Abstract
A comprehensive review of necrotizing enterocolitis (NEC) is provided; including history, biological basis and frequently asked questions. In addition, a system of improved NEC classification is explained in detail (consisting of five NEC subsets and four NEC-like diseases), to aid the bedside clinician in therapy and prevention. The authors offer opinion for therapeutics in italics at the end of each definition.
Keywords: necrotizing enterocolitis, neonate, premature infant, classification
A BRIEF HISTORY OF NEC
Much of our clinical understanding of NEC comes from the pre-surfactant era. The first reports are from the 1950s (in German), and the first English reports are from the 1960s.1–4 The use of Bell’s staging criteria originates from the 1970s when NEC still predominantly occurred in term and near term infants.5 Seminal breakthroughs occurred in this decade (including CPAP and total parental nutrition) and with them a “new NEC” emerged in increasingly premature infants that survived because of these technologies. Some suggested that NEC was not a single disease entity, but rather a disease spectrum, perhaps in part because NEC had expanded into these new survivors.6–8 Some groups documented increased NEC with surfactant introduction, but eventually NEC in older infants declined and the new NEC became static, essentially resetting incidence at a level comparable to the presurfactant era.
The debate about whether or not NEC was a singular disease or a spectrum persisted but the practicality of Bell’s staging criteria moved the field towards an inclusive approach and became the de facto definition of NEC. A variety of acquired neonatal intestinal diseases (ANIDs) were in-advertently included in NEC cohorts and datasets. However, in the late 1980s, clinicians gradually realized that A) some patients were simply not as sick as the others and B) combining heterogeneous causes of ANIDs into datasets obstructed progress. The first modification of the prevailing NEC definition was the exclusion of Bell’s stage I (Table 1).9,10
Table 1.
Comparison of NEC/ANID Classification System and Their Purposes
| Bell’s Original Classification of NEC |
Modified Bell’s Classification of NEC |
NEC vs SIP Differentiation |
NEC Reductionism (NEC Subsets) |
|---|---|---|---|
| Created to estimate mucosal to serosal progression of disease | Stage I was dropped to reduce inclusion of non-specific ileus | (Gordon’s Classification) The only staging is medical vs surgical | This expansion takes NEC from ANIDs & reduces it into clinical subsets |
| Stage I = ileus | NEC = pneumatosis and/or portal air |
|
|
| Stage II = pneumatosis | Stage II = pneumatosis | SIP = pneumoperitoneum without pneumatosis |
|
| Stage IIIa = pneumatosis + systemic illness | Stage IIIa = pneumatosis + systemic illness | When in doubt, time of onset should be considered (SIP vs NEC) |
|
| Stage IIIb = Stage IIIa + intestinal perforation | Stage IIIb = Stage IIIa + intestinal perforation | Surgical or pathology findings can overturn clinical diagnoses |
|
| Focus was on clinical staging of NEC | Focus on datasets that contain NEC vs ileus due to sepsis | Focus is on getting clean NEC data | Focus is on identifying risk factors to facilitate quality improvement & prevention |
In the post surfactant era, a new kind of ANID emerged. Spontaneous intestinal perforation (SIP) was first described in a term infant in 1956 in the New England Journal of Medicine, but in the post surfactant era an extremely premature version of it emerged and became rampant during the early postnatal steroid trials.11–13 It took years for investigators to determine there was a robust relationship between early postnatal steroid therapy and SIP.14–18 Subsequent research confirmed SIP to be a separate disease entity from NEC.19 This prompted a rethinking of the prevailing NEC definition (i.e. modified Bell’s staging). SIP most commonly presents as pneumo-peritoneum without pneumatosis but was, and in some datasets still is, being miss-classified as Bell’s stage IIIb.20 Because of this, it has been suggested that Bell’s staging be abandoned in favor of a new ANID taxonomy.21 The goal of such a system (Gordon’s classifications), was not to stage the severity of disease (other than surgery and mortality), but to focus on the different etiologies of the two known ANIDs at the time of diagnosis. This manuscript furthers this concept, by coupling Gordon’s classifications with NEC reductionism (the science of dividing NEC into reproducible subgroups) and provides an up-to-date review of NEC ontogeny.22
WHAT IS NEC AND WHAT IS NOT?
Crucial to NEC research is the ability to investigate NEC through databases. In order for this to occur, clinicians and investigators must agree on a common reliable definition of NEC. The challenge faced today is that SIP can be a substantial contaminant of NEC databases if it is not removed. SIP predominantly affects premature infants, whereas NEC affects newborns of all gestational ages although it is more frequent at lower gestational ages (Figure 1).23–32 Finally, SIP has distinct, non-ischemic histopathology.23 Cases show thinned or segmental necrosis of the muscularis interna (consistent with mesenteric-specific atrophy) but good mucosal integrity. In contrast, coagulative necrosis of the mucosa with focal hemorrhage is a universal finding in NEC and is presumed to correlate with ischemia (Figure 2).33 Moreover, animal models demonstrate fundamentally opposing etiologies for SIP versus NEC.34,35 If we are to understand NEC, SIP must be excluded from NEC datasets.
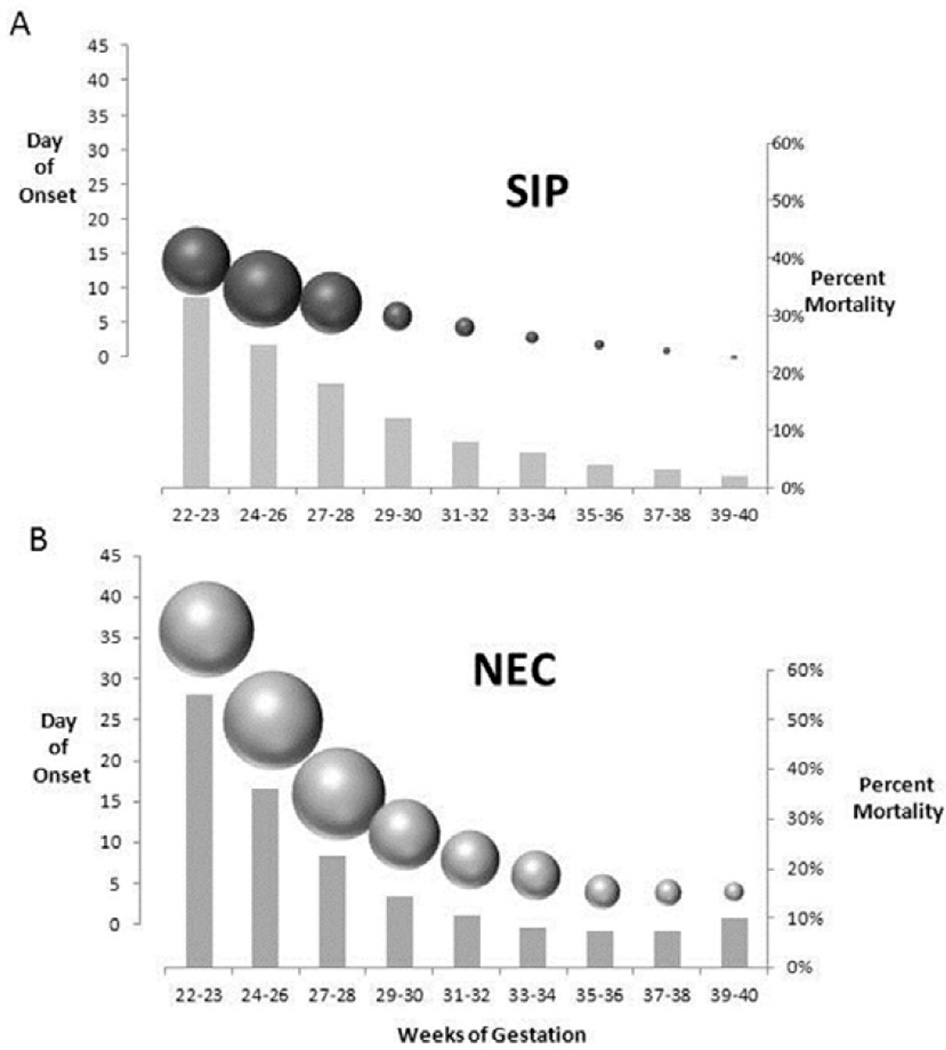
Figure 1.
Summation / consolidation of data about SIP and NEC, including time of presentation, percent mortality and incidence of disease stratified by gestational age. A: SIP, the left-sided y-axis equals day of onset indicated by point at the sphere centers. The sphere diameters indicate the percentage of the population of SIP who presented on that day of life (presented as estimated means based on multiple studies). The spheres are arrayed along the x-axis in the underlying graph to show the approximated gestational distribution. The percent mortality stratified by gestational age is indicated by the bars with the values indicated on the right-sided y-axis. B: NEC, left-sided y-axis equals day of onset indicated by the sphere centers. The sphere diameters indicate the percent of the population of NEC that presented on that day of life (mean for the gestational age group), and correlate with the x-axis below. The gray columns indicate the percent mortality (right side y-axis). On average, NEC can be seen to occur later in life at all gestational ages than SIP. NEC also has a higher mortality when compared across gestational strata. It is apparent in this comparison of SIP and NEC, that the timing of presentation, incidence and trends in mortality are different across gestational strata. Data for the extrapolations these figures are based upon are summarized data from references 24–29. In cases where precise data was unavailable in the units needed (example, if the data was available by birth weight instead of gestational age), the data was extrapolated using the assumption that the population as a whole was appropriate for gestational age and the datasets were representative of the same disease entity. For example, in the case where pooled averages of two birth weight groups were the only data available for timing and incidence from a national dataset, gestational spread of a single center report was used to re-apportion the values into the gestational distribution shown (SIP). Likewise, two single center reports were used to overlay the timing of NEC onset onto the national dataset of incidence and mortality.
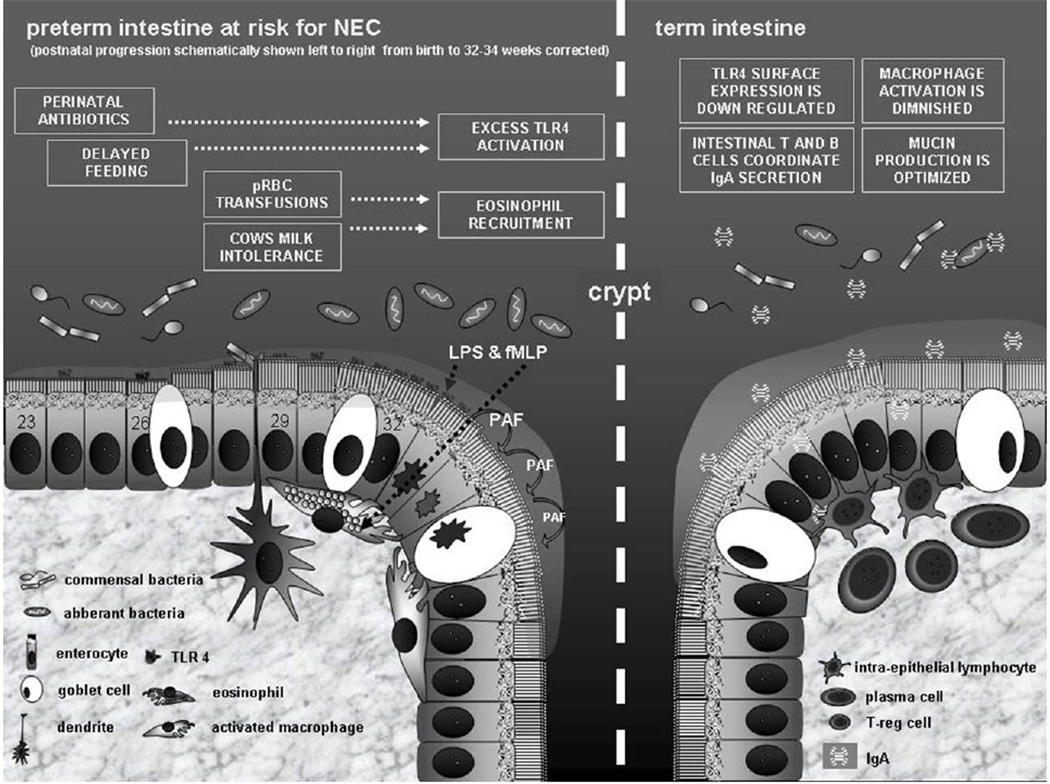
Figure 2.
Schematic illustrating proposed differences in NEC risk between preterm and term intestine at the cellular level of the mucosa. At the top of the schematic, risk modifiers and their resultant effectors are shown in boxes. Immediately above the mucosa, bacteria are shown in appropriate abundance and in relation to the boxed narrative above and the corrected gestation time line below (indicated by the numbers in the intestinal epithelial cells). Innate immune system effectors of cellular apoptosis / necrosis can be seen at or after 32 weeks corrected gestation, consistent with Sartwell’s model of incubation. In the term intestine, adaptive immune constituents abrogate NEC risk via active modulation of lumenal flora and down regulation of the innate immune system. Abbreviations / legend: TLR4 (toll like receptor-4 – principle mediator of enterocyte apoptosis in NEC genesis), fMLP (a bacterial metabolite that acts as a chemoattractant for eosinophils), T reg cells (T regulatory cells), PAF (platelet activating factor), LPS (lipopolysacharide – bacterial metabolite that activates TLR4, pRBC (packed red blood cells).
The differentiation of NEC from SIP at the bedside (first published by Gordon et al.), has been validated to have the best sensitivity and specificity for NEC and should be the modern standard for creating clean NEC datasets (Table 1).21,23,36 At the time of this publication, The Intermountain Healthcare, and the NICHD databases (although not all individual NICHD sites) have definitions consistent with Gordon’s classifications. The Pediatrix Corporation uses diagnostic categories consistent with Gordon’s classifications, (but there is no verification of diagnostic accuracy at the level of data entry). The Vermont Oxford Network and the Canadian Neonatal Network have well defined and verified definitions of NEC but these do not effectively exclude SIP (Gordon, personal communication).
SIP is not our only confounder. Mucosal necrosis is a common endpoint, with many etiologies. Equally important to our mission to reduce NEC is the understanding of spurious triggers of mucosal necrosis. NEC, as neonatology has known it for half a century requires three basic pre-conditions: 1) a recently colonized and naïve bowel (i.e. a neonatal bowel), 2) a substantial amount of food in that bowel, 3) a trigger event that compromises the integrity of the mucosal barrier. In recent years we have seen the emergence of entities that we (the authors of this review), describe as “NEC-like diseases” (NLDs). These entities lack one or more of the NEC pre-requisites. This is not to say that such entities are not important or that they should not be tracked. Rather, each entity is associated with a unique risk factor that has altered the ground rules for NEC. Such risk factors must be identified and understood, but collectively, NLDs obscure our understanding of true NEC and need to be excluded from NEC datasets (we review these later in the manuscript).
WHAT IS THE BIOLOGICAL BASIS OF NEC VULNERABILITY?
When does NEC occur?
A fundamental issue with the newborn bowel is that its immunity is dependent upon the innate system rather than the adaptive until feeding is successfully established. In that developmental interim, toll-like receptors survey the lumen for macromolecules specific to pathogens. If they are activated, they induce apoptosis to entrap the pathogen in the dying enterocyte. Toll-like receptor 4 (TLR4) is especially important, as it senses lipopolysaccharide (LPS).37,38 TLR4 is known to gradually increase in expression with increasing gestational age, until term birth, when its abundance falls precipitously and its surface expression is actively down regulated (Figure 2). TLR4 expression also increases in response to LPS. The more TLR4 is expressed, the more vigorous the apoptosis and severity of NEC. TLR4 may be the biologic “clock” that sets the timing of NEC onset by an infant’s gestation at birth (illustrated in Figures 1 and 2). This observation may also explain why NEC incidence is highest in the lowest gestation survivors. TLR4 expression is attenuated by breast milk, potentially explaining one of breast milk feedings many benefits.35
What determines the severity of NEC?
Mucosal Injury
Several studies indicate that the preterm intestine may have a pro-inflammatory bias, which may be particularly evident in epithelial cells and resident macrophages. Unlike the adult intestine, intestinal epithelial cells (IECs) in the preterm intestine express HLA-I and HLA-DR and could serve as non-professional antigen-presenting cells,39–44 express a large repertoire of innate response receptors, and can produce a significant local acute inflammatory response. Upon exposure to bacterial products such as lipopolysaccharide (LPS), fetal IECs produce more interleukin (IL)-8 than ileal/colonic epithelial cells from adult subjects.45–48,35 Fetal IECs can also produce tumor necrosis factor, IL-1, IL-6, and platelet-activating factor (PAF).49 This inflammatory hyper-responsiveness of fetal IECs correlates with increased nuclear factor kappa B (NF-κB)-mediated gene transcription,50 which, in turn, is related to a developmental deficiency of the inhibitor of kappa B (IκB).51 NF-κB signals undergo gradual down regulation in IECs following birth at term gestation.52
Bacteria that breach the gut epithelial barrier are normally eliminated by resident macrophages in the lamina propria.53,54 Macrophages first appear in the developing intestine at 11–12 weeks of gestation and increase rapidly during the second trimester.41,42,55–58 In the adult intestine, macrophages display a profound inflammatory anergy to bacterial products,54 a unique adaptation that maintains the normal absence of inflammation in the gut mucosa in spite of the close physical proximity to luminal bacteria.54,59–62 We have shown recently that this inflammatory down regulation of intestinal macrophages is mediated by transforming growth factor (TGF)-β, particularly its isoform TGF-β2. In the mid-gestation intestine, which is developmentally deficient in TGF-β2, the non-inflammatory differentiation of gut macrophages is not yet complete. In the event of a preterm delivery, bacterial colonization/translocation before the resident macrophages have undergone specific differentiation may increase the risk of mucosal inflammation and NEC.
Morbidity and Mortality
Some NEC subsets appear to be more severe in their morbidity and mortality than others. For example, transfusion associated NEC has been found to be more severe than other forms of NEC, whereas cow’s milk associated NEC appears to be less severe.63 These subsets may originate using mechanisms other than TLR4 as the initial trigger, likely yielding different baselines of severity. In two small single center datasets, NEC with lymphocytosis was found to have high mortality in extremely preterm infants.30,64 More research is needed to verify and understand these findings.
Why do some infants seem intrinsically vulnerable to NEC?
Although genetic factors are less likely to play a causative role in NEC, epidemiological studies have identified several candidate genes that may modify disease severity and/or outcome. Bhandari et al.65 recorded NEC in either 1 or both of the twins in 9 (14%) of 63 pairs of monozygotic twins and in 29 (15%) of 189 pairs of dizygotic twins. After controlling for covariates, genetic factors did not account for any variance in liability for NEC. However, in other studies, the severity of NEC and its outcome was associated with single nucleotide polymorphisms in several genes, including the IL-4 receptor (+1902G; protective),66 IL-18 (−607A; increased severity),68 vascular endothelial growth factor (+450C; increased risk),69 and carbamoyl-phosphate synthetase 1 (T450N; increased risk).67 More recently, Sampath et al.70 followed a cohort of 271 preterm infants, where 15 infants (5.6%) developed NEC. They identified the NFKB1 (g.−24519delATTG) variant in all infants with NEC but only in 65% of infants without NEC (p = 0.003). In contrast, the NFKBIA (g.−1004A>G) variant was present in 13.3% of infants with NEC but in 49% of infants without NEC (P = 0.007).
How do environmental factors influence the risk of NEC?
There are a variety of environmental factors that increase the risk of NEC (bacterial skew and overgrowth, hypoxia-ischemia, transfusion, cow’s milk allergy, and delayed feeding to name the ones that traditional research and NEC reductionism have identified). Platelet activating factor (PAF) is an important acute mediator in the pathway of mucosal necrosis.71,72 PAF is a soluble phospholipid, generated from existing cell membranes. It is released into the extracellular space by enterocytes in response to a wide variety of metabolic stresses (including hypoxia and inflammation). It is thought to act as an autocrine and paracrine signal, crossing into the synthesizing and adjacent cells then stimulating de novo production of TLR4, (thus increasing both the risk and severity of NEC through these local mechanisms of amplification).
What is the role of bacteria in NEC?
Fewer than 15% of blood cultures obtained on patients with NEC are positive.27 Thus systemic infection is not a common characteristic of NEC. In addition, conventional techniques for stool culture are suboptimal because 80% of the human colonic microbiota is not detected. Recently, taxonomic classification and molecular profiling of microbiota has become possible by sequencing the 16S small subunit bacterial ribosomal RNA gene, allowing identification of previously undetectable microbes.73 From these studies we’ve learned that bacteria outnumber human cells 10:1 and their genome out-sizes the human genome by a factor of 100. The microbiome is essential for the normal development of a balanced immune system, but conversely disturbances in immune regulation can modify the microbiome. Although species composition differs greatly between individuals, incredible functional overlap exists and different bacterial types may provide the same metabolic function. Mapping bacterial species diversity with their function is becoming a new way to define the host-microbiome interaction within “enterotypes”.74,75
Previous studies performed on stool samples have shown an association of NEC with delayed gut colonization after birth, prolonged empiric use of antibiotics, lower microbial diversity, and higher risk of colonization with pathogenic bacteria in the week leading up to the disease.76–78 However, results of these studies are not consistent and are limited by the heterogeneity of methods. More importantly, stool samples may not entirely represent the bacteria that exist at the point of injury in the small and large bowel. Bacterial adherence may be important in NEC, as studies in patients with inflammatory bowel diseases have demonstrated increased bacterial attachment to the intestinal epithelium surface compared to controls. In healthy adults, mucosa-associated microbial communities at different sites of the colon are quite distinct from those in stool.79,80 Similar studies in preterm infants are difficult to perform as intestinal tissue samples can only be obtained from surgical resections rather than endoscopic biopsies. If a microbial signature in stool samples could be reliably used to identify patients at risk for NEC, modern high-throughput technology might allow cost-effective strategies for microbiome screening of NEC. Unfortunately, that technology is not on the immediate horizon due to the complexities described.
To conceptualize the role of enteric bacteria, one must understand how the neonatal bowel senses them. TLR4 senses LPS, but a similar innate immune receptor, TLR9 has antagonistic effects on apoptosis and senses the CpG repeats within the DNA of primarily commensal bacteria.35 Thus colonization with lactobacillus and bifidobacterium species reduce the risk of NEC via TLR9 signaling, and down-regulate TLR4 expression. In contrast, PAF senses metabolic stress, up-regulates TLR4 and therefore can indirectly sense bacterial burden. A simplified mental model of the neonatal intestinal innate immune system’s ability to sense the commensal versus the total bacterial burden is a teeter-totter. PAF sits on one seat and TRL9 on the other. There is ground under TRL9 and an abyss under PAF. TLR4 sits in the middle and if it loses its balance in the direction of PAF, disease ensues. One other entity may come into play that can sway the balance toward the necrotic abyss. Eosinophils have the capacity to sense N-formyl-methionylleucyl-phenylalanine (fMLP – a bacteria specific metabolite) and may be recruited to the intestine as an additional form of innate defense when there is mucosal barrier dysfunction such as in cow’s milk associated (and perhaps transfusion associated) NEC.81 To complete the analogy, the playground is dismantled when adaptive immunity grows up.
Another fundamental problem for the preterm neonate is early suppression / alteration of intestinal floral with antibiotics. Many preterm infants are treated empirically with antibiotics at birth (and their mothers are treated ante- and perinatally). We know that these antibiotics suppress intestinal flora and that such alterations are associated with an increased risk of NEC.82,83 There is also compelling evidence that lack of antimicrobial exposure and/or reduced duration of exposure reduces the risk of NEC.84,85 More recently, antibiotic choice has come into the spotlight. Ampicillin and gentamicin are the most commonly used initial antibiotics. A large before and after retrospective study found a 10-fold reduction in NEC when the participating centers switched to pipericillin-tazobactam as their antibiotic for initial therapy of presumed sepsis.86 Thus it seems that a substantial portion of NEC might be related to perinatal antibiotic exposure.
Breast milk contains lactobacillus and helps to drive the flora towards normal colonization. Probiotics are live commensal organisms that are introduced by nasogastric tube as a means to colonize the gastrointestinal tract with “normal” flora. Probiotics have been demonstrated to reduce the incidence of NEC, but the FDA has yet to approve these agents for use in neonates and many diverse concerns have been voiced for this reluctance.87–90 This debate is at times heated, as there are staunch advocates in favor of probiotic use who favor expediated use, versus critics who are pushing for more rigorous trials. The timing, dosing and composition of probiotics have been variable in past studies, making it challenging to find a consensus therapy. Prebiotics are complex sugars added to formulas that have been demonstrated to promote normal flora growth in neonates. These agents are currently available in some commercial term formulas and will likely be available in preterm formulas in the future.
Mother’s milk is a natural and inexpensive means to appropriately colonize the neonate that has other benefits not found with probiotics or prebiotics. Mother’s milk should be the first choice for driving appropriate intestinal colonization. Pasteurized donor breast milk should be the second choice (because of its prebiotic, bacterial static and anti-inflammatory effects).91,92
Why is the preterm intestine more vulnerable to NEC?
As mentioned above, NEC vulnerability in the preterm infant is mediated by many mechanisms related to the transition from innate to adaptive immune function. Although the exact sequence of events and the specific roles and parts of the intestinal immune system require additional clarification, the common pathway includes mucosal barrier disruption with invasion of luminal bacteria secondary to an innate and injurious inflammatory host response (See Figure 2 for Summary Schematic).
Innate Immunity
Over the last decade, most research on NEC pathogenesis has been focused on the first line of defense against invading organisms, the innate arm of the immune system. NEC has been associated with disrupted tight junctions, reduced mucin-producing goblet cells, and fewer Paneth cells.93–95 However, a large body of work provides compelling evidence that immature signaling through toll-like receptors, in particular the imbalance of signaling between TLR4 and TLR9 is one of the more important drivers of the disease.35 TLR4 activation increases apoptosis and reduces enterocyte proliferation and migration.96–98 Enterocyte apoptosis is one of the first histopathologic events in experimental models of NEC99 and is caused by TLR4 activation in immature small intestinal tissue (but not in immature colonic or adult small intestinal tissues).96 Specific vulnerability of the preterm infant to NEC may be mediated by failed down-regulation of TLR4 signaling activity, which is characteristic in the gut of older children.100 TLR4 signaling pathways are continuously up-regulated in intestinal tissues from premature neonates and rats with NEC compared to controls and absence of TLR4 is protective in experimental NEC model.101–103 Up-regulation of TLR4 in NEC models can be reduced experimentally both by polyunsaturated fatty acids104 and through activation of TLR9.37 Novel compounds are in development to specifically suppress TLR4-mediated intestinal inflammation.38
Physical separation between bacteria and intestinal epithelial cells is critical for limiting immune activation and maintaining mutual host-bacterial relationship. A 50µm zone of separation composed of a viscous gel-like mucus layer covers the epithelium and inhibits bacterial access to the mucosal surface (Figure 2). This “demilitarized zone” is maintained by Myd88-dependent secretion of bactericidal C-peptide lectin RegIIIγ by intestinal Paneth cells.105 RegIIIγ limits bacterial colonization of the small intestinal mucosal surface with Gram-positive bacteria and lack of RegIIIγ in mice results in innate immune activation. Especially in the preterm intestine, where mucus production is limited and local defense mechanisms may not be as effective, perturbed spatial relationships between colonizing microbes and host cells is thought to be an important factor in NEC vulnerability.
Adaptive Immunity
The role of T cells in the pathogenesis of NEC has been understudied. This may be explained in part by suboptimal animal models, including the observed attenuated mucosal T cell response in a piglet model of NEC90 and the delayed ontogeny of T cell responses in mice compared to humans.106 However, we have known for a long time that T cells are present in the human fetal ileum at early gestation, that they accumulate following chorioamnionitis and they are capable of activation in vitro.107–109 The in-utero antigen-challenged preterm infant exhibits precocious lymphoid development and the potential for a vigorous host response upon appropriate antigenic triggers.110 In addition to alteration in effector T cell function, there may also be a role for deficient immune regulation in NEC.
To balance the effects of immune activation, the vertebrate immune system employs regulatory cell subsets that protect the host’s own tissues from immune-mediated destruction. One major protective subset is FOXP3+ T regulatory cells (Treg), professional immune suppressor cells that are critical in preventing excessive innate and adaptive immune activation in the intestinal tract.111–115 Patients lacking this subset of regulatory immune cells, due to X-chromosome linked mutations of FOXP3 (IPEX syndrome), exhibit early development of enteropathy, similar to inflammatory bowel disease.116 Excessive innate immune activation in the preterm intestine can suppress Treg function.38,117 Treg act through several distinct mechanisms including: a) secretion of inhibitory cytokines (e.g. IL-10, IL-35, TGF-β), b) granzyme and perforin-dependent cytolysis of T effector cells, c) metabolic disruption of T effector cells leading to apoptosis, and d) inhibition of dendritic cell maturation and function.118
While functional Treg are generally absent in the newborn mouse, they are abundant in the intestinal mucosa at early gestational ages in preterm infants.119 Associated with profound local inflammatory signals, patients with surgical NEC exhibit decreased ratios of Treg to effector T cells in the ileum mucosa compared to gestational-age matched non-NEC controls.120 Although insufficiently studied in clinical trials, nutritional supplements such as Vitamin A metabolites, probiotics or their components can enrich Treg numbers in inflamed regions, induce immune homeostasis and ameliorate experimental colitis.121–123 The potential to accelerate Treg development exists, and might truncate the NEC window of vulnerability, but more research is needed.
Despite some evidence that enteral immunoglobulin preparations can reduce the risk for NEC124 and the protective effects of secretory IgA antibodies in human milk, the role and function of B cells in the developing small intestine and a possible relationship to NEC has not been adequately established. An elegant study recently showed that intestinal epithelial cells up-regulate expression of immune and inflammatory genes when the adaptive immune system fails to control the microbiota.125 The absence of B cells and/or IgA resulted in a functional switch from metabolic to immunologic gene expression resulting in metabolic dysfunction. These studies support a new mechanism for the well-known anti-inflammatory properties of IgA.126 Treg provide survival signals to lamina propria IgA-producing B cells in part through production of TGF-β and are therefore critical for induction and maintenance of intestinal IgA responses.127 Thus Treg not only regulate the acute host-response to avoid injury but can also provide long-term immune homeostasis by actively shaping the intestinal microbiome via enhancement of local adaptive immunity and dampened inflammation.128
Why does the risk of NEC diminish after infants are post term?
The transition from innate to adaptive immunity appears to be both the moment of greatest risk to the normal preterm infant as well as the finish line toward relative NEC immunity. TLR4 signaling and surface expression decrease as neonates approach term gestation.37 Around the same time, macrophage activation diminishes.39 Many of the environmental triggers that have been associated with NEC and one of the NLDs seem to be linked to aberrant intestinal immune phenomenon. These may include transfusion associated NEC and cow’s milk associated NEC (both of which are associated with eosinophilia), and IVIG associated NLD, which occurs most commonly in infants with ABO incompatibility. Infants who navigate this window without intestinal immune aberrancy and/or aberrant bacterial growth have a greatly diminished risk of NEC.
NEC REDUCTIONISM
In this section, we describe five subsets of NEC that have been identified in multiple datasets (Figure 3). These entities should not be regarded as discrete, but rather as overlapping triggers along the spectrum of things that cause NEC. This is because patients in datasets have a Heisenberg uncertainty-like quality – they can only be assigned to one subset at a time. At the bedside, with any given case of NEC, more than one etiology may be in play.
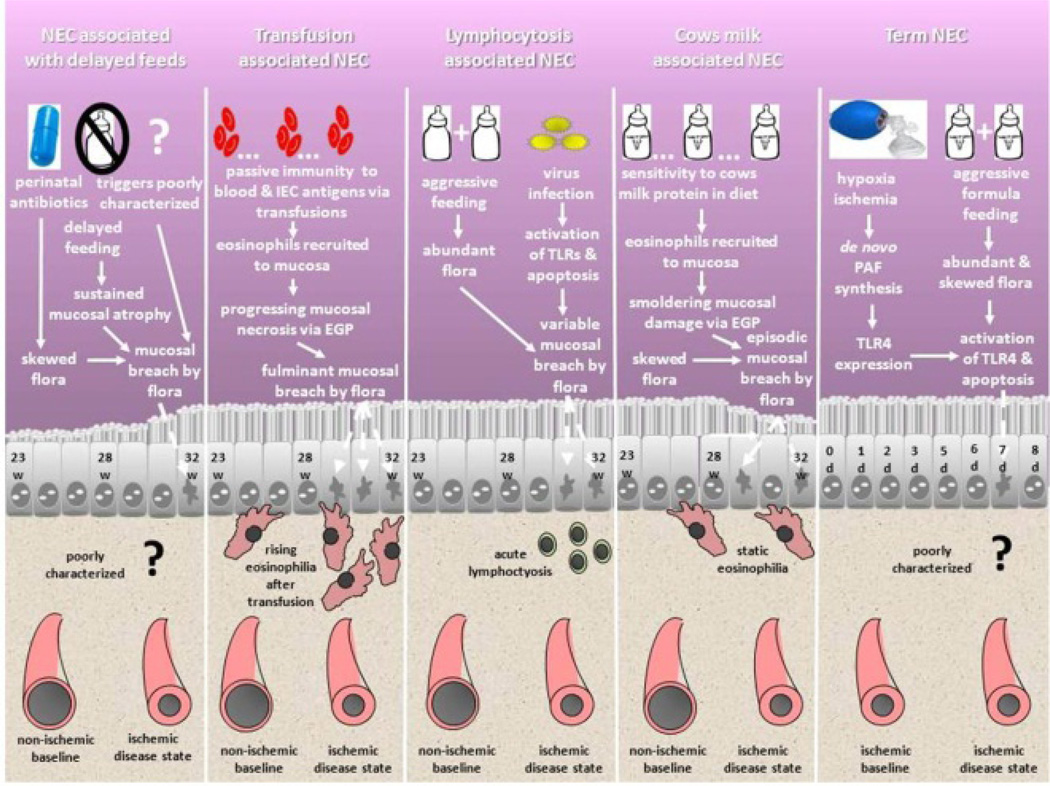
Figure 3.
Schematic of NEC subset ontogeny. Five NEC subsets are shown. At the top of the chart, clinical risk factors and pathways of ontogeny are described. Within the intestinal epithelial cell layers, most common time windows of presentation and mucosal health are characterized. In the bottom portion of the schematic, characteristic findings at presentation on the CBC and the intrinsic vascular tone within the lamina propria of the villi are characterized. Each subset has a novel ontogeny in comparison to the others. NEC after delayed feeds may ultimately be found to be a subcomponent of the other subsets (particularly transfusion and lymphocytosis associated NEC subsets), since rigorous investigation of potential triggers at the time of presentation have not been reported. Term NEC can also rightly be called “ischemia-triggered NEC” and can rarely occur in preterm / late preterm infants when the described precedent conditions are fulfilled. EGP = eosinophil granule protein.
Term NEC / Ischemic NEC
NEC in the term / late preterm infant occurs early, relative to premature infants, with the majority presenting by the end of the first week of life.129–132 Risk factors include formula feeding (versus breast milk), rapid advance of feeding volumes, chorioamnionitis, polycythemia with hyperviscosity, cyanotic heart disease and/or a precedent hypoxic-ischemic event or an illicit maternal drug history (especially cocaine) that leads to mesenteric ischemia. Term NEC is thought to be secondary to events that principally end in mesenteric bowel ischemia. This is presumed to cause acute elevation of PAF and de novo TLR4 expression in enterocytes, since hypoxia/ischemia is the principle trigger for NEC in rodent models (which are typically term for their species). There is sufficient feeding in this specific subset to allow heavy bacterial colonization, (but probably not overgrowth – relative to preterm infants (Figure 3)) and this is likely sufficient to activate newly synthesized TLR4 and induce a cascade of apoptosis that progresses to mucosal necrosis. Term NEC has a much lower surgery and mortality rate than preterm NEC.130 We caution that although term NEC most commonly occurs in older gestation infants, rarely a preterm infant will acquire NEC that is precipitated predominately by mesenteric ischemia (i.e. ischemic NEC). Such infants will not fit with Sartwell’s model of timing but will have a precedent severe hypoxic event.
Reasonable preventative measures for the term / ischemic NEC subset are exclusive breast milk feeding and slower feeding advances for infants with precedent hypoxic/ischemic events, congenital heart disease, polycythemia or maternal history of drug abuse. Use of banked breast milk for infants with especially concerning maternal drug abuse histories is an alternative.
NEC Associated with Packed Red Blood Cell Transfusions (NECw/pRBCs)
NECw/pRBCs has been demonstrated to affect preterm infants relatively late in life, consistent with Sartwell’s model of disease and in line with preterm NEC in general (Figure 1). It was robustly authenticated in a multicenter retrospective study by Christensen et al. in 2010. Since then more than a dozen publications have appeared, including a meta-analysis summarizing this amazing two years of literature.133,134 It is one of the most severe forms of NEC in preterm infants with a high rate of surgery and mortality. The timing of NECw/pRBCs is typically within 48 hours of transfusion and commonly within 12 hours.
Recent reports from a center with a two decade history of using O-donor blood demonstrated that blood type AB infants have a higher NEC mortality rate than other blood types (and risk was highest if infants were born to a mother with A or AB blood).135 A multi-center retrospective report found that patients with NECw/pRBCs had eosinophilia spikes upwards immediately following the transfusions associated with NEC (and an earlier single center study documented neonatal eosinophilia to be most common with transfusion and NEC).136,137 These findings suggest that AB blood group epitopes, known to be expressed on the neonatal enterocyte,138 may be vulnerable to serum antibodies in blood products (or perhaps to multiple exposures of low level antibody). Our current favored hypothesis is that enterocytes become over-whelmed by these passive antibodies, allowing them to opsonize surface antigens, initiate apoptosis and attract eosinophils. A function of eosinophils is to sense opsonized parasites. Eosinophils migrate toward fMLP (a bacterial specific metabolite) and can be recruited to the intestine when there is mucosal barrier dysfunction.139 Eosinophils release eosinophil granule protein to trigger apoptosis. However, preterm infants in the vulnerable window of Sartwell’s model have increased TLR4 expression/signaling, potentially resulting in a synergistic progression to mucosal necrosis. We stress that this is a working hypothesis. No histology from surgical NECw/pRBCs has been published thus far. We will undoubtedly need to refine this model after such studies become available.
One of the most important observations about NECw/pRBCs is that it has variable incidence. Several authors have described the baseline incidence as roughly one third of all preterm NEC cases.140–142 Two studies evaluating withholding of feeds during transfusions (to reduce the effects of post prandial blood flow) found reduced rates of NEC with this practice.143,144 Although such data is limited, these findings are encouraging for the prospects of prevention.
Strategies for preventing NECw/pRBCs might include: 1) eliminating or avoiding “late” transfusions by minimizing unnecessary blood draws, 2) use of erythropoiesis stimulating factors and hematinics to avoid transfusion, 3) typing and washing all pRBCs; 4) withholding feedings during transfusions; 5) switching to continuous feedings when a transfusion may be needed soon, and 6) standardizing transfusion criteria (more research in the area of standardization is needed and should include NIRS and Doppler ultrasound of mesenteric artery flow).
NEC Associated with Cow’s Milk Intolerance
There is limited data on this entity beyond published case reports.145–148 The most useful of the case reports show that neonatal disease is generally not associated with robust IGE hypersensitivity but rather the eosinophils are being recruited to the bowel by interleukin-5 elevation.148 Challenge with cow’s milk antigen causes them to release eosinophil granule protein.149 Pneumatosis intestinalis occurs principally in premature infants. Within the Pediatrix dataset, a search for infants with NEC who demonstrated eosinophilia at the time of presentation yielded a subset of infants with lower mortality, suggesting a more benign form of NEC.145 Unfortunately no feeding data was available. A follow up study in the Intermountain Healthcare dataset was more fruitful.130 Neonates with bloody stools at presentation were evaluated for eosinophilia, associated risk factors and outcomes. One subgroup was NECw/pRBCs (who had severe outcomes), but the other half was a group with feeding difficulty, high formula and fortifier exposure but relatively benign outcomes. A little less than half of these infants developed pneumatosis. This would be the precise demographic for cow’s milk intolerance evolving into NEC. Switching to elemental formula was found to be curative in all cases where it was tried.
One of the more interesting findings in the Intermountain Healthcare dataset was mild, indolent eosinophilia with cow’s milk associated NEC.130 In older infants, the hallmark of cow’s milk allergy is eosinophils in the stool. Our hypothesis is that neonatal cow’s milk associated NEC is on the same spectrum, except that they have not yet acquired the full capacity to develop true allergy and so they smolder and trigger intermittent episodes of mucosal necrosis when their intestines are over-challenged with excess antigen. Like NECw/pRBCs, we hypothesize that eosinophils are a crucial component in NEC associated with cow’s milk intolerance, only with this disease subset, the immune aberrancy has been actively mediated by the host rather than passively acquired by transfusion.
Prevention of this entity entails exclusive use of human breast milk, introduction of fortifiers after reaching the minimum volume for full feeds (120 mls/kg/day); and when appropriate, use of human based fortifiers. NICUs with higher Caucasian and Oriental prevalence will likely have a higher incidence of this NEC subset based on the racial prevalence of eosinophilia noted in the Pediatrix dataset and the fact that cow’s milk allergy is more common in Caucasians and Orientals.128 Therapy involves exclusive use of elemental formula, because mother’s milk can have sufficient dietary cow antigen to sustain the allergic response once they have become symptomatic.
NEC Associated with Contagion and/or Lymphocytosis
NEC is known to be seasonal.149,150 The timing of peak incidence varies from region to region but generally is higher in the Winter and early Spring, coincident with community enteral viruses associated with NEC in case reports and series.151–162 However, there are very few dataset-based studies with good quality showing the prevalence of virus-associated NEC other than rotavirus.163 Rough estimates from the seasonality data would place the impact of virus-suspected NEC cases at about ten to fifteen percent above baseline of all NEC.
Another line of evidence that has been emerging more recently is that of lymphocytosis on the CBC at time of NEC diagnosis. Recent single center studies have demonstrated much higher total estimates.30,64 If the percent lymphocyte count is used (>40%), then 61% of all NEC cases in that institution might be associated with viruses.64 When the absolute lymphocyte count was used in another single center study, 25% were thought to be potentially viral in nature.30 In both studies a high lymphocyte count was associated with increased mortality in preterm infants. Infants who have lymphocytosis associated NEC are most often preterm and typically acquire NEC consistent with Sartwell’s model. This observation suggests that the lymphocytosis may be more about the timing than the pathogen (if indeed there is one in each case). One of the fundamental difficulties with this research is that it has been unclear what “normal” lymphocyte numbers should be for preterm infants at any given gestation or at any given point of time during their life in the NICU. That should change with a recent publication from the Intermountain Healthcare System, which provides reference ranges for lymphocytes on CBCs by gestation and age of postnatal life.164
Prevention of NEC acquired during contagion entails the usual infectious disease strategies employed to prevent spread to others: quarantine of infected infants (preferably in an isolation room), hand washing, glove and gowning, sanitary disposal of diapers in a separate area from other patients, dedicated rather than shared nursing, and visitation policies that address hygiene and communicability of potential pathogens. In addition, bowel decompression and prophylactic antibiotics to suppress bowel flora should also be considered. Some units have begun employing these strategies as preventive measures when infants present with lymphocytosis and ileus. These measures seem logical to us.
NEC Associated with Extreme Prematurity and Delayed Feeding
Perhaps the most challenging NEC subset to define is that of the preterm infant with delayed feeding / feeding intolerance. We know that this entity exists, because it keeps showing up as the NEC control group in surgical NEC or our fulminant NEC cohort studies165,166 (where it is the majority of cases), but no specific definition exists. Recently, a well powered analysis of nutrition practices demonstrated that early nutrition optimization (including early enteral feeding), decreased the risk of NEC.167 Biologically speaking, Douglas Burrin’s lab established that total parental nutrition causes immediate and lasting mucosal hypoplasia in the absence of enteral feeding.168 In contrast, tiny amounts of enteral feeding can stimulate mucosal growth and elemental diets with no biological components are sufficient to do so.169,170 Mucosal restitution (the process of mucosal renewal through cell proliferation), is dependent upon establishing successful enteral feeding. If restitution becomes imbalanced, mucosal barrier function fails and NEC ensues. Anecdotally, and based on limited retrospective data, this form of NEC may present at lower feeding volumes than other subsets.
Like all other forms of true NEC, this subset is hypothesized to present at a time that is consistent with the Sartwell’s model, however data confirming this postulate is not robust and needs to be investigated further. Another weakness of this subset is that there may be substantial overlap with the other forms of preterm NEC, since its triggers have yet to be characterized.
Early introduction of breast milk with a gradual standardized advance is the best current feeding practice for prevention of NEC in the preterm infant and likely has the greatest impact on NEC associated with delayed feeding.
NEC-Like Diseases (NLDs)
In defining NLDs the first tenet is to understand why they are not “true” NEC. If one recalls the three preconditions of NEC (a naïve bowel, food in the bowel and a trigger event), these criteria are relatively simple to satisfy. We offer one additional requirement, namely that true NEC behaves according to Sartwell’s model of disease onset. This dramatically improves the rigor of such a definition, allowing us to remove entities that are different because some event or agent has been introduced to the patient that has changed their baseline vulnerability.
NLD Associated with IVIG Administration for Red Blood Cell Hemolysis
There are three reports with a total of 16 cases of IVIG associated NLD in the literature.171–173 Eleven of these received the IVIG for ABO incompatibility whereas five had rH disease. None of those with rH disease required surgery but seven of the ABO cases did and one of them died. Although a small sample, this is a significant difference (p < 0.05) in morbidity by blood type. It seems likely that IVIG associated NLD might be similar in nature to NECw/pRBCs. One important difference is: infants with IVIG associated NLD were often not fed or minimally fed. In other words, they failed to meet the feeding criteria for NEC. Another important difference is that they occur too early to be consistent with Sartwell’s model. We postulate that this disease may represent the extreme example of opsonization effect, using anti-AB immunoglobulin in the IVIG to opsonize AB blood group type antigens expressed on the enterocytes with sufficient vigor to induce apoptosis, necrosis and pneumatosis without first acquiring substantial enteral flora. We do not know if these infants had eosinophilia.
A sensible strategy for reducing NLD associated with IVIG is to abstain from IVIG use in infants with ABO disease (which can usually be managed without it).
NLD Associated with Use of Simply Thick
In July of 2011, the FDA issued a warning regarding Simply Thick™ following 15 cases of NEC that had been reported to them.174 Shortly thereafter they closed one of the three plants that make the product. That plant was closed due to lack of adequate sterility. Thus far the only description of this entity in the literature is a case series from Wake Forrest.175 The report describes three preterm infants with colonic NEC acquired well after the period predicted by Sartwell’s model (either at discharge or post discharge at home). The infants were all on Simply Thick™ for reflux. Simply Thick™ is made from natural gum, which is nearly impossible to digest in the absence of endogenous bacteria growing in the thickening product itself. If gum is fermented into injurious short chain fatty acids then absorbed directly across the colonic mucosa, a colonic version of NEC may ensue in infants who would otherwise be out of the window of susceptibility.
We recommend that no natural gum thickeners be used in the neonatal population, since it is always possible that a company utilizing natural gum could have a failure in the sterility of its processing. Synthetic thickeners should not have this risk.
NLD Associated with Gastroschisis
Gastroschisis is an abdominal wall birth defect that results in prolonged delays in feeding due to bowel dysmotility. Dated reports estimate the incidence of “NEC” in gastroschisis infants to be 10 times that of their gestational contemporaries.176,177 Anecdotal experience with gastroschisis indicates that it does not adhere to Sartwell’s model and that infants have often been on full feeds for extended periods before they manifest the one ileus episode that yields pneumatosis. Many infants with gastroschisis are NPO for prolonged periods, likely leading to significant mucosal atrophy. In trying to understand this association, we believe that chronic dysmotility increases floral skew and overgrowth, subsequently resulting in NEC. Little is known about the maturation of adaptive immunity in infants with gastroschisis (it is possible that they do not mature as other infants do because of the prolonged exposure of the bowel to amniotic fluid). Clearly more research is needed, but “NEC” associated with gastroschisis should be considered an NLD on the basis of a common congenital defect that is associated with exponential risk (for gestation) and discontinuity with Sartwell’s model.
At this time, we do not have any unique insight for the prevention of NLD associated with gastroschisis. An awareness of heightened risk may be sufficient to help clinicians reduce its incidence until better data is available.
Typhlitis
Few people would categorize typhlitis (neutropenic enterocolitis) as NEC, but if one considers this carefully, it has many of the same properties (including food in the bowel, a trigger event and the finding of pneumatosis). Typhlitis is not NEC because the bowel is not naïve (or neonatal) and it does not fit with Sartwell’s model. The bowel has been reconditioned to the innate immune state by chemotherapy. Not surprisingly, it occurs most commonly in patients with leukemia and lymphoma, in whom the adaptive immune system is specifically damaged.178,179 We include typhlitis as a NLD so that our classification system will be complete, but offer no opinion on its management.
WHAT IS THE FUTURE OF NEC RESEARCH?
By classifying NEC by its risk factors and triggers, five subsets can be established. The evidence is stronger for some than others and much work is needed, but this reorganization maps definitive strategies for NEC prevention. Hopefully one day NEC will be like central line infections. Each unit will count the numbers of days, if not the months and years, between NEC cases. For some it is already happening.180,181 Such institutions generally have the benefit of having mostly inborn patients and thus full control of clinical support decisions. It was once like this with central line infections, until we developed universal definitions and NICUs were able to compare infection data with transparency.182 NEC reductionism will set and then reset new research priorities as we systematically work to minimize the incidence within the individual NEC subsets. Basic research will continue to refine our understanding of NEC subsets and further validate them. In the past the NICHD has held think tanks on NEC research at pivotal junctures (and some of the authors of this review have been participants).183,184 Perhaps it is time to do so again.
Surgical NEC, adjusted for inflation since the time of the original publication,185 is now estimated to add an additional $234,603 per patient and 60 additional hospital days (with 41% mortality). Medical NEC adds an additional $92,858 per patient and 22 additional hospital days (with 22% mortality). In a single year the VON documented nearly 5000 cases of NEC in its NICUs in the United States.186 The Pediatrix Corporation documented over 10,000 cases in the United States over 12 years in all NICU admits.29 No one knows how many cases of NEC there are a year in the United States, but likely it is above 10,000. If true, we are spending more than $1.5 billion dollars a year on NEC with hospital costs alone. Much of this is borne by the American taxpayer through the Medicaid program. We must re-engage in a concerted national effort to reduce this impact.
PUTTING ALL THIS INFORMATION TOGETHER AT THE BEDSIDE
One of the challenges of a review of this magnitude is information overload and discerning key points to take to the bedside. The first message is that NEC reductionism improves one’s ability to predict the types and timing of triggers that precipitate NEC. The second is that preterm NEC almost always involves multiple phenomena. For example, imagine a 24 week gestation infant who is now corrected to 32 4/7 weeks, stable on room air and tolerating full volume bolus feeds of formula, who suddenly re-develops apnea and has a non-specific distal ileus. The infant has a lymphocytosis of 53% on the CBC and a hematocrit of 24.4%. This patient actually has five concurrent risk factors for NEC: 1) this patient fits the timing of Sartwell’s model for disease onset, 2) The baby is anemic and this may or may not be related to the apnea. If one’s first act is to transfuse, this may increase the risk of NEC (especially if the infant’s blood type is AB and it has already had multiple transfusions that were each associated with transient eosinophilia). 3) the patient is being bolus fed, which creates postprandial blood flow effects (and thus relative mesenteric ischemia), 4) the infant has a lymphocytosis, and an ileus, consistent with an enteral-viral infectious trigger, 5) the infant is not on breast milk as its primary nutrition source and thus does not have many of its NEC protective effects. Reasonable interventional strategies in such an infant might include: stopping feeds, a 48 hour rule out with antibiotics to suppress luminal flora (or longer if pneumatosis becomes evident on x-ray) and quarantine in isolation. Delay or avoidance of transfusion by return to nasal cannula support for apnea might be one way to deal with anemia/apnea (versus typed and matched transfusion while NPO). The decision to use a Replogle tube would depend upon the extent of the ileus. If it appears more obstructive in nature than global, glycerin might be a reasonable alternative. The point we are trying to make is that the support strategies chosen should best address as many of the underlying NEC risk factors as possible. Individual clinicians will undoubtedly choose a variety of interventions, but this may not matter if they are addressing the right issues. The addition of Sartwell’s model and NEC reductionism to our clinical lexicon shifts the focus from just clinical support of the immediately apparent clinical issues - to support integrated with NEC prevention.
Clinicians who are cognizant of the multifactorial risks for NEC may reduce its morbidity and mortality in their units. At Tulane-Lakeside Hospital in New Orleans (a level III regional NICU), where the concept of NEC reductionism has been employed for more than two and half years, there has not been a single death or bowel resection associated with NEC since 2009 (total patients admitted = 604 and counting, with no true NEC, 3 cases of SIP and 2 cases of NLD associated with gastroschisis).181 Anecdotes are not data, but the reader deserves to know if the architect of NEC reductionism can generate meritorious outcomes with it.
CONCLUSION
The separation of NEC from SIP (Gordon’s classification) and the subsequent reduction of NEC into subgroups (NEC reductionism) together represent an improved operational framework for more accurately assessing NEC incidence and origin. Basic science has better elucidated the biologic basis of NEC vulnerability and the application of Sartwell’s model has helped translate these findings, such that we now better understand when infants are at greatest risk to acquire NEC during their hospital course. The time is now to bring these advances to the bedside. When a clinician can accurately identify and understand NEC, they can work to prevent it.
ACKNOWLEDGEMENTS
This work was supported in part by the NIH Grants K08HD061607 (to JHW) and HD59142 (to AM). We thank Dr Marc Rothenberg for sharing his expertise on eosinophils.
Footnotes
Author Disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Rabl R. Necrotizing enterocolitis in premature infants. Beitr Pathol Anat. 1957;117:266–282. [PubMed] [Google Scholar]
- 2.Eroess A, Loerinczi K, Nemeth N. Enterocolitis necroticans in newborn infants. Kinderarztl Prax. 1959;27:403–406. [PubMed] [Google Scholar]
- 3.Berdon WE, Grossman H, Baker DH, Mizrahi A, Barlow O, Blanc WA. Necrotizing enterocolitis in the premature infant. Radiology. 1964;83:879–887. doi: 10.1148/83.5.879. [DOI] [PubMed] [Google Scholar]
- 4.Mizrahi A, Barlow O, Berdon W, Blanc WA, Silverman WA. Necrotizing enterocolitis in the premature infant. J Pediatr. 1965;66:697–705. doi: 10.1016/s0022-3476(65)80003-8. [DOI] [PubMed] [Google Scholar]
- 5.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis in the absence of pneumatosis intestinalis. Am J Dis Child. 1982;136:618–620. doi: 10.1001/archpedi.1982.03970430050014. [DOI] [PubMed] [Google Scholar]
- 7.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. Am J Dis Child. 1981;135:603–607. doi: 10.1001/archpedi.1981.02130310009005. [DOI] [PubMed] [Google Scholar]
- 8.Grosfeld JL, Cheu H, Schlatter M, West KW, Rescorla FJ. Changing trends in necrotizing enterocolitis. Experience with 302 cases in two decades. Ann Surg. 1991;214:300–306. doi: 10.1097/00000658-199109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luig M, Lui K, et al. NSW & ACT NICUS Group. Epidemiology of necrotizing enterocolitis–Part I: Changing regional trends in extremely preterm infants over 14 years. J Paediatr Child Health. 2005;41:169–173. doi: 10.1111/j.1440-1754.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 10.Battaglia JD. Neonatal surgery: changing patterns 1972–1980. J Pediatr Surg. 1982;17:666–669. doi: 10.1016/s0022-3468(82)80130-9. [DOI] [PubMed] [Google Scholar]
- 11.Kliegman RM, Hack M, Jones P, Fanaroff AA. Epidemiologic study of necrotizing enterocolitis among low-birth-weight infants. Absence of identifiable risk factors. J Pediatr. 1982;100:440–444. doi: 10.1016/s0022-3476(82)80456-3. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter A. Spontaneous pneumoperitoneum in the newborn;report of a case. N Engl J Med. 1956;254:694–696. doi: 10.1056/NEJM195604122541504. [DOI] [PubMed] [Google Scholar]
- 14.Aschner JL, Deluga KS, Metlay LA, Emmens RW, Hendricks-Munoz KD. Spontaneous focal gastrointestinal perforation in very low birth weight infants. J Pediatr. 1988;113:364–367. doi: 10.1016/s0022-3476(88)80285-3. [DOI] [PubMed] [Google Scholar]
- 15.Gordon P, Rutledge J, Sawin R, Thomas S, Woodrum D. Early postnatal dexamethasone increases the risk of focal small bowel perforation in extremely low birth weight infants. J Perinatol. 1999;19:573–577. doi: 10.1038/sj.jp.7200269. [DOI] [PubMed] [Google Scholar]
- 16.Gordon PV, Young ML, Marshall DD. Focal small bowel perforation: an adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J Perinatol. 2001;21:156–160. doi: 10.1038/sj.jp.7200520. [DOI] [PubMed] [Google Scholar]
- 17.Garland J, Colleen A, Thomas P, Whitehead V, Brand J, Winston J, et al. A three-day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomized trial. Pediatrics. 1999;104:91–99. doi: 10.1542/peds.104.1.91. [DOI] [PubMed] [Google Scholar]
- 18.Stark AR, Carlo WA, Tyson JE, Papile L-A, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. N Engl J Med. 2001;344:95–101. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 19.Vermont Oxford Network Steroid Study Group. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics. 2001;108:741–748. doi: 10.1542/peds.108.3.741. [DOI] [PubMed] [Google Scholar]
- 20.Sinkin R, Harry D, Horgan M, Gallaher K, Cox C, Maniscalco W, et al. Early dexamethasone – attempting to prevent chronic lung disease. Pediatrics. 2000;105:542–548. doi: 10.1542/peds.105.3.542. [DOI] [PubMed] [Google Scholar]
- 21.Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. 2009;65:138–144. doi: 10.1203/PDR.0b013e31818c7920. [DOI] [PubMed] [Google Scholar]
- 22.Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK the Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 23.Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? J Perinatol. 2007;27:661–671. doi: 10.1038/sj.jp.7211782. [DOI] [PubMed] [Google Scholar]
- 24.Gordon PV. What progress looks like in NEC research. J Perinatol. 2011;31:149. doi: 10.1038/jp.2010.164. [DOI] [PubMed] [Google Scholar]
- 25.Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol. 2006;26:185–188. doi: 10.1038/sj.jp.7211439. [DOI] [PubMed] [Google Scholar]
- 26.Attridge JT, Herman AC, Gurka MJ, Griffin MP, McGahren ED, Gordon PV. Discharge outcomes of extremely low birth weight infants with spontaneous intestinal perforations. J Perinatol. 2006;26:49–54. doi: 10.1038/sj.jp.7211407. [DOI] [PubMed] [Google Scholar]
- 27.Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012;32:199–204. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 29.González-Rivera R, Culverhouse RC, Hamvas A, Tarr PI, Warner BB. The age of necrotizing enterocolitis onset: an application of Sartwell’s incubation period model. J Perinatol. 2011;31:519–523. doi: 10.1038/jp.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon P, Thibeau S, Pennier C, Ginsberg H, Lunyong V, Cortez M, et al. Is lymphocytosis an adjunct predictor of NEC mortality in low gestation infants? e-J Neonatal Res. 2011;2:29–36. [Google Scholar]
- 31.Gordon PV, Herman AC, Marcinkiewicz M, Gaston BM, Laubach VE, Aschner JL. A neonatal mouse model of intestinal perforation: investigating the harmful synergism between glucocorticoids and indomethacin. J Pediatr Gastroenterol Nutr. 2007;45:509–519. doi: 10.1097/MPG.0b013e3181558591. [DOI] [PubMed] [Google Scholar]
- 32.Israel EJ, Schiffrin EJ, Carter EA, Freiberg E, Walker WA. Prevention of necrotizing enterocolitis in the rat with prenatal cortisone. Gastroenterology. 1990;99:1333–1338. doi: 10.1016/0016-5085(90)91158-3. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki PT, Caniano DA, Hammond S, Giannone PJ, Besner GE, Reber KM, Nankervis CA. Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr. 2007;150:40–55. doi: 10.1016/j.jpeds.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Bohnhorst B, Kuebler JF, Rau G, Gluer S, Ure B, Doerdelmann M. Portal venous gas detected by ultrasound differentiates surgical NEC from other acquired neonatal intestinal diseases. Eur J Pediatr Surg. 2011;21:12–17. doi: 10.1055/s-0030-1265204. [DOI] [PubMed] [Google Scholar]
- 35.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6:e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, Hackam DJ. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res. 2011;69:183–188. doi: 10.1203/PDR.0b013e3182093280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Good M, Sodhi C, Siggers R, Prindle T, Branca M, Russo A, et al. Epithelial Growth Factor Attenuates the Severity of Experimental Necrotizing Enterocolitis and Inhibits Toll-Like Receptor 4 Signaling in Enterocytes. E-PAS. 2011:2720–2723. [Google Scholar]
- 39.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology. 2011;140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emami CN, Mittal R, Wang L, Ford HR, Prasadarao NV. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J Surg Res. 2012;172(1):18–28. doi: 10.1016/j.jss.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maheshwari A. Role of cytokines in human intestinal villous development. Clin Perinatol. 2004;31:143–155. doi: 10.1016/j.clp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Rognum TO, Thrane S, Stoltenberg L, Vege A, Brandtzaeg P. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res. 1992;32:145–149. doi: 10.1203/00006450-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Braegger CP, Spencer J, MacDonald TT. Ontogenetic aspects of the intestinal immune system in man. Int J Clin Lab Res. 1992;22:1–4. [PubMed] [Google Scholar]
- 44.MacDonald TT, Spencer J. Ontogeny of the gut-associated lymphoid system in man. Acta Paediatr Suppl. 1994;83:3–5. doi: 10.1111/j.1651-2227.1994.tb13219.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoyne GF, Callow MG, Kuo MC, Thomas WR. Presentation of peptides and proteins by intestinal epithelial cells. Immunology. 1993;80:204–208. [PMC free article] [PubMed] [Google Scholar]
- 46.Maheshwari A, Lacson A, Lu W, Fox SE, Barleycorn AA, Christensen RD, Calhoun DA. Interleukin-8/CXCL8 forms an autocrine loop in fetal intestinal mucosa. Pediatr Res. 2004;56:240–249. doi: 10.1203/01.PDR.0000133196.25949.98. [DOI] [PubMed] [Google Scholar]
- 47.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daig R, Rogler G, Aschenbrenner E, Vogl D, Falk W, Gross V, et al. Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut. 2000;46:350–358. doi: 10.1136/gut.46.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11(5):369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Rumbo M, Schiffrin EJ. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell Mol Life Sci. 2005;62:1288–1296. doi: 10.1007/s00018-005-5033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci U S A. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, Vu HL, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80:492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 55.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maheshwari A, Zemlin M. Ontogeny of the intestinal immune system. Haematologica Reports. 2006;10:18–26. [Google Scholar]
- 57.MacDonald TT, Spencer J. Ontogeny of the mucosal immune response. Springer Semin Immunopathol. 1990;12:129–137. doi: 10.1007/BF00197501. [DOI] [PubMed] [Google Scholar]
- 58.Harvey J, Jones DB, Wright DH. Differential expression of MHC- and macrophage-associated antigens in human fetal and postnatal small intestine. Immunology. 1990;69:409–415. [PMC free article] [PubMed] [Google Scholar]
- 59.Spencer J, MacDonald TT, Isaacson PG. Heterogeneity of non-lymphoid cells expressing HLA-D region antigens in human fetal gut. Clin Exp Immunol. 1987;67:415–424. [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 61.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 62.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 63.Christensen RD, Lambert DK, Gordon PV, Baer VL, Gerday E, Henry E. Neonates presenting with bloody stools and eosinophilia can progress to two different types of necrotizing enterocolitis. J Perinatol. 2011 Nov 10; doi: 10.1038/jp.2011.163. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Hair AB, Swanson JR, Attridge JT. Lymphocytosis at necrotizing enterocolitis presentation is associated with higher mortality, increased feeding volumes and more formula exposure in an 11 year, single center retrospective study. e-J Neonatal Res. 2011;2:37–43. [Google Scholar]
- 65.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 66.Treszl A, Heninger E, Kalman A, Schuler A, Tulassay T, Vasarhelyi B. Lower prevalence of IL-4 receptor alpha-chain gene G variant in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg. 2003;38:1374–1378. doi: 10.1016/s0022-3468(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 67.Heninger E, Treszl A, Kocsis I, Derfalvi B, Tulassay T, Vasarhelyi B. Genetic variants of the interleukin-18 promoter region (−607) influence the course of necrotising enterocolitis in very low birth weight neonates. Eur J Pediatr. 2002;161:410–411. doi: 10.1007/s00431-002-0968-y. [DOI] [PubMed] [Google Scholar]
- 68.Banyasz I, Bokodi G, Vasarhelyi B, Treszl A, Derzbach L, Szabo A, et al. Genetic polymorphisms for vascular endothelial growth factor in perinatal complications. Eur Cytokine Netw. 2006;17:266–270. [PubMed] [Google Scholar]
- 69.Moonen RM, Paulussen AD, Souren NY, Kessels AG, Rubio-Gozalbo ME, Villamor E. Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res. 2007;62:188–190. doi: 10.1203/PDR.0b013e3180a0324e. [DOI] [PubMed] [Google Scholar]
- 70.Sampath V, Le M, Lane L, Patel AL, Cohen JD, Simpson PM, et al. The NFKB1 (g.−24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res. 2011;169:e51–e57. doi: 10.1016/j.jss.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:100–106. doi: 10.1053/j.semperi.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soliman A, Michelsen KS, Karahashi H, Lu J, Meng FJ, Qu X, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS One. 2010;15:e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stearns JC, Lynch MD, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD. Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1:170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moshfegh A, Lothian C, Halldén G, Marchini G, Lagercrantz H, Lundahl J. Neonatal eosinophils possess efficient Eotaxin/IL-5- and N-formyl-methionyl-leucyl-phenylalanine-induced transmigration in vitro. Pediatr Res. 2005;58(1):138–142. doi: 10.1203/01.PDR.0000156230.94757.47. [DOI] [PubMed] [Google Scholar]
- 82.Bonnemaison E, Lanotte P, Cantagrel S, Thionois S, Quentin R, Chamboux C, Laugier J. Comparison of fecal flora following administration of two antibiotic protocols for suspected maternofetal infection. Biol Neonate. 2003;84:4–10. doi: 10.1159/000073639. [DOI] [PubMed] [Google Scholar]
- 83.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ. NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chong E, Reynold J, Shaw J, Forur L, Delmore P, Bloom BT, Gordon PV. Results of a two Center, before and after study of pipericillin-tazobactam versus ampicillin and gentamicin as therapy for suspected sepsis at birth in neonates less than 1500 grams. E-PAS. 2012 doi: 10.1038/jp.2012.169. 3852.645. [DOI] [PubMed] [Google Scholar]
- 87.Alfaleh K, Anabrees J, Bassler D. Probiotics reduce the risk of necrotizing enterocolitis in preterm infants: a meta-analysis. Neonatology. 2010;97:93–99. doi: 10.1159/000235684. [DOI] [PubMed] [Google Scholar]
- 88.Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol. 2011;45(Suppl S):133–138. doi: 10.1097/MCG.0b013e318228b799. [DOI] [PubMed] [Google Scholar]
- 89.Mihatsch WA. What is the power of evidence recommending routine probiotics for necrotizing enterocolitis prevention in preterm infants? Curr Opin Clin Nutr Metab Care. 2011;14:302–306. doi: 10.1097/MCO.0b013e3283454e78. [DOI] [PubMed] [Google Scholar]
- 90.Millar M, Wilks M, Fleming P, Costeloe K. Should the use of probiotics in the preterm be routine? Arch Dis Child Fetal Neonatal Ed. 2012;97:F70–F74. doi: 10.1136/adc.2009.178939. [DOI] [PubMed] [Google Scholar]
- 91.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 92.Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2007;17 doi: 10.1002/14651858.CD002971.pub2. CD002971. [DOI] [PubMed] [Google Scholar]
- 93.Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, McElroy SJ. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5:522–532. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McElroy SJ, Prince LS, Weitkamp JH, Reese J, Slaughter JC, Polk DB. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 96.Richardson WM, Sodhi CP, Russo A, Siggers RH, Afrazi A, Gribar SC, et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qureshi FG, Leaphart C, Cetin S, Li J, Grishin A, Watkins S, et al. Increased expression and function of integrins in enterocytes by endotoxin impairs epithelial restitution. Gastroenterology. 2005;128:1012–1022. doi: 10.1053/j.gastro.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 99.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 100.Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr. 2001;32:449–453. doi: 10.1097/00005176-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 101.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 103.Chan KL, Wong KF, Luk JM. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World J Gastroenterol. 2009;15:4745–4752. doi: 10.3748/wjg.15.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu J, Jilling T, Li D, Caplan MS. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2007;61:427–432. doi: 10.1203/pdr.0b013e3180332ca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anttila A, Kauppinen H, Koivusalo A, Heikkila P, Savilahti E, Rintala R. T-cell-mediated mucosal immunity is attenuated in experimental necrotizing enterocolitis. Pediatr Surg Int. 2003;19:326–330. doi: 10.1007/s00383-003-1004-7. [DOI] [PubMed] [Google Scholar]
- 107.Adinolfi M, Lessof MH. Development of humeral and cellular immunity in man. J Medical Genetics. 1972;9:86. doi: 10.1136/jmg.9.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spencer J, Dillon SB, Isaacson PG, MacDonald TT. T cell subclasses in fetal human ileum. Clin Exp Immunol. 1986;65:553–558. [PMC free article] [PubMed] [Google Scholar]
- 109.MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolfs TG, Buurman WA, Zoer B, Moonen RM, Derikx JP, Thuijls G, et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS One. 2009;4:e5837. doi: 10.1371/journal.pone.0005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silverstein AM, Lukes RJ. Fetal response to antigenic stimulus. I. Plasmacellular and lymphoid reactions in the human fetus to intrauterine infection. Laboratory Investigation. 1962;11:918–932. [PubMed] [Google Scholar]
- 112.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;12:437–446. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 113.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;9:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Veltkamp C, Ruhwald R, Giesem T, Autschbach F, Kaden I, Veltkamp R, et al. CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm Bowel Dis. 170:3939–3943. doi: 10.1097/00054725-200606000-00002. 206. [DOI] [PubMed] [Google Scholar]
- 115.Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- 116.Wildin RS, Ramsdell F, Peake J, Feng TT, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature Genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 117.Neu J, Chen M, Beierle E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:137–144. doi: 10.1053/j.sempedsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Vignail DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weitkamp JH, Rudzinski E, Koyama T, Correa H, Alberty JB, Polk DB. Ontogeny of FOXP3+ regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr Dev Pathol. 2009;12:443–449. doi: 10.2350/08-09-0533.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, et al. Necrotizing enterocolitis is characterized by disrupted immune regulation and diminished mucosal regulatory (FOXP3+) / effector (CD4+, CD8+) T cell ratios. Gut. 2012 Jan 20; doi: 10.1136/gutjnl-2011-301551. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopeckny J, et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eibl MM, Wolf HM, Fürnkranz H, Rosenkranz A. Prophylaxis of necrotizing enterocolitis by oral IgA-IgG: review of a clinical study in low birth weight infants and discussion of the pathogenic role of infection. N Engl J Med. 1988;319:1–7. [Google Scholar]
- 125.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–1593. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defense of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19260. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2:119–129. doi: 10.1016/j.chom.2007.06.010. 200. [DOI] [PubMed] [Google Scholar]
- 129.Christensen RD, Lambert DK, Schmutz N, Stoddard RA. Fatal bowel necrosis in two polycythemic term neonates. Fetal Pediatr Pathol. 2008;27:41–44. doi: 10.1080/15513810802028597. [DOI] [PubMed] [Google Scholar]
- 130.Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. 2007;27:437–438. doi: 10.1038/sj.jp.7211738. [DOI] [PubMed] [Google Scholar]
- 131.Maayan-Metzger A, Itzchak A, Mazkereth R, Kuint J. Necrotizing enterocolitis in full-term infants: case-control study and review of the literature. J Perinatol. 2004;24:494–499. doi: 10.1038/sj.jp.7211135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg. 2003;38:1039–1042. doi: 10.1016/s0022-3468(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 133.Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL, et al. Is“transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50:1106–1112. doi: 10.1111/j.1537-2995.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 134.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–540. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 135.Thomson T, Habeeb O, Dechristopher PJ, Glynn L, Yong S, Muraskas J. Decreased survival in necrotizing enterocolitis is significantly associated with neonatal and maternal blood group: the AB isoagglutinin hypothesis. J Perinatol. 2012;32:626–630. doi: 10.1038/jp.2011.150. [DOI] [PubMed] [Google Scholar]
- 136.Christensen RD, Lambert DK, Gordon PV, Baer VL, Gerday E, Henry E. Neonates presenting with bloody stools and eosinophilia can progress to two different types of necrotizing enterocolitis. J Perinatol. 2011 Nov 10; doi: 10.1038/jp.2011.163. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 137.Juul SE, Haynes JW, McPherson RJ. Evaluation of eosinophilia in hospitalized preterm infants. J Perinatol. 2005;25:182–188. doi: 10.1038/sj.jp.7211226. [DOI] [PubMed] [Google Scholar]
- 138.Singh R, Visintainer PF, Frantz ID, 3rd, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–182. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moshfegh A, Lothian C, Halldén G, Marchini G, Lagercrantz H, Lundahl J. Neonatal eosinophils possess efficient Eotaxin/IL-5- and N-formyl-methionyl-leucyl-phenylalanine-induced transmigration in vitro. Pediatr Res. 2005;58:138–142. doi: 10.1203/01.PDR.0000156230.94757.47. [DOI] [PubMed] [Google Scholar]
- 140.Couselo M, Aguar M, Ibáñez V, Mangas L, García-Sala C. Relation between packed red blood cell transfusion and severity of necrotizing enterocolitis in premature infants. Cir Pediatr. 2011;24:137–141. [PubMed] [Google Scholar]
- 141.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–641. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 142.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–409. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 143.El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. 2011;31:183–187. doi: 10.1038/jp.2010.157. [DOI] [PubMed] [Google Scholar]
- 144.Perciaccante JV, Young TE. Necrotizing enterocolitis associated with packed red blood cell transfusions in premature neonates. E-PAS. 2008 5839.8. [Google Scholar]
- 145.Gordon PV, Clark R. In response to the case report of allergic enterocolitis in a preterm neonate: how prevalent is systemic eosinophilia with NEC? J Perinatol. 2011;31:297–298. doi: 10.1038/jp.2010.192. [DOI] [PubMed] [Google Scholar]
- 146.Srinivasan P, Brandler M, D’Souza A, Millman P, Moreau H. Allergic enterocolitis presenting as recurrent necrotizing enterocolitis in preterm neonates. J Perinatol. 2010;30:431–433. doi: 10.1038/jp.2009.153. [DOI] [PubMed] [Google Scholar]
- 147.Osterlund P, Smedberg T, Hakulinen A, Heikkilä H, Järvinen KM. Eosinophil cationic protein in human milk is associated with development of cow’s milk allergy and atopic eczema in breast-fed infants. Pediatr Res. 2004;55:296–301. doi: 10.1203/01.PDR.0000106315.00474.6F. [DOI] [PubMed] [Google Scholar]
- 148.Coviello C, Rodriquez DC, Cecchi S, Tataranno ML, Farmeschi L, Mori A, Buonocore G. Different clinical manifestation of cow’s milk allergy in two preterm twins newborns. J Matern Fetal Neonatal Med. 2012;25:132–133. doi: 10.3109/14767058.2012.663171. [DOI] [PubMed] [Google Scholar]
- 149.Snyder CL, Hall M, Sharma V, St Peter SD. Seasonal variation in the incidence of necrotizing enterocolitis. Pediatr Surg Int. 2010;26:895–898. doi: 10.1007/s00383-010-2675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meinzen-Derr J, Morrow AL, Hornung RW, Donovan EF, Dietrich KN, Succop PA. Epidemiology of necrotizing enterocolitis temporal clustering in two neonatology practices. J Pediatr. 2009;154:656–661. doi: 10.1016/j.jpeds.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Birenbaum E, Handsher R, Kuint J, Dagan R, Raichman B, Mendelson E, et al. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am J Perinatol. 1997;14:469–473. doi: 10.1055/s-2007-994182. [DOI] [PubMed] [Google Scholar]
- 152.Castro R. Echovirus 7 infection and necrotizing enterocolitis-like symptoms in a premature infant. J Perinatol. 2000;20:558–561. doi: 10.1038/sj.jp.7200467. [DOI] [PubMed] [Google Scholar]
- 153.Rousset S, Moscovici O, Lebon P, Barbet JP, Helardot P, Mace B, et al. Intestinal lesions containing coronavirus-like particles in neonatal necrotizing enterocolitis: an ultrastructural analysis. Pediatrics. 1984;73:218–224. [PubMed] [Google Scholar]
- 154.Gessler P, Bischoff GA, Wiegand D, Essers B, Bossart W. Cytomegalovirus-associated necrotizing enterocolitis in a preterm twin after breastfeeding. J Perinatol. 2004;24:124–126. doi: 10.1038/sj.jp.7211042. [DOI] [PubMed] [Google Scholar]
- 155.Yolken RH, Franklin CC. Gastrointestinal adenovirus: an important cause of morbidity in patients with necrotizing enterocolitis and gastrointestinal surgery. Pediatr Infect Dis. 1985;4:42–47. [PubMed] [Google Scholar]
- 156.Johnson FE, Crnic DM, Simmons MA, Lilly JR. Association of fatal Coxsackie B2 viral infection and necrotizing enterocolitis. Arch Dis Child. 1977;52:802–804. doi: 10.1136/adc.52.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parvey LS, Ch’ien LT. Neonatal herpes simplex virus infection introduced by fetal-monitor scalp electrodes. Pediatrics. 1980;65:1150–1153. [PubMed] [Google Scholar]
- 158.Boccia D, Stolfi I, Lana S, Moro ML. Nosocomial necrotising enterocolitis outbreaks: epidemiology and control measures. Eur J Pediatr. 2001;160:385–391. doi: 10.1007/s004310100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lodha A, de Silva N, Petric M, Moore AM. Human torovirus: a new virus associated with neonatal necrotizing enterocolitis. Acta Paediatrica. 2005;94:1085–1088. doi: 10.1111/j.1651-2227.2005.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 160.Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, et al. Mother-to-child transmission of Chikungunya virus infection. J Pediatr Infect Dis. 2007;26:811–815. doi: 10.1097/INF.0b013e3180616d4f. [DOI] [PubMed] [Google Scholar]
- 161.Turcios-Ruiz RM, Axelrod P, St John K, Bullitt E, Donahue J, Robinson N, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008;153:339–344. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bagci S, Eis-Hubinger AM, Franz AR, Bierbaum G, Heep A, Schildgen O, et al. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis. 2008;27:347–350. doi: 10.1097/INF.0b013e318162a17a. [DOI] [PubMed] [Google Scholar]
- 163.Sharma R, Garrison RD, Tepas JJ, 3rd, Mollitt DL, Pieper P, Hudak ML, et al. Rotavirus-associated necrotizing enterocolitis: an insight into a potentially preventable disease? J Pediatr Surg. 2004;39:453–457. doi: 10.1016/j.jpedsurg.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 164.Christensen R, Baer V, Gordon P, Henry E, Whitaker C, Andres R, Bennett S. Reference ranges for lymphocyte counts of neonates: associations between abnormal counts and outcomes. J Pediatr. doi: 10.1542/peds.2011-2661. (In Press). [DOI] [PubMed] [Google Scholar]
- 165.Thompson A, Bizzarro M, Yu S, Diefenbach K, Simpson BJ, Moss RL. Risk factors for necrotizing enterocolitis totalis: a case-control study. J Perinatol. 2011;31:730–738. doi: 10.1038/jp.2011.18. [DOI] [PubMed] [Google Scholar]
- 166.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ehrenkranz RA, Das A, Wrage LA, Poindexter BB, Higgins RD, Stoll BJ, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res. 2011;69:522–529. doi: 10.1203/PDR.0b013e318217f4f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, et al. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134:1467–1474. doi: 10.1093/jn/134.6.1467. [DOI] [PubMed] [Google Scholar]
- 169.Bowling TE, Silk DB. Intestinal responses induced by enteral feeding. Nutrition. 1995;11:304–307. [PubMed] [Google Scholar]
- 170.Zonta S, Doni M, Alessiani M, Lovisetto F, Vigano J, Mazzilli M, et al. Elemental enteral nutrition preserves the mucosal barrier and improves the trophism of the villi after small bowel transplantation in piglets. Transplant Proc. 2007;39:2024–2027. doi: 10.1016/j.transproceed.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 171.Figueras-Aloy J, Rodríguez-Miguélez JM, Iriondo-Sanz M, Salvia-Roiges MD, Botet-Mussons F, Carbonell-Estrany X. Intravenous immunoglobulin and necrotizing enterocolitis in newborns with hemolytic disease. Pediatrics. 2010;125:139–144. doi: 10.1542/peds.2009-0676. [DOI] [PubMed] [Google Scholar]
- 172.Krishnan L, Pathare A. Necrotizing enterocolitis in a term neonate following intravenous immunoglobulin therapy. Indian J Pediatr. 2011;78:743–744. doi: 10.1007/s12098-010-0334-4. [DOI] [PubMed] [Google Scholar]
- 173.Navarro M, Negre S, Matoses ML, Golombek SG, Vento M. Necrotizing enterocolitis following the use of intravenous immunoglobulin for haemolytic disease of the newborn. Acta Paediatr. 2009;98:1214–1217. doi: 10.1111/j.1651-2227.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 174.U.S. Food and Drug Administration. FDA safety alerts for human products: Simply Thick ®. [Retrieved on 19 August 2012]; from http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm256257.htm.
- 175.Woods CW, Oliver T, Lewis K, Yang Q. Development of necrotizing enterocolitis in premature infants receiving thickened feeds using SimplyThick®. J Perinatol. 2012;32:150–152. doi: 10.1038/jp.2011.105. [DOI] [PubMed] [Google Scholar]
- 176.Oldham KT, Coran AG, Drongowski RA, Baker PJ, Wesley JR, Polley TZ., Jr The development of necrotizing enterocolitis following repair of gastroschisis: a surprisingly high incidence. J Pediatr Surg. 1988;23:945–946. doi: 10.1016/s0022-3468(88)80392-0. [DOI] [PubMed] [Google Scholar]
- 177.Jayanthi S, Seymour P, Puntis JW, Stringer MD. Necrotizing enterocolitis after gastroschisis repair: a preventable complication? J Pediatr Surg. 1998;33:705–707. doi: 10.1016/s0022-3468(98)90191-9. [DOI] [PubMed] [Google Scholar]
- 178.El-Matary W, Soleimani M, Spady D, Belletrutti M. Typhlitis in children with malignancy: a single center experience. J Pediatr Hematol Oncol. 2011;33:e98–e100. doi: 10.1097/MPH.0b013e3181eda606. [DOI] [PubMed] [Google Scholar]
- 179.Moran H, Yaniv I, Ashkenazi S, Schwartz M, Fisher S, Levy I. Risk factors for typhlitis in pediatric patients with cancer. J Pediatr Hematol Oncol. 2009;31:630–634. doi: 10.1097/MPH.0b013e3181b1ee28. [DOI] [PubMed] [Google Scholar]
- 180.Lafayette General Medical Center. The Pavillion for women and children. Retrieved from http://www.lafayettegeneral.com/pavilion/Level-III-Neonatal-Intensive-Care-Unit-1/Key-Performance-Indicators-3. [Google Scholar]
- 181.Benjamin J, Chong E, Reynolds J, Gordon PV. Detailed analysis of NEC risks across a decade in a low incidence NICU: Can we drive the incidence of NEC toward zero? e-J Neonatol Res. 2012;2:182–189. [Google Scholar]
- 182.Li S, Bizzarro MJ. Prevention of central line associated bloodstream infections in critical care units. Curr Opin Pediatr. 2011;23:85–90. doi: 10.1097/MOP.0b013e328341d1da. [DOI] [PubMed] [Google Scholar]
- 183.Kliegman RM, Walker WA, Yolken RH. Necrotizing enterocolitis: research agenda for a disease of unknown etiology and pathogenesis. Pediatr Res. 1993;34:701–708. doi: 10.1203/00006450-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 184.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 185.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109:423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 186.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–1075. doi: 10.1016/j.jpedsurg.2009.02.013. [DOI] [PubMed] [Google Scholar]