Abstract
Background
There is on going controversy on the effect of experimentally induced hypertension on nociception. The effect of salt-loading-induced hypertension on pain was studied in male rats.
Method
Twenty-four male Sprague-Dawley rats (160–280 g) were divided into two groups. Group A (n = 12) was treated with normal-feed diet (control), while group B (n = 12) was treated with 8% salt-loaded diet for 10 weeks. After 10 weeks of the treatment, six rats each from groups A and B were used for blood pressure measurement, while the remaining six rats were used for both the tail-flick and formalin tests. Thermal and chemical pain test were assessed using tail immersion test (tail flick) and formalin test pain paradigms at onset of salt-loading diet and after 10 weeks of salt loading.
Results
Chronic administration of salt-loading diet caused significant increases (P < 0.001) in systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure. Moreover, salt-loading-induced hypertension was found to significantly reduce pain sensitivity in the tail-immersion test (P < 0.001) and in the early and late phase of the formalin test (P < 0.01). However, the hypoalgesia was higher in the late phase (94.8%) than in the early phase (56.8%) of the formalin test.
Conclusion
The present study suggests that high salt-loading-induced hypertension causes hypoalgesia in rats, which might be due more to reduction in inflammatory response.
Keywords: formalin test, tail-flick test
Video abstract
Introduction
Hypertension, which is a persistent increase in arterial blood pressure is often referred to as a “silent” killer, as it is well established that individuals with high blood pressure rarely experience subjective symptoms of their disorder.1 High blood pressure increases the risk of heart diseases and stroke, the first and third most common causes of death among African Americans.
According to the International Association for the Study of Pain, pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.2 Decreased sensitivity to noxious stimulation has been reported in hypertensive humans. Although investigations using human populations have been relatively few in number,3 these studies have consistently confirmed the presence of a decrease in pain threshold in hypertensive compared with normotensive controls.4–6 Even in normotensive humans, pain threshold has been shown to be positively correlated with resting systolic blood pressure levels.7 However, a number of studies suggest that sustained blood pressure elevation might not be a necessary factor in nociceptive threshold differences observed in hypertensive animals and pain sensitivity differences in hypertensive humans. Sitsen and de Long8 found that the reduction in pain perception in spontaneous hypertensive rats did not occur in rats which had experimentally induced hypertension by renal artery clip and deoxycorticosterone-salt treatment. Furthermore, it has been observed that significant blood pressure reductions following antihypertensive treatment did not produce significant pain threshold differences in humans.5 These last two findings seem to cast doubt on the relationship between hypertension and hypoalgesia, seeming to indicate that the observed hypoalgesia in hypertension is due to genetic factors and not to the hypertension per se. While hypertension can be experimentally induced by salt loading, constriction of renal blood flow, denervation of carotid sinus and aortic arch, just to mention a few, this study was designed to investigate the effect of salt-loading-induced hypertension on nociception in rats.
Materials and method
Animals and experimental design
Sex differences in pain perception have been reported in numerous studies, with pain thresholds and pain tolerance being lower in females than in males. Previous studies on the estradiol modulation of nociception produced equivocal results, with some demonstrating longer latencies,9,10 while another reported hyperalgesia.11 Moreover, the estrous cycle in female rats has been shown to affect pain perception.12,13 Therefore, we chose to investigate the effects of salt-loading hypertension on nociception in male rats.
Twenty-four male Sprague Dawley rats (160–280 g) were purchased from the Institute of Medical Research and Training, University of Ibadan College Hospital, Nigeria and were acclimated to their new environment. They were housed in plastic cages (n = 3/cage), and fed a standard laboratory diet (Bova Jay Feeds Nig. Ltd, Ogbomoso, Nigeria) with free access to tap water ad libitum. The rats were kept under conditions of uniform humidity and temperature on a 12-hour light–dark cycle. Study protocol and animal use were approved prior to the beginning of the study by our institutional research and ethical committee. All necessary protocols were followed to ensure the correct welfare of the animals.
Experimental protocol
After 2 weeks acclimatization to their new environment, animals were randomly divided in a blinded fashion into two treatment groups (A and B) consisting of 12 rats per group. Group A were fed with a normal diet for 10 weeks (control group); group B were fed with an 8% NaCl diet for 10 weeks (salt-loaded group). After 10 weeks of the treatment, six rats from each group were used for blood pressure measurement. In the remaining six rats in each group, both tail-flick and formalin tests were completed a day before the commencement of the treatment and again a day after the 10-week treatment, as described below.
Blood pressure measurement
Anesthesia was induced by an intraperitoneal injection of 0.6 mL/100 g bodyweight of 25% urethane. The animals were allowed to breathe room air (25°C) spontaneously through a small tracheotomy. The left femoral artery was cannulated and connected to a Grass 7D polygraph through a pressure transducer to monitor blood pressure.
Pain assessment method
The tail-flick test was done a week before the commencement of the treatment and repeated a day after the 10-week treatment. Animals were handled for 3 minutes and habituated to the testing room for 1 hour on two occasions before the day of testing and again on the day of testing. Each rat was removed from its home cage and gently restrained in a towel, and its tail was immersed in 54°C water.14 The latency to flick the tail was recorded three times, each time separated by 10 seconds, and the average of the three measures was calculated. The responses were also analyzed as a repeated measure to observe whether there was any difference. All tail-flick testing was performed between 9:00 AM and 1:00 PM.
Formalin test
The formalin test was done in the same animals on a different foot before and after the treatment at least 7 days after the tail-flick test. Tail-flick testing 1 week before was not expected to affect formalin pain responses because other studies have reported no effect of repeated (formalin) testing at 1 week intervals.15–17 Animals were habituated to the 30 cm3 transparent Plexiglas observation box for 30 minutes on two occasions before the day of testing and immediately before testing. Each rat was removed from the observation box and restrained in a towel, and 50 μL of 1.5% formaldehyde was injected under the plantar surface of the left hind paw. Each rat was placed in the observation box, and the pain behavior within the first 5 minutes of intraplantar formalin injection was recorded as the early formalin score, while the pain behavior between 20 and 40 minutes of the formalin injection was recorded as the late phase. Below the floor of the box, a mirror at a 45° angle facilitated viewing of the injected paw. The behavior was scored as a 2 if the rat licked, bit, or shook the injected paw; as a 1 if the rat elevated the paw from the floor; or as a 0 if any part of the paw other than the tips of the digits was in contact with the box. The score was entered into a computer that recorded the last score entered once every half second. A mean pain score (a weighted sum of the durations of each behavior) was calculated as the sum of the scores divided by the number of scores in the time period. All formalin testing was performed between 9:00 AM and 2:00 PM.
Statistical analysis
Data were analyzed using SPSS version 16.0 (IBM Corporation, Armonk, NY, USA). All values given were the mean ± standard error of the mean of the variables measured. Significance was assessed by analysis of variance followed by a post-hoc Tukey multiple range test for multiple comparisons. P-values of 0.05 or less were taken as statistically significant.
Results
Figure 1 shows the effects of salt-loaded diet on the blood pressure of rat. The systolic blood pressure in the salt-loaded rats (205.50 ± 2.32 mmHg) was significantly higher (P < 0.001) than in the control (126.80 ± 12.54 mmHg). The diastolic blood pressure in the salt-loaded rats (162.30 ± 0.56 mmHg) was significantly higher (P < 0.001) than in the control (88.80 ± 12.60 mmHg). The mean arterial blood pressure in the salt-loaded rats (175.83 ± 0.82 mmHg) was significantly higher (P < 0.001) than in the control (101.12 ± 1.02 mmHg).
Figure 1.
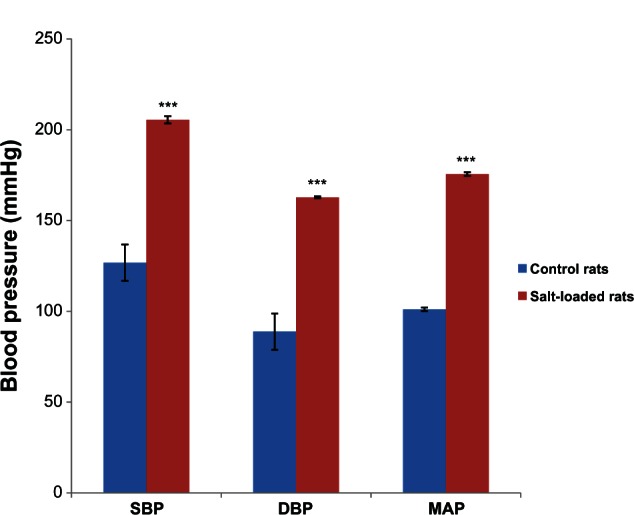
Effects of a salt-loaded diet on blood pressure in rats.
Notes: Values are expressed as mean ± standard error of the mean (n = 6); ***P < 0.001 versus control.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Figure 2 shows the effects of salt-loading hypertension on the tail-flick latency period in rats. The baseline latency periods in the control (3.29 ± 0.20 seconds) and salt-loaded rats (3.89 ± 0.30 seconds) were not significantly different (P > 0.05). However, 10 weeks after administration of the salt-loaded diet, the tail-flick latency period in the salt-loaded rats (8.93 ± 0.18 seconds) was significantly higher (P < 0.001) than its baseline (3.89 ± 0.25 seconds) and the control (3.86 ± 0.17 seconds).
Figure 2.
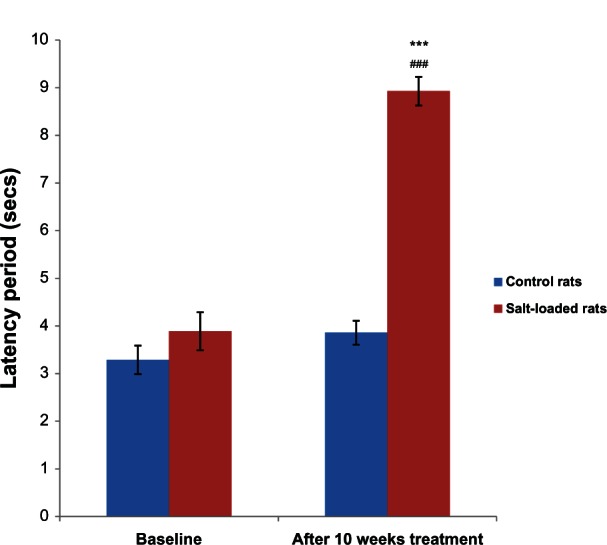
Effects of salt-loading hypertension on the latency period in the tail-flick test in rats.
Notes: Values are expressed as mean ± standard error of the mean (n = 6); ***P < 0.001 versus control, ###P < 0.001 versus baseline.
Figure 3 shows the effect of salt-loading hypertension on the early phase formalin score in rats. The baseline formalin score in the control (78.00 ± 2.47 pain score/second) and salt-loaded rats (74.53 ± 3.32 pain score/second) were not significantly different (P . 0.05). However, 10 weeks after administration of the salt-loaded diet, the formalin score in the salt-loaded rats (32.02 ± 1.85 pain score/second) was significantly lower (P < 0.001) than its baseline (74.53 ± 3.32 pain score/second) and the control (78.14 ± 2.75 pain score/second).
Figure 3.
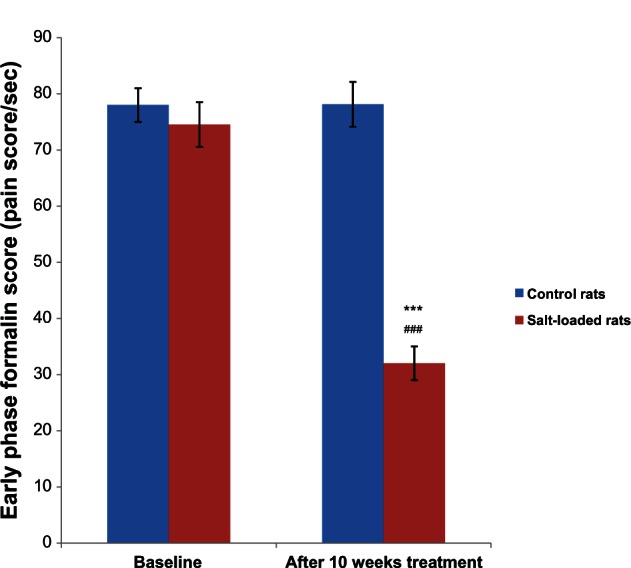
Effects of salt-loading hypertension on the early phase formalin score in rats.
Notes: Values are expressed as mean ± standard error of the mean (n = 6); ***P < 0.001 versus control, ###P < 0.001 versus baseline.
Figure 4 shows the effects of salt-loading hypertension on the late phase formalin score in rats. The baseline formalin score in the control (200.38 ± 8.50 pain score/second) and salt-loaded rats (195.00 ± 0.30 pain score/second) were not significantly different (P > 0.05). However, 10 weeks after administration of the salt-loaded diet, the formalin score in the salt-loaded rats (10.69 ± 0.40 pain score/second) was significantly lower (P < 0.001) than its baseline (195.00 ± 0.30 pain score/second) and the control (200.38 ± 0.71 pain score/second).
Figure 4.
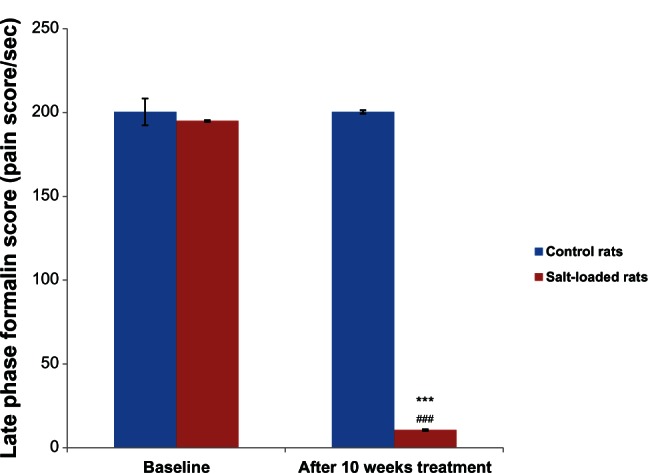
Effects of salt-loading hypertension on the late phase formalin score in rats.
Notes: Values are expressed as mean ± standard error of the mean (n = 6). ***P < 0.001 versus control, ###P < 0.001 versus baseline.
Discussion
The increased blood pressure after administration of the salt-loaded diet in the present study is consistent with the previous study.18 Administration of the salt-loaded diet significantly increased systolic diastolic and mean arterial blood pressure in the rats. The salt-loaded rats also had reduced formalin score and increased tail-flick latency, suggesting that the salt-loaded hypertensive rats had lower pain perception frequency and higher threshold compared with the controls. The finding of higher tolerance for pain in the salt-loaded hypertensive group above that of normal diet-fed control group, though at variance with the findings of Sitsen and de Long,8 is consistent with previous studies that reported a close association between experimentally induced high blood pressure and hypoalgesia.19–22 The fact that formalin score and latency period were not significantly different in the two groups before administration of salt-loaded diet further suggested that the observed hypoalgesia after its treatment was undoubtedly induced by salt-loading hypertension. Although individual differences may account for higher pain threshold in some individuals, hypertension has consistently been associated with hypoalgesia.5,23 Hypoalgesia associated with high blood pressure has been attributed to many factors. Several lines of evidence point to a role of the baroreflex system reducing or interrupting sino-aortic afferent input in raised blood pressure and high levels of endogenous opiate activity in the pituitary, hypothalamus and spinal cord.23
The association of hypoalgesia with hypertension assumed great significance with the finding of greater susceptibility to “silent” myocardial ischemia in hypertensive patients with coronary heart disease.24 Thus, patients who have coronary heart disease with concomitant hypertension are more likely to have a sudden massive myocardial infarction. The situation of hypertensive patients is more precarious as they are more susceptible to left ventricular hypertrophy,25,26 which is in itself a predisposing factor to coronary heart disease.
The greater hypoalgesia in the late phase of the formalin test (98.4%) above that of the early phase (56.8%) in the salt-loading hypertensive rats is of particular interest. Although both early and late phase have nociceptive effect and travel through the same pathways (ie, Aδ and C fibers), the early phase is thought to act by direct chemical stimulation of nociceptors,27 while late phase response acts through inflammation.22 The results may reflect reduced activity in chemical sensitivity of afferents, Aδ and C fibers that mediate the second phase response.21 In the tail-immersion pain test, there was increase in pain tolerance in salt-loaded rats. However, tail immersion test is a thermal test which travels via the Aδ fiber in which the tolerance may be due to sensitivity of endogenous opiate.23
Although the underlying mechanisms responsible for this form of hypertension-associated hypoalgesia remain to be fully identified, several hypotheses have been proposed. Several anatomic, physiological, and pharmacological findings support the hypothesis of a relationship between the various central mechanisms that are involved in pain modulation and in arterial blood pressure regulation; various studies have described an association, or at least a partial overlap, between the brain circuits involved in the control of blood pressure and pain perception.28–30 The observation that hypoalgesia can be suppressed in the experimental animal by naloxone,31–33 but not by its exclusively principally acting analog, N-methyl-naloxone,33 clearly indicates an involvement of the endogenous opioid systems within the central nervous system. A further aspect of possible importance is represented by the role of the carotid sinus and cardiopulmonary baroreceptor pathways. In fact, hypoalgesia can be induced by baroreceptor activation in the hypertensive rats, and it can be attenuated by carotid sinus baroreceptor denervation and by reducing the cardiopulmonary baroreceptor afferent input.31,34,35
Previous epidemiological studies have shown that circulating inflammatory molecules are increased in hypertensive patients, and their levels predict the onset of hypertension.36–40 Moreover, treatment of animal models of hypertension with angiotensin-II receptor blockers reduces the level of inflammatory activation in the vessels.41,42 Though this study did not measure plasma inflammatory molecules, which is one of its limitation, we did however observe a lower inflammatory response in the salt-loaded hypertensive rats compared with the control. Another limitation of this study is the inability to report some health conditions of the animals, such as whether they have motor dysfunction, which could also account for the lack of response in the formalin tests. Further studies that will extend the time of this experiment and assess myocardial damage, in addition to incorporating these limitations, are needed.
Conclusion
The present study suggests that high salt-loading-induced hypertension causes hypoalgesia in rats and that this might be due more to reduction in inflammatory response. More studies need to be done to determine the mode of action of salt-loading hypertension-induced hypoalgesia, especially on its potential anti-inflammatory effect.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Ditto BF, France CR. Risk of hypertension and pain sensitivity in women. Int J Behav Med. 1997;4:117–130. doi: 10.1207/s15327558ijbm0402_2. [DOI] [PubMed] [Google Scholar]
- 2.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 3.Zamir N, Shubber E. Altered pain perception in hypertensive humans. Brain Res. 1980;201:471–474. doi: 10.1016/0006-8993(80)91055-0. [DOI] [PubMed] [Google Scholar]
- 4.Ghione S, Rosa C, Panattoni E, Nuti M, Mezzasalma L, Giruliano G. Comparison of sensory and pain threshold in tooth pulp stimulation in normotensive man and essential hypertension. J Hypertens. 1985;3:5113–5115. [PubMed] [Google Scholar]
- 5.Ghione S, Rosa C, Mezzasalma L, Panattoni E. Arterial hypertension is associated with hypalgesia in humans. Hypertension. 1988;12:491–497. doi: 10.1161/01.hyp.12.5.491. [DOI] [PubMed] [Google Scholar]
- 6.Ghione S. Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 7.Bruehl S, Carlson CR, Mccubbin JA. The relationship between pain sensitivity and blood pressure in normotensives. Pain. 1992;48:463–467. doi: 10.1016/0304-3959(92)90099-W. [DOI] [PubMed] [Google Scholar]
- 8.Sitsen JMA, de Long W. Hypoalgesia in genetically hypertensive rats (SHR) is absent in rats with experimental hypertension. Hypertension. 1983;5:185–190. doi: 10.1161/01.hyp.5.2.185. [DOI] [PubMed] [Google Scholar]
- 9.Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behav Brain Res. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. 2007;8:494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Gomez M, Cruz Y, Salas M, Hudson R, Pacheco P. Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav. 1994;55:651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 13.Molina N, Bedran-De-Castro MTB, Bedran-De-Castro JC. The role of opioids in the analgesic response of rats during the estrous cycle. Braz J Med Biol Res. 1990;23:1157–1159. [PubMed] [Google Scholar]
- 14.d’Amore A, Chiarotti F, Renzi P. High-intensity nociceptive stimuli minimize behavioral effects induced by restraining stress during the tail-flick test. J Pharmacol Toxicol Methods. 1992;27:197–201. doi: 10.1016/1056-8719(92)90041-x. [DOI] [PubMed] [Google Scholar]
- 15.Matthies BK, Franklin KB. Effects of partial decortication on opioid analgesia in the formalin test. Behav Brain Res. 1995;67:59–66. doi: 10.1016/0166-4328(94)00104-n. [DOI] [PubMed] [Google Scholar]
- 16.Matthies BK, Franklin KB. Formalin pain is expressed in decerebrate rats but not attenuated by morphine. Pain. 1992;51:199–206. doi: 10.1016/0304-3959(92)90261-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosland JH, Tjølsen A, Mæhle B, Hole K. The formalin test in mice: effect of formalin concentration. Pain. 1990;42:235–242. doi: 10.1016/0304-3959(90)91167-H. [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC, Colemen TG. Long-term regulation of the circulation: interrelationship with body fluid volumes. In: Reeves EB, Guyton AC, editors. Physical Bases of Circulatory Transport: Regulation and Exchange. Philadelphia: WB Saunders; 1967. pp. 179–201. [Google Scholar]
- 19.Fillingim RB, Maixner W. The Influence of resting pressure and gender on pain responses. Psychosom Med. 1996;58:326–332. doi: 10.1097/00006842-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Oyadeyi AS, Afolabi AO, Ajao FO, Ibironke GF. Resting blood pressure and blood pressure reactivity: contributions to experimental pain report in healthy males. World J Med Sci. 2006;1(2):90–92. [Google Scholar]
- 21.Oyadeyi AS, Ajao FO, Afolabi AO, Azeez OM, Udoh US. The formalin test: effects of different injection sites on the pattern of nociceptive responses. Nig J Health Biomed Sci. 2006;5(1):48–50. [Google Scholar]
- 22.Oyadeyi AS, Afolabi AO, Ajao FO, Ibironke GF. Reduced formalin nociceptive responses in a rat model of post-surgical pain. American-Euroasian Journal of Scientific Research. 2007;2(1):29–32. [Google Scholar]
- 23.Zamir N, Simantov R, Segal D. Pain sensitivity and opioid activity in genetically and experimentally hypertensive rats. Brain Res. 1980;184:299. doi: 10.1016/0006-8993(80)90800-8. [DOI] [PubMed] [Google Scholar]
- 24.Droste C, Roskman H. Experimental pain measurement in patients with asymptomatic myocardial ischemia. J Am Coll Cardiol. 1983;1:940–945. doi: 10.1016/s0735-1097(83)80214-9. [DOI] [PubMed] [Google Scholar]
- 25.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable silent killer? JAMA. 2004;292(19):2396–2398. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 26.Devereux RB, Pickering TG, Harshfield GA, et al. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation. 1983;68:470–476. doi: 10.1161/01.cir.68.3.470. [DOI] [PubMed] [Google Scholar]
- 27.Dubussion R, Dennis SG. Pain signaling systems in the dorsal and ventral spinal cord. Pain. 1977;4:97–132. doi: 10.1016/0304-3959(77)90126-9. [DOI] [PubMed] [Google Scholar]
- 28.Friedman R, Murphy D, Persons W, McCaughran JA., Jr Genetic predisposition to hypertension, elevated blood pressure and pain sensitivity: a functional analysis. Behav Brain Res. 1984;12:75–79. doi: 10.1016/0166-4328(84)90205-5. [DOI] [PubMed] [Google Scholar]
- 29.Ghione S. Hypertension-associated hypalgesia. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 30.Rosa C, Vignocchi G, Panattoni E, Rossi B, Ghione S. Relationship between increased blood pressure and hypoalgesia: additional evidence for the existence of an abnormality of pain perception in arterial hypertension in humans. J Hum Hypertens. 1994;8:119–126. [PubMed] [Google Scholar]
- 31.Maixner W, Touw KB, Brody MJ, Gebhart GF, Long JP. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res. 1982;237:137–145. doi: 10.1016/0006-8993(82)90562-5. [DOI] [PubMed] [Google Scholar]
- 32.Saavedra JM. Naloxone reversible decrease in pain sensitivity in young and adult spontaneously hypertensive rats. Brain Res. 1981;209:245–249. doi: 10.1016/0006-8993(81)91189-6. [DOI] [PubMed] [Google Scholar]
- 33.Sitsen JM, de Jong W. Observations on pain perception and hypertension in spontaneously hypertensive rats. Clin Exp Hypertens. 1984;6:1345–1356. doi: 10.3109/10641968409039601. [DOI] [PubMed] [Google Scholar]
- 34.Dworkin BR, Elbert T, Rau H, et al. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proc Nat Acad Sci USA. 1994;91:6329–6333. doi: 10.1073/pnas.91.14.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG. Baroreceptor activation reduces reactivity to noxious stimulation: Implication for hypertension. Science. 1979;205:1299–1301. doi: 10.1126/science.472749. [DOI] [PubMed] [Google Scholar]
- 36.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 37.Schillaci G, Pirro M, Gemelli F, et al. Increased C-reactive protein concentrations in never-treated hypertension: the role of systolic and pulse pressures. J Hypertens. 2003;21:1841–1846. doi: 10.1097/00004872-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Stumpf C, John S, Jukic J, et al. Enhanced levels of platelet P- selectin and circulating cytokines in young patients with mild arterial hypertension. J Hypertens. 2005;23:995–1000. doi: 10.1097/01.hjh.0000166840.63312.12. [DOI] [PubMed] [Google Scholar]
- 39.Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004;17:568–573. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- 40.Yan JC, Ma GS, Wu ZG, Kong XT, Zong RQ, Zhan LZ. Increased levels of CD40-CD40 ligand system in patients with essential hypertension. Clin Chim Acta. 2005;355:191–196. doi: 10.1016/j.cccn.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Sanz-Rosa D, Oubina MP, Cediel E, et al. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-kappaB/IkappaB system. Am J Physiol Heart Circ Physiol. 2005;288:H111–H115. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- 42.Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:RA194–RA205. [PubMed] [Google Scholar]


