are constitutively expressed in MSCs [26], we first tested whether canonical Wnt ligands can up-regulate sensory neuron markers in MSCs after neural induction. Incubation of MSCs in neural induction medium supplemented with human recombinant Wnt1 (1–400 ng/mL) for 7 days resulted in a significant up-regulation of Ngn1, NeuroD, Brn3a and P2X3, and the effects of Wnt1 were dose-dependent (Fig. 1). Wnt1 at 10 ng/mL or less had little effect on gene expression levels, whereas Wnt1 at 100 and 400 ng/mL induced robust up-regulation of all sensory neuron markers examined. Recombinant Wnt3a exhibited similar but lesser effects than Wnt1 (supplemental Fig. 2). Interestingly, when MSCs were cultured in maintenance medium, Wnt1 did not induce any of Ngn1, NeuroD or Brn3a (supplemental Fig. 3). We also tested the effects of Wnt1 on AMPA receptors (GluR1–4) and found that all AMPA receptors were significantly up-regulated in MSCs grown in neural induction medium containing Wnt1 (Fig. 1 and supplemental Fig. 4A). Consistent with the RT-PCR data, Brn3a, GluR4 and VGLUT2 proteins were detected in MSCs grown in neural induction medium with Wnt1, but not in those grown in maintenance medium with Wnt1 (Fig. 2A–F). In contrast to the sensory neuron marker genes, expression of pan-neural marker genes, such as Tau and TUJ1, was not altered by Wnt1 in neurally-induced MSCs (Fig. 1). Likewise, expression of dopaminergic- (TH, Nurr1), GABAergic neuronal subtype markers (Grik2, Viaat), Glial markers (Glial fibrillary acidic protein (GFAP), Integrin beta 4 (Itgb4)) was unresponsive to Wnt1 (supplemental Fig. 4B). Flow cytometric analysis was performed to quantitatively compare expression of CD24 (pan-neural surface marker; [27] and GluR2 between MSCs grown in the presence or absence of Wnt1 (Fig. 2G and H). The percentage of MSCs expressing CD24 increased dramatically after culture in neural induction medium for 7 days, but there was no significant difference in the percentage of CD24-positive cells between MSCs grown in neural induction medium only and those grown in neural induction medium containing Wnt1. In contrast, the percentage of GluR2-positive cells in neurally-induced MSCs grown in the presence of Wnt1 (53.0%) was much greater than undifferentiated MSCs (2.2%) as well as neurally-induced MSCs in the absence of Wnt1 (12.9%). Since CD24 is expressed not only in neurons, but also in granulocytes [28, 29], we tested a possible enrichment of granulocyte precursors in our culture using the hematopoietic markers, CD34, 117 and Sca1 (supplemental Fig. 5). The percentage of neurally-induced MSCs expressing CD34, 117 or Sca1 was less than 5% and there was no difference in the percentage between cells grown with or without Wnt1, ruling out the possibility that Wnt1 promotes hematopoetic cell proliferation or differentiation.
Figure 1. Wnt1 induces sensory neuron marker and AMPA receptor genes in neurally-induced MSCs.
qRT-PCR analysis for sensory neuron markers (Ngn1, NeuroD, Brn3a and P2X3), glutamate receptors (GluR2 and GluR4) and pan-neural markers (Tau and TUJ1) in MSCs grown in neural induction medium with recombinant human Wnt1 (1–400 ng/mL) for 7 days. Values are mean ± SD. *P<0.05, **P<0.001
Figure 2. Sensory and glutamatergic neural markers are expressed in neurally-induced MSCs in the presence of Wnt1.
Immunohistochemical characterization of MSCs grown in maintenance medium (A–C) and neural induction medium containing Wnt1 for 7 days (D–F). (A–F) TUJ1, NF and VGULT2 (VG2) immunofluorescence is shown in green. Synp, Brn3a and GluR4 immunofluorescence is shown in red. Cells are counterstained with DAPI (blue). Scale bar: 100 μm. (G and H) Flow cytometry profiles of CD24-PE and GluR2-Alexa 568 fluorescence in undifferentiated MSCs (MSCs) and MSCs at neural induction day 7 without (MSCs-NI7) or with Wnt1 (MSCs−NI7+Wnt1).
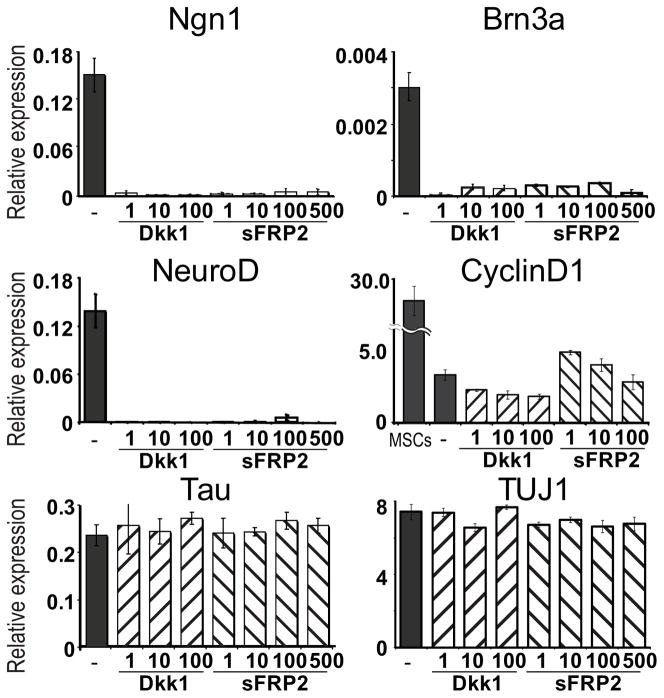
To test whether Wnt1-induced up-regulation of the sensory marker genes in MSCs can be suppressed by specific canonical Wnt inhibitors, MSCs were incubated in neural induction medium supplemented with Wnt1 (100 ng/mL) and either Dkk1 (1–100 ng/mL) or sFRP2 (1–500 ng/mL) for 7 days (Fig. 3). Both Dkk1 and sFRP2 efficiently suppressed Wnt1-induced up-regulation of Ngn1, NeuroD and Brn3a. Furthermore, Wnt1-induced up-regulation of Cyclin D1, a known Wnt target, was suppressed by Dkk1 or sFRP2 at both transcriptional and protein levels (Fig. 3; supplemental Fig. 6). To test whether Dkk1 and/or sFRP2 have any toxic effects on cell viability or can silence genes nonspecifically, we examined expression of Tau and TUJ1, whose expression is not regulated by Wnt1. No significant changes in expression levels of these neural marker genes were observed in cells treated either with Dkk1 or sFRP2 even at the highest concentration used.
Figure 3. Wnt antagonists suppress Wnt1-induced induction of Ngn1, NeuroD and Brn3a in MSCs.
q RT-PCR for Ngn1, NeuroD, Brn3a, CyclinD1, Tau and TUJ1 in MSCs grown in neural induction medium with recombinant human Wnt1 and either Dkk1(1–100 ng/mL) or sFRP2 (1–500 ng/mL) for 7 days.
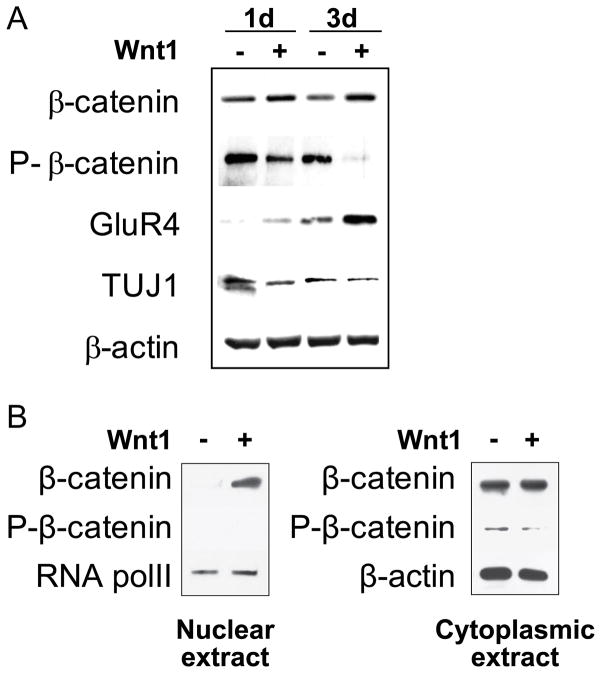
The canonical Wnt signaling pathway regulates β-catenin stability through Fz and LRP co-receptors. When Wnt ligands bind Fz and LRP, phosphorylation of β-catenin is inhibited, causing β-catenin stabilization and translocation into the cellular nucleus. Using Western blot analysis, we evaluated changes in total and phosphorylated β-catenin expression in MSCs in response to Wnt1 stimulation (Fig. 4A). MSCs were cultured in neural induction medium in the presence or absence of Wnt1 for 1 or 3 days. Expression of total β-catenin was increased by Wnt1, while expression of phospho-β-catenin was decreased. Furthermore, nuclear and cytoplasmic protein fractions were extracted from neurally-induced MSCs that had been grown in the presence or absence of Wnt1 for 3 days (Fig. 4B). Total β-catenin in the nucleus was significantly higher in MSCs grown in the presence of Wnt1 than those devoid of Wnt1. In contrast, expression of phosphorylated β-catenin in the cytoplasm was significantly lower in MSCs exposed to Wnt1 compared to those not exposed to Wnt1. These results indicate that stimulation of the canonical Wnt signaling pathway in neurally-induced MSCs promotes dephospholation of cytoplasmic β-catenin and its translocation into the nucleus. Consistent with our qRT-PCR results (Fig. 1), an increase in the GluR4 level and no changes in the TUJ1 level were observed when MSCs were grown in the presence of neural induction medium containing Wnt1 (Fig. 4A).
Figure 4. β-catenin in MSCs is dephosphorylated and translocated in response to Wnt1 after neural induction.
(A)Western blot analysis for total β-catenin and phosphorylated β-catenin (P-β-catenin), GluR4 and TUJ1 in MSCs at 1, 3 days after neural induction with (+) or without (−) recombinant human Wnt1. β-actin is used as a loading control. (B) Expression of β-catenin and P-β-catenin in the nuclear and cytoplasmic extracts from neurally-induced MSCs with Wnt1 for 3 days. RNA polII was used as an internal control for the nuclear protein fraction.
Tlx3 is up-regulated directly by canonical Wnt signaling
T cell leukemia 3 (Tlx3) is a selector transcription factor promoting an excitatory glutarmatergic neuronal phenotype over an inhibitory GABAergic phenotype during dorsal spinal cord development [21, 30, 31]. We recently demonstrated that forced expression of Tlx3 is sufficient to induce ectopic expression of glutamatergic neuron markers in embryonic stem cell (ESC)-derived neurons [32]. Moreover, Tlx3 expressing ESC-derived neurons exhibit excitatory neuronal functions, such as action potentials and excitatory post synaptic currents. Based on the function of Tlx3 as a master selector gene [30] as well as the overlapping set of genes up-regulated by Wnt1 and by Tlx3 (Fig. 1, [32]), we hypothesized that the effects of canonical Wnt signaling on expression of an array of sensory and glutamatergic marker genes in MSCs may be mediated, at least in part, by Tlx3. To validate this hypothesis, we first tested if Tlx3 expression in MSCs can be regulated by canonical Wnt signaling. Incubation of MSCs in neural induction medium containing Wnt1 increased the Tlx3 mRNA level in a dose-dependent manner and the Wnt1-induced up-regulation of Tlx3 was suppressed by Dkk1 as well as by sFRP2 (Fig. 5A, B).
To determine whether TCF3/4, Wnt-activated DNA binding proteins, interact with the regulatory regions of Tlx3, we conducted chromatin immunoprecipitation assays. DNA from the immunoprecipitated complex with an anti-TCF3/4 antibody was analyzed by PCR using 6 primer pairs that span potential regulatory regions containing a consensus TCF binding sequence (A/T A/T CAAA) [21] and could therefore serve as a binding site for TCF3/4. A promoter region of Cyclin D1 was used as a positive control. Positive binding of TCF3/4 to a region approximately 540 bp upstream of the Tlx3 start codon (region 3) was detected in neurally-induced MSCs grown in the presence of Wnt1, but not in those in the absence of Wnt1 (Fig. 5C–D). Sequence analysis of PCR products for the region 3 validated that the immunoprecipitated chromatin indeed contained sequences in this promoter region. All other Tlx3 regulatory regions containing a consensus TCF binding sequence exhibited a lack of binding with TCF3/4. To determine whether the TCF binding site that we have identified (region 3 in Fig. 5C) is functional, we compared the activities of the Tlx3 promoter (nt −2577 to +272) containing either an intact (TTTGTT) or mutated (TTTGGC) sequence (Fig. 5E). The Tlx3 promoter was cloned into a pGL3 luciferase reporter vector and, using a site-specific mutagenesis kit, another reporter construct with a mutation in the TCF binding motif was generated. MSCs were transfected with these reporter constructs and incubated in neural induction medium containing Wnt1 (400 ng/mL) for 4 days. Consistent with the results from chromatin immunoprecipitation assays, the Tlx3 promoter activity was significantly reduced with a mutation in the region 3 TCF binding site, when compared to a wild-type (Fig. 5F). Notably, the promoter activity of the Tlx3 mutant was significantly higher than that of the negative control pGL3-basic vector lacking any promoter sequence. This could be attributed to nuclear factor Y (NFY)-dependent basal promoter activation since the mutant Tlx3 promoter contains NFY-binding sites which are critical for basal Tlx3 expression [33].
Ectopic expression of Tlx3 in MSCs induces a sensory neuron phenotype
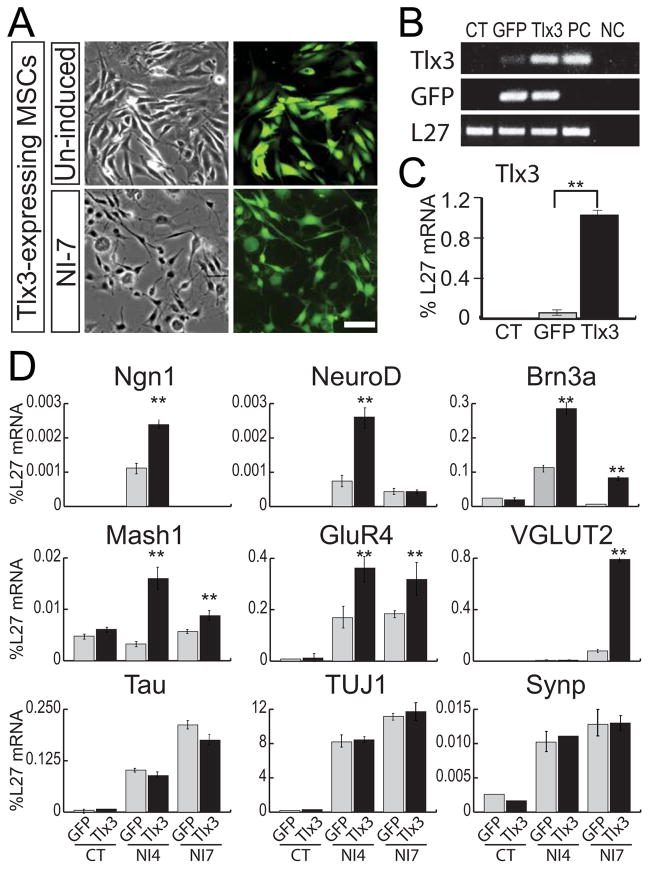
To test whether Tlx3 can regulate expression of those genes which are regulated by Wnt1, MSCs, which do not constitutively express Tlx3, were transfected with a Tlx3 expression vector (pBud-eGFP-cTlx3) or a control vector (pBud-eGFP). MSCs stably expressing the Tlx3 expression construct or control construct were subjected to neural induction. Despite dramatic changes in cell morphology, intense GFP fluorescence was maintained in virtually 100% of neurally-induced MSCs, indicating that trangene expression was not silenced by neural induction (Fig. 6A). Consistent with this, high-level Tlx3 expression was detected in MSCs expressing pBud-eGFP-cTlx3, but not in untransfected MSCs or MSCs expressing pBud-eGFP (Fig. 6B–C).
Figure 6. Forced expression of Tlx3 results in up-regulation of sensory and glutamatergic marker genes in neurally-induced MSCs.
(A) Forced expression of Tlx3 in MSCs. Virtually 100% of MSCs expressing pBud-eGFP-cTlx3 in the figure are GFP-positive and remain stably exhibiting GFP expression after neural induction. Scale bar: 100 μm. (B and C) RT-PCR analysis for GFP and Tlx3 in neurally-induced MSCs expressing pBud-eGFP (GFP) or pBud-eGFP-cTlx3 (Tlx3). PC; E10 otocyst, NC; no cDNA. (D) qRT-PCR analysis for Ngn1, NeuroD, Brn3a, Mash1, GluR4, VGLUT2, Tau, TUJ1 and Synp in MSCs expressing the Tlx3 expression vector (Tlx3) in comparison with those expressing the control vector (GFP). Undifferentiated MSCs (CT) or MSCs grown in neural induction medium for 4 days (NI4) or 7 days (NI7). **P<0.001
qRT-PCR analyses were performed to evaluate changes in gene expression in neurally-induced MSCs in response to ectopic Tlx3 expression. Pan-neural markers, such as Tau, TUJ1 and Synp, were induced after neural induction, however, no significant differences in their expression levels were observed in the presence or absent of Tlx3. In contrast, expression levels of Ngn1, NeuroD, Brn3a, Mash1 and GluR4 in Tlx3-expressing MSC-derived cells were significantly higher than in control MSC-derived cells at neural induction day 4 (Fig. 6D). In addition, Brn3a, Mash1, GluR4 expression remained elevated in cells expressing Tlx3 at neural induction day 7, and a notable up-regulation of the glutamate transporter VGLUT2 was observed in MSC-derived cells expressing Tlx3, but not in those expressing the control vector. The down-regulation of Ngn1 and NeuroD at neural induction day 7 as compared to day 4 (Fig. 6D) is consistent with transient expression of these proneural genes in neural progenitor cells in vivo. Furthermore, the delayed up-regulation of GluR4 and VGLUT2 in neurally-induced MSCs expressing Tlx3 coincides with that observed during embryonic neural differentiation.
Wnt1 promotes engraftment and differentiation of MSCs in an animal model of auditory neuropathy
The positive effects of Wnt1 on MSC differentiation in vitro prompted us to test its effect on MSCs in vivo. We hypothesized that engraftment and neuronal differentiation would be enhanced if MSCs are implanted into the cochlea of animals bearing selective loss of auditory sensory neurons and subsequently exposed to neural induction medium and Wnt1 in situ. GFP-positive MSCs isolated from TgN(ACTbEGFP) mice were subjected to pre-induction and then implanted into eight Mongolian gerbils, deafened by ouabain [25, 34]. A modified ALZET osmotic pump was used to continuously infuse (1) neural induction medium for 3 days followed by Wnt1 and BDNF for 25 days (experimental group), (2) neural induction medium followed by BDNF alone (control 1 group) or (3) physiological saline for 28 days (control 2 group) into the implanted cochlea (Fig. 7A). After 28 days post-implantation, a very small number of MSCs were found in the cochlea of control animals that had received physiological saline (Fig. 7B, F). In contrast, a significantly greater number of GFP-positive MSC-derived cells were found in the modiolus of animals that had received neural induction medium and the number of engrafted cells was even greater in animals that had received neural induction medium followed by Wnt1 (Fig. 7C–G). To test whether the observed difference in the number of engrafted cells between the experimental and the control 1 groups is due to the effect of Wnt1 on cell proliferation, we performed in vitro BrdU-incorporation assays (supplemental Fig. 7). The percentages of BrdU-positive cells in neurally-induced MSCs in the presence and absence of Wnt1 after 24 hrs exposure to 10 μM BrdU were 2.36% and 2.26%, respectively, and these values were much lower than the percentage of BrdU-positive cells (76%) in undifferentiated MSCs (11). Strikingly, GFP-positive MSCs that had been injected into the basal cochlear turn were found throughout all cochlear turns and many were found in areas surrounding the spiral ganglion at 28 days post-implantation, thus indicating extensive migration of the MSCs throughout the sensorineural tissues of the cochlea. Numerous GFP-positive cells were observed areas surrounding the spiral ganglion, while only several MSCs were detected within the ganglion (Fig. 7D). Some of the GFP-positive cells exhibited spherical cell bodies with long neurites and expressed several neural markers, including neurofilament and TUJ1 (Fig. 7G).
Figure 7. Wnt1 promotes engraftment and differentiation of MSCs in the gerbil cochlea with selective loss of spiral ganglion neurons.
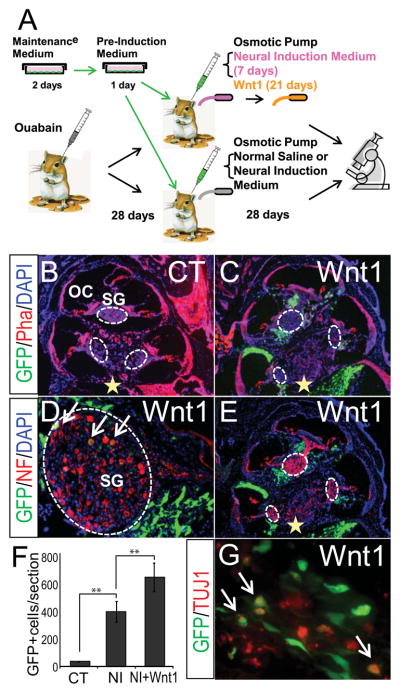
(A) Experimental design in in vivo experiments. (B–G) GFP-positive MSCs transplanted into the modiolus of the gerbil cochlea that has received physiological saline (B, control) or neural induction medium followed by Wnt1 (C, D, E, G). The number of average GFP-positive profiles per section for the control (CT) and neural induction medium (NI) and Wnt1 group (NI+Wnt1) (F). GFP-positive MSCs (green) are found not only in areas surrounding the spiral ganglion (SG) in the cochlea, but also in the sensory epithelia of the macula in the saccule. Cellular nuclei are stained with DAPI (blue). Cochlear structures are visualized with rhodamine conjugated phalloidin (Pha, red) (B and C). Neurofilament (NF) and TUJ1 immunofluorescence are shown in red (D, F and G). Yellow star indicates the stem cell Injection site. OC, organ of Corti. **P<0.001
Discussion
MSCs in the bone marrow possess several unique properties that make them a particularly attractive source of somatic stem cells for cell-replacement therapy. MSCs have a high self renewal potential, genetic stability and relative ease of collection from patients’ own bone marrow. Furthermore, two other extraordinary characteristics have been recently identified, including one in our present study, which makes MSCs an excellent source of cells for autologous transplantation therapy: MSCs exhibit remarkable abilities to migrate towards sites of tissue injury and have strong immunosuppressive properties [35, 36]. In the present study, we sought to determine the identity of microenvironment-derived soluble proteins that promote specification of MSCs towards a sensory neuron phenotype.
We have demonstrated that Wnt1 and −3a secreted from the microenvironment surrounding the embryonic inner ear can instructively promote expression of several sensory neuron markers, including Ngn1, NeuroD, Brn3a, P2X3, GluR1–4, in MSCs that are exposed to neural induction signals (Fig. 1 and supplemental Fig. 2). Wnt ligands are secreted glycoproteins, but are known to be highly hydrophobic and thus tend to adhere the plasma membrane of the secreting cells [14]. Despite this adherent property, we were able to detect both Wnt1 and Wnt3a proteins in conditioned medium prepared from the E10 mouse cervical tissues containing the otocyst, hindbrain and somite (T. Kondo unpublished observations). This is consistent with previous reports, in which Wnt4 secreted from COS cells exert attractive cues for responsive neurons that were located more than 300 μm from Wnt4-secreting cells [37, 38], suggesting that Wnt proteins can function as classical diffusible signaling proteins albeit for lesser spatial ranges. Although we did not measure electrophysiological properties of neurally-induced MSCs used in this study, recent studies revealed that MSC-derived cells are capable of generating action potentials, as well as expressing sodium and voltage-gated calcium channels [39–41], indicating the competence of MSCs to give rise to functional neurons.
We also presented evidence that the regulation of the sensory marker genes by canonical Wnt signaling is mediated, at least in part, by the master selector gene Tlx3. Wnt1 was able to induce Tlx3 expression in MSCs in a dose-dependent manner (Fig. 5A). When Tlx3 was introduced in MSCs that do not constitutively express Tlx3, these cells exhibited significantly higher levels of Ngn1, NeuroD, Brn3a and GluR4 than those lacking Tlx3 after neural induction (Fig. 6). Importantly, the up-regulation of the sensory neuron marker genes in MSCs by Wnt1 or Tlx3 was context-dependent. Both Wnt1 and Tlx3 instructively promoted expression of the target genes only after MSCs were exposed to neural induction medium, while they had no effect on gene expression in untreated MSCs. The context-dependent regulation of gene expression in MSCs by Tlx3 is consistent with our previous results with mouse embryonic stem cells [32]. One potential mechanism involved in this process is epigenetic transcriptional regulation mediated by Pbx3, a member of the TALE family of DAN-binding proteins. Pbx proteins are known to function as transcription co-factors and modulate DNA binding affinity and specificity of HOX proteins, such as Tlx3 [42, 43]. Since most HOX proteins exhibit promiscuous DNA binding properties despite their specific transcriptional activity, it is believed that Pbx proteins enhance target site selectivity by interacting with HOX proteins on the promoter loci of their target genes [44, 45]. The transcriptional complexes formed by Pbx and Hox proteins recruit a variety of co-regulators. It is possible that some transcriptional co-activators, such as a histone acetyltransferase, might be recruited upon neural induction to the Tlx3-Pbx3 heterodimer on the Ngn1 promoter, thereby inducing chromatin modifications and evoking transcriptional activation of Ngn1. Alternatively, Sox2 might be involved in the context-dependent Tlx3 actions. In the adult hippocampus, canonical Wnt signaling activates NeuroD expression in neural stem cells. The regulatory region of NeuroD has binding sites for TCF/LEF and Sox2, some of which form a Sox2/LEF hybrid binding motif. Wnt signaling directly control NeuroD transcription through TCF/LEF, but that the Sox2/LEF element serves as a dual switch either repression by Sox2 or activation by TCF/LEF [21]. Despite a lack of the Sox2/LEF hybrid binding site, a Sox2 binding site was detected in the 3′-regulatory region of Tlx3. Since Sox2 is expressed in undifferentiated MSCs, but not after neural induction, it is possible that loss of Sox2 after neural induction might result in dis-inhibition of TCF-mediated transcriptional activation of Tlx3, thereby indirectly activating its target genes.
We have identified Tlx3 as a direct down-stream target for canonical Wnt signaling in MSCs that are grown in neural induction medium containing Wnt1. Ectopic expression of Tlx3 in MSCs up-regulated the same set of genes as those which were up-regulated by Wnt1 (Figs. 1, 6). An increase in nuclear β-catenin and a reduction in cytoplasmic phosphorylated β-catenin were observed concomitantly with the specific binding of TCF3/4 with the Tlx3 promoter. Our sequence search identified a total of 6 sites in the Tlx3 regulatory regions that contain consensus TCF binding motif(s). Of these putative TCF binding sites, only a region located approximately 530 bp upstream of the Tlx3 coding region turned out to exhibit a binding with TCF3/4 and a mutation in this promoter region resulted in impaired Tlx3 promoter activity. The identification of the functional TCF binding site reveals that Tlx3 is a novel Wnt target gene.
It is interesting to note that stimulation of the canonical Wnt pathway has little effect on the expression levels of GATA3 or Sox10, both of which are up-regulated by a combination of Shh and RA [11]. Conversely, Shh and RA failed to induce expression of Brn3a and NeuroD in MSCs [11], which are up-regulated by Wnt1 or Wnt3a (Fig. 1 and supplemental Fig. 2). Several lines of evidence indicate that some of the genes induced either by Wnt1 or Shh+RA in our preparations are complementary expressed in neural progenitors during development. In the dorsal spinal cord, neural crest-derived progenitors lose Sox10 expression and give rise to Brn3a-positive sensory neurons [16]. In the inner ear, NeuroD-positive progenitor cells lose NeuroD expression and differentiate into GATA3-positive auditory sensory neurons [46]. Thus, timing and balance of Wnt proteins and Shh+RA applications may be a key for instructing neurally-induced MSCs to differentiate into cell populations with a specific phenotype.
Positive effects of Wnt1, when combined with neural induction medium, on engraftment of MSC-derived cells were observed in the cochlea of animals with selective loss of spiral ganglion neurons (Fig. 7). Since MSCs become post-mitotic after neural induction and since Wnt1 has no effect on cell growth rate of neurally-induced MSCs (supplemental Fig. 7; [11]), the observed increase in the number of engrafted cells is most likely due to the effect of Wnt1 on cell survival. This is consistent with a recently recognized function of Wnt/β-catenin signaling for survival and maintenance of neural progenitor cells [47–50]. The fact that Wnt1-induced up-regulation of CyclinD1 did not accompany an increase in cell proliferation in neurally-induced MSCs seems paradoxical: However, the expression level of CyclinD1 in neurally-induced MSCs in the presence of Wnt1 was only 15% of undifferentiated MSCs (Fig. 3). The extent of MSC migration was also robust, as partially differentiated MSCs implanted into the basal turn of the cochlea were found to be present in the apical turn 4 weeks after transplantation. This, however, is probably due to an innate characteristic of MSCs, rather than trophic effects of Wnt1, as cell engraftment was observed only in the basal turn of the cochlea that had received mouse embryonic stem cells (A. Matsuoka unpublished observations). Despite the enhanced migration and engraftment, the rate of engrafted MSCs expressing pan-neural markers was low (less than 10%). This could be attributed to (1) the short infusion period (3 days) with neural induction medium, and (2) the concentration of neural induction medium was not high enough to promote neural induction, although we used a medium that contained 5-times higher concentrations of reagents used for our in vitro experiments. Furthermore, Wnt1, known to exert diverse functions depending on developmental contexts, might not have promoted neuronal differentiation as neural induction was incomplete in our cell populations. Wnt proteins have been shown to promote neurogenesis, neuronal cell fate specification, axon growth and synaptic number/transmission depending on cellular and developmental contexts [16, 38, 51, 52]. In our in vivo experimental paradigm, it would be extremely difficult to control precise timing and doses of Wnt(s) that MSCs are exposed to while undergoing neural differentiation. Since Wnt signaling-mediated sensory neuron specification in MSCs is mediated by Tlx3, Tlx3-expressing cells will provide a novel strategy to conditionally generate excitatory glutamatergic neurons in vivo. Consistent with this, 50–75% of mouse ESCs overexpressing Ngn1 were shown to differentiate into neurons with a glutamatergic phenotype in deafened guinea pig’s cochleae [53]. Since our results indicate that Tlx3 controls Ngn1 expression in MSCs, stimulating the Tlx3-Ngn1 pathway would likely yield stem cell-derived neurons to replace auditory neurons in the inner ear. Further investigation is required to determine the optimal conditions for neural induction from embryonic and somatic stem cells in vivo.
Conclusions
We have identified Tlx3 as a novel target for the canonical Wnt signaling that promotes neuronal differentiation from bone marrow-derived somatic stem cells. TCF3/4, Wnt-activated DNA binding proteins, interact with a regulatory region of Tlx3 in neurally induced MSCs and a mutation in the TCF binding motif reduces Wnt-dependent Tlx3 promoter activity. Thus, Wnt-induced acquisition of a sensory neuron phenotype in neuraIly-induced MSCs appears to be mediated by Tlx3. In addition, Wnt1 promotes engraftment and survival of transplanted MSCs in the inner ear of animals bearing selective loss of sensory neurons. These results suggest that activating the Wnt-Tlx3 pathway could confer neurally-competent MSCs with a sensory neuron phenotype and promote their survival.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health grant R01 DC007390 (to E. H.) and the Ruth and Lynn Townsend Professorship in Communication Disorders to the University of Michigan (to J.M.M).
We would like to thank Angela Thompson, Diane Prieskorn, Leon Carter and Zhenyun Yang for their expert technical assistance. This work was supported by a National Institutes of Health grant R01 DC007390 (to E. H.) and the Ruth and Lynn Townsend Professorship in Communication Disorders to the University of Michigan (to J.M.M.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contribution:
Takako Kondo: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing
Akihiro J. Matsuoka: Conception and design, Collection and/or assembly of data, Data analysis and interpretation
Atsushi Simomura: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing
Karl R. Koehler: Collection and/or assembly of data, Data analysis and interpretation Rebecca J. Chan: Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript
Josef M. Miller: Data analysis and interpretation, Final approval of manuscript Edward F. Srour: Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript
Eri Hashino: Conception and design, Financial Support, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
References
- 1.Jori FP, Napolitano MA, Melone MA, et al. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem. 2005;94:645–655. doi: 10.1002/jcb.20315. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae JS, Han HS, Youn DH, et al. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;25:1307–1316. doi: 10.1634/stemcells.2006-0561. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuhuber B, Timothy Himes B, Shumsky JS, et al. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 7.Yu K, Ge J, Summers JB, et al. TSP-1 secreted by bone marrow stromal cells contributes to retinal ganglion cell neurite outgrowth and survival. PLoS ONE. 2008;3:e2470. doi: 10.1371/journal.pone.0002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang EJ, Liu W, Fritzsch B, et al. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier J, Quina LA, Eng SR, et al. Brn3a target gene recognition in embryonic sensory neurons. Dev Biol. 2007;302:703–716. doi: 10.1016/j.ydbio.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Lei L, Eng SR, et al. Brn3a regulation of TrkA/NGF receptor expression in developing sensory neurons. Development. 2003;130:3525–3534. doi: 10.1242/dev.00582. [DOI] [PubMed] [Google Scholar]
- 11.Kondo T, Johnson SA, Yoder MC, et al. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci U S A. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 13.Grigoryan T, Wend P, Klaus A, et al. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes & development. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Hari L, Brault V, Kleber M, et al. Lineage-specific requirements of beta-catenin in neural crest development. The Journal of cell biology. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HY, Kleber M, Hari L, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science (New York, NY. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama T, Mohamed OA, Taketo MM, et al. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- 18.Freter S, Muta Y, Mak SS, et al. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- 19.Salero E, Hatten ME. Differentiation of ES cells into cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2997–3002. doi: 10.1073/pnas.0610879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otero JJ, Fu W, Kan L, et al. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131:3545–3557. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- 21.Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro- elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitteti BR, Cheng YH, Poteat B, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta- catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuoka AJ, Kondo T, Miyamoto RT, et al. In vivo and in vitro characterization of bone marrow-derived stem cells in the cochlea. Laryngoscope. 2006;116:1363–1367. doi: 10.1097/01.mlg.0000225986.18790.75. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka AJ, Kondo T, Miyamoto RT, et al. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117:1629–1635. doi: 10.1097/MLG.0b013e31806bf282. [DOI] [PubMed] [Google Scholar]
- 26.Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433:1–7. doi: 10.1016/j.gene.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Pruszak J, Ludwig W, Blak A, et al. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells. 2009;27:2928–2940. doi: 10.1002/stem.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elghetany MT, Patel J. Assessment of CD24 expression on bone marrow neutrophilic granulocytes: CD24 is a marker for the myelocytic stage of development. Am J Hematol. 2002;71:348–349. doi: 10.1002/ajh.10176. [DOI] [PubMed] [Google Scholar]
- 29.Droin N, Jacquel A, Hendra JB, et al. Alpha-defensins secreted by dysplastic granulocytes inhibit the differentiation of monocytes in chronic myelomonocytic leukemia. Blood. 2010;115:78–88. doi: 10.1182/blood-2009-05-224352. [DOI] [PubMed] [Google Scholar]
- 30.Cheng L, Arata A, Mizuguchi R, et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- 31.Cheng L, Samad OA, Xu Y, et al. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- 32.Kondo T, Sheets PL, Zopf DA, et al. Tlx3 exerts context-dependent transcriptional regulation and promotes neuronal differentiation from embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:5780–5785. doi: 10.1073/pnas.0708704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borghini S, Vargiolu M, Di Duca M, et al. Nuclear factor Y drives basal transcription of the human TLX3, a gene overexpressed in T-cell acute lymphocytic leukemia. Mol Cancer Res. 2006;4:635–643. doi: 10.1158/1541-7786.MCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 34.Schmiedt RA, Lang H, Okamura HO, et al. Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Shi J, Lu CC, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 38.Wolf AM, Lyuksyutova AI, Fenstermaker AG, et al. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J Neurosci. 2008;28:3456–3467. doi: 10.1523/JNEUROSCI.0029-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox LE, Shen J, Ma K, et al. Membrane properties of neuron-like cells generated from adult human bone-marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1831–1841. doi: 10.1089/scd.2010.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho KJ, Trzaska KA, Greco SJ, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 41.Wislet-Gendebien S, Hans G, Leprince P, et al. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 42.Asahara H, Dutta S, Kao HY, et al. Pbx-Hox heterodimers recruit coactivator- corepressor complexes in an isoform-specific manner. Mol Cell Biol. 1999;19:8219–8225. doi: 10.1128/mcb.19.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Chang CP, Brocchieri L, Shen WF, et al. Pbx modulation of Hox homeodomain amino-terminal arms establishes different DNA-binding specificities across the Hox locus. Mol Cell Biol. 1996;16:1734–1745. doi: 10.1128/mcb.16.4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peltenburg LT, Murre C. Specific residues in the Pbx homeodomain differentially modulate the DNA-binding activity of Hox and Engrailed proteins. Development. 1997;124:1089–1098. doi: 10.1242/dev.124.5.1089. [DOI] [PubMed] [Google Scholar]
- 46.Lawoko-Kerali G, Rivolta MN, Lawlor P, et al. GATA3 and NeuroD distinguish auditory and vestibular neurons during development of the mammalian inner ear. Mech Dev. 2004;121:287–299. doi: 10.1016/j.mod.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Liu C, Wang Y, Smallwood PM, et al. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seitz R, Hackl S, Seibuchner T, et al. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi Y, Hirotsu T, Iwata R, et al. A trophic role for Wnt-Ror kinase signaling during developmental pruning in Caenorhabditis elegans. Nat Neurosci. 2009;12:981–987. doi: 10.1038/nn.2347. [DOI] [PubMed] [Google Scholar]
- 50.Davidson KC, Jamshidi P, Daly R, et al. Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol Cell Neurosci. 2007;36:408–415. doi: 10.1016/j.mcn.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Castelo-Branco G, Wagner J, Rodriguez FJ, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gogolla N, Galimberti I, Deguchi Y, et al. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 53.Reyes JH, O’Shea KS, Wys NL, et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.