Abstract
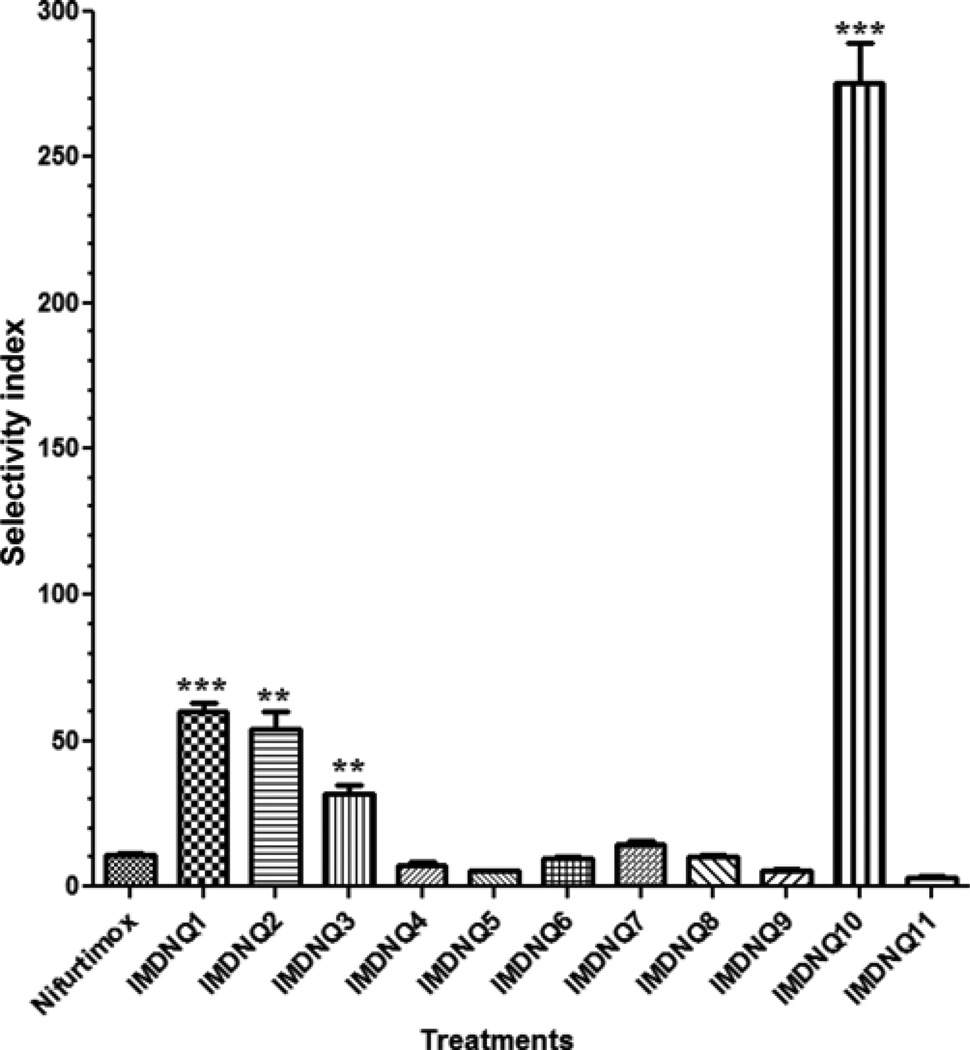
The antitrypanosomal activities, cytotoxicity, and selectivity indices of eleven imido-substituted 1,4-naphthoquinone derivatives and nifurtimox have been studied. Compared to nifurtimox (IC50 = 10.67 µM), all the imido-naphthoquinone analogs (IMDNQ1-IMDNQ11) are more potent on Trypanosoma cruzi with IC50 values ranging from 0.7 µM to 6.1 µM (p < 0.05). Studies of the cytotoxic activities of these compounds on a Balb/C 3T3 mouse fibroblast cell line revealed that four of these compounds, IMDNQ1, IMDNQ2, IMDNQ3, and IMDNQ10 displayed selectivity indices of 60.25, 53.97, 31.83, and 275.3, respectively, rendering them significantly (p < 0.05) more selective in inhibiting the parasite growth than nifurtimox (selectivity index = 10.86).
Keywords: Imido-substituted 1, 4-naphthoquinone, Trypanosoma cruzi, Cytotoxicity, Chagas disease, Epimastigotes, Fibroblasts
INTRODUCTION
Chagas disease is a tropical disease caused by the protozoan Trypanosoma cruzi and transmitted by triatomine bugs. It commonly occurs in poor and rural areas of Central and South America. Chagas disease is expanding beyond its endemic area as a result of migration from and to the endemic countries (Hanford et al., 2007; Hotez, 2008; Schofield and Kabayo, 2008). American trypanosomiasis therapy mostly depends on drugs that were developed decades ago, requires long term administration, and is not available to all patients due to its high cost. Two drugs, Nifurtimox and Benznidazole (Fig. 1), are currently used to treat only the acute phase of the infection where parasites (try-pomastigotes) are detectable in the peripheral blood (Andrade et al., 2004; Schofield and Kabayo, 2008). Both drugs have gastrointestinal and neurological side effects which may worsen as the patient ages (Nagel and Nepomnaschy, 1983; Ferreira and Ferreira, 1986; Melo and Ferreira, 1990; Coura and de Castro, 2002). Consequently, alternative drugs with a more selective mode of action are being investigated. Several classes of drug-like molecules have been studied for their antitrypanosomal activity. One of the most interesting is the quinone family of compounds. This class of compounds incorporates several diverse structural types including the naphthoquinones, which are known to possess a number of useful biological activities including antiviral, antifungal, antineoplastic, antihypoxic, anti-ischemic, antiplatelet, anti-inflammatory, and antiallergic activities (Kartoflitskaya et al., 1997; Huang et al., 1998; Tandon et al., 2004; Copeland et al., 2007). For instance, the naturally occurring naphthoquinone, lapachol (Fig. 1), and some of its derivatives have been found to show trypano-cidal activity against T. cruzi (Salas et al., 2008). Also, some naphthofuranquinones synthesized from 2-hydroxy-3-allyl-1,4-naphthoquinone were found to be active against epimastigote and trypomastigote forms of T. cruzi (Silva et al., 2006). In a recent study, a series of naphthoquinones were assessed for their try-panocidal activity and 2,3-diphenyl-1,4-naphthoquin-one (DPNQ) was found to be effective against T. cruzi epimastigotes at a low micromolar concentration (LD50 = 2.5 µM) by inhibiting T. cruzi lipoamide dehydro-genase (TcLipDH) (Ramos et al., 2009). Previously, Bakare et al. (2003) and Berhe et al. (2008) reported a series of imido-substituted 1,4-naphthoquinones as a unique class of mitogen activated protein kinase kinase 1 (MEK1) inhibitors with a number of them showing anticancer activities. In pursuit of potent and more selective antitrypanosomal agents, several imido-sub-stituted 1,4-naphthoquione (IMDNQ) derivatives (Fig. 2) have been identified as a new class of anti-try-panosomal agent. This new class of naphthoquinones has not been previously investigated as an antitry-panosomal agent. The present research reports on the in vitro antitrypanosomal activities of eleven imido-substituted 1,4-naphthoquinone analogs on T. cruzi epimastigotes.
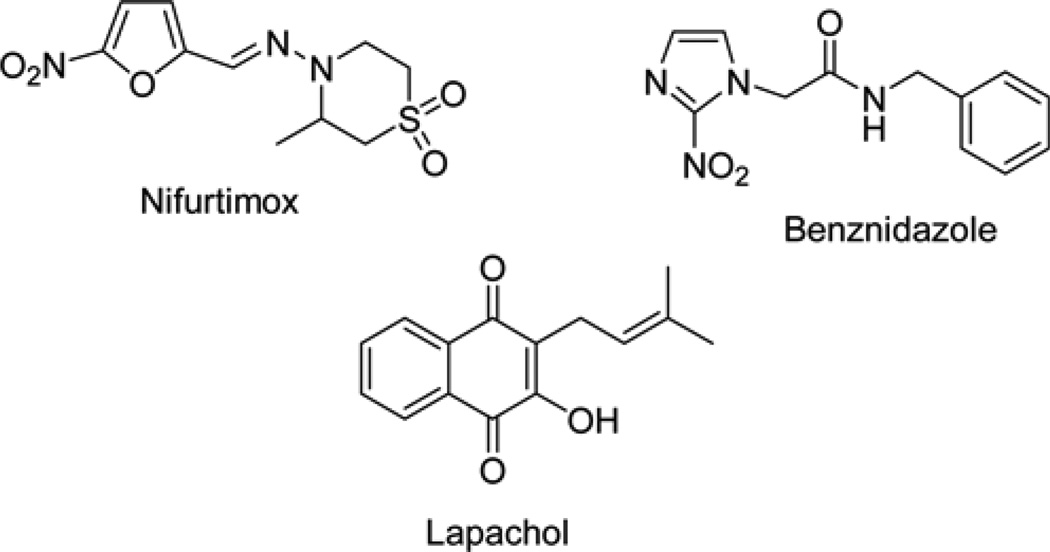
Fig. 1.
Structures of nifurtimox, benznidazole and the naturally occurring naphthoquinone compound lapachol
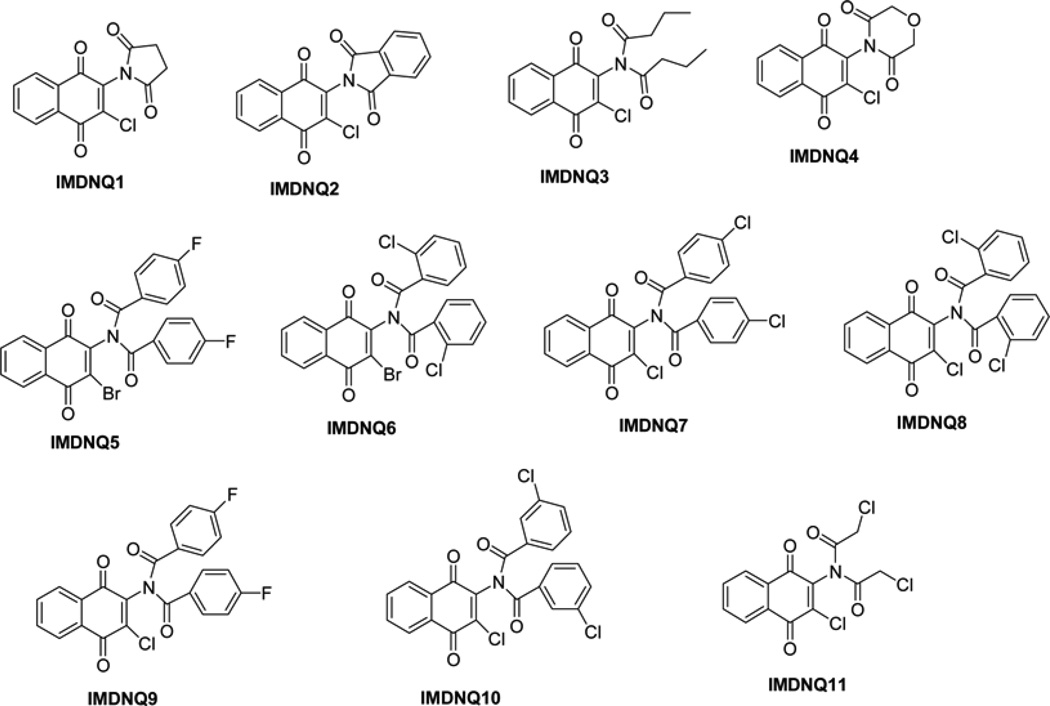
Fig. 2.
Structures of imido–substituted 1,4-naphthoquinone derivatives
MATERIALS AND METHODS
Chemical compounds
Eleven imido-substituted 1, 4-naphthoquinones (Fig. 2) were used in this study.
Chemistry
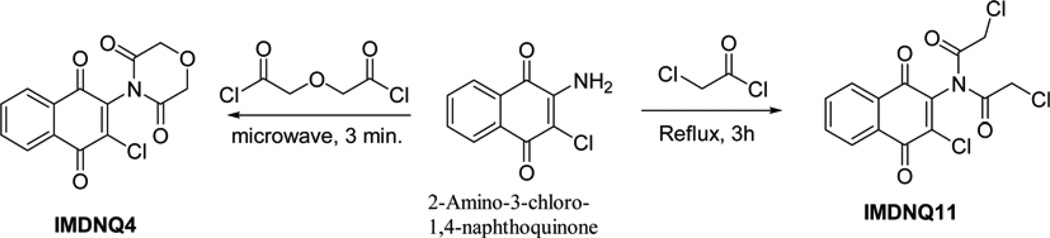
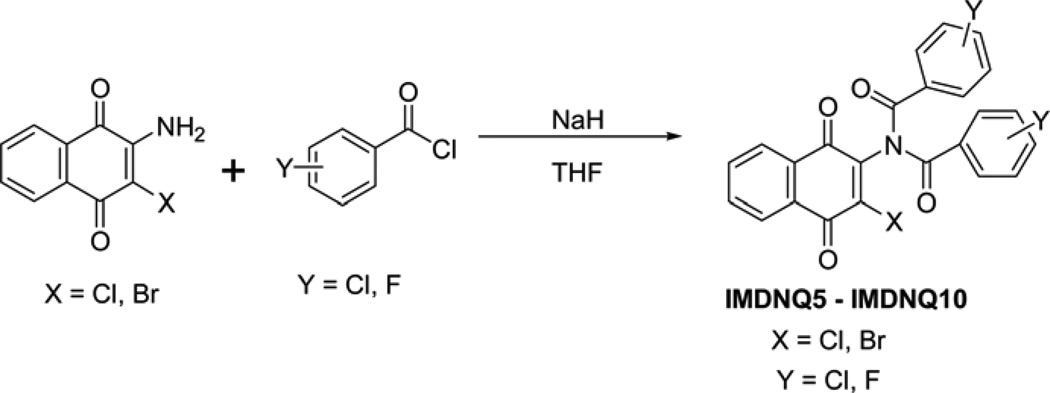
The succinimidyl (IMDNQ1), phthalimidyl (IMDNQ2), and dibutytryl (IMDNQ3) derivatives were synthesized from 2-amino-3-chloro-1,4-naphthoquinone and the appropriate acid chloride as previously described (Bakare et al., 2003; Berhe et al., 2008). The morpholine dione analog (IMDNQ4) was synthesized by microwave irradiation of a mixture of 2-amino-3-chloro-1,4-na-phthoquinone and diglycolyl chloride as depicted in scheme 1 (Berhe et al., 2008). On the other hand, the bis-(chloroacetyl)-derivative (IMDNQ11) was prepared by heating 2-amino-3-chloro-1,4-naphthoquinone in excess 2-chloroacetyl chloride at high temperatures as shown in scheme 1 (Bakare et al., 2003; Berhe et al.,2008). The diarylimido-substituted naphthoquinones IMDNQ5 to IMDNQ10 were synthesized via sodium hydride facilitated bis-acylation of 2-amino-3-chloro-1,4-naphthoquinone or 2-amino-3-bromo-1,4-naphtho-quinone as shown in scheme 2. All reactions were carried out using laboratory grade materials and solvents. Melting points were determined in open capillary tubes on a Mel-Temp melting point apparatus and are uncorrected. The IR spectra were recorded on a Perkin Elmer PE 100 spectrometer with an Attenuated Total Reflectance (ATR) window. The 1H- and 13C-NMR spectra were obtained on a Bruker Avance 400 MHz spectrometer in deuterated chloroform (CDCl3). Chemical shifts are in ä units (ppm) with TMS (0.00 ppm) or CHCl3 (7.26 ppm), as the internal standard for 1H-NMR, and CDCl3 (77.00 ppm) for 13C-NMR. Electro-spray ionization mass spectrometry was recorded on a Thermo LTQ Orbitrap XL mass spectrometer and compounds dissolved in acetonitrile containing 0.1% formic acid. The known intermediates were prepared according to procedures that are reported in the literature. 2-Amino-3-bromo-1,4-naphthoquinone was prepared by refluxing commercially available 2,3-dibromo-1,4-naphthoquinone with ammonia/ammonium hydroxide mixture in ethanol.
Scheme 1.
Synthesis of imido-substituted naphthoquinone derivatives IMDNQ4 and IMDNQ11
Scheme 2.
Synthesis of diaryimido-naphthoquinone derivatives IMDNQ5 to IMDNQ10
Antitrypanosomal activity assay
T. cruzi epimastigotes (Tulahuen CL98 strain) were cultured in liver infusion trypose (LIT) medium supplemented with 10% fetal bovine serum (FBS) at 28°C, with an inoculum of 1 × 105 cells/mL. Different concentrations ranging from 0.39–100 µM of the chemical compounds were added. All assays were carried out in triplicate. Parasites were counted after 48 h using hemocytometers and the concentration that inhibits the parasite’s proliferation by 50% (IC50) was calculated for each compound. Nifurtimox (one of two commercial drugs for Chagas disease) was used as a reference drug.
Cytotoxicity assay
The cytotoxic effects of the imido-substituted 1, 4-naphthoquinones on Balb/C 3T3 mouse fibroblasts (clone A31) were quantitatively assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. MTT is a yellow tetra-zolium salt that is reduced to purple formazan crystals by metabolically active cells. Cells (2.2 × 104 cells/well) were seeded in a 96-well plate in Improved Minimum Essential Medium (IMEM). Serial dilutions (0.39–100 µM) of the compounds were added. After 48 h incubation at 37°C in the presence 5% CO2, the medium was aspirated and the cells were washed twice to remove traces of chemical compounds. IMEM without phenol containing 10% (v/v) of 3 mg/mL MTT was added to each well. The plates were incubated for 4 h at 37°C followed by aspiration. The plates were dried for 1 h in a 37°C incubator. 100 µL of 0.04 N HCl in isopropanol was added to each well and incubated at room temperature (27°C) in the dark for 2 h to dissolve the formazan crystals. The absorbance was measured spectrophotometrically at 570 nm in a plate reader. The concentration that reduces cell viability by 50% (IC50) was calculated for each compound. The toxicity for mammalian cells and the activity against T. cruzi were compared by calculating the selectivity index (SI), which is the ratio of IC50 for fibroblast cells/ IC50 for parasites.
Statistical analysis
All experiments were carried out in triplicate, and the means and standard errors (S.E.) were determined. Data were analyzed by one way ANOVA and Tukey’s multiple comparison test using GraphPad PRISM software version 5.00. p < 0.05 was considered significant.
General procedure for the synthesis of arylim-ido-substituted naphthoquinones (IMDNQ5 – IMDNQ10)
2-Amino-3-chloro-1,4-naphthoquinone (1.47 mmol) or the 3-bromo-analog was dissolved in THF (15 mL). NaH (3.08 mmol) was added and the mixture was stirred at room temperature for 15 min. Appropriate acid chloride (3.08 mmol) was added drop wise, and the resulting mixture was stirred at room temperature for 24 h. The THF was then evaporated under vacuum and ice-cooled water added to the residual mixture. The resulting aqueous mixture was extracted with CH2Cl2 (2 × 30 mL) and the combined organic phase was washed with water (3 × 15 mL) and saturated NaCl solution (15 mL) and dried over anhydrous MgSO4. The crude product was purified by triturating in hot ethanol followed by recrystallization in ethyl acetate and/or column chromatography on silica gel.
N-(3-Bromo-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-4-fluoro-N-(4-fluorobenzoyl)-benzamide (IMDNQ 5)
Yellow solid (66%); m.p. 170–172°C; IR (cm−1) 1719.23, 1670.56, 1596.69, 1505.57; 1H-NMR (CDCl3) 7.05 (t, 4H, J = 12.0 Hz), 7.77–7.88 (m, 6H), 8.08–8.15 (m, 1H), 8.21–8.27 (m, 1H); 13C-NMR (CDCl3) 115.57, 115.79, 127.4, 127.71, 130.18, 130.30, 130.33, 130.85, 131.35, 131.44, 134.46, 134.50, 138.52, 146.90, 165.03, 169.91, 176.96, 177.97; ESI MS m/z 517.9785 ([M+Na]+ calcd 517.9815).
N-(3-Bromo-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-2-chloro-N-(2-chlorobenzoyl)-benzamide (IMDNQ 6)
Yellow crystal (34%); m.p. 232–234°C; IR (cm−1) 1728.78, 1688.76, 1671.10, 1588.22, 1468.70; 1H-NMR (CDCl3) 7.13 (d, 2H, J = 7.9 Hz), 7.21 (dd, 2H, J = 1.6, 7.9 Hz), 7.28 (dd, 2H, J = 1.3, 7.5 Hz), 7.78–7.86 (m, 2H), 7.94 (d, 2H, 5.2 Hz), 8.15–8.21 (m, 1H), 8.22–8.28 (m, 2H); 13C-NMR (CDCl3) 126.65, 127.66, 128.08, 129.63, 130.49, 130.81, 131.23, 132.25, 134.68, 134.80, 140.78, 145.81, 167.57, 177.26, 177.81; ESI MS m/z 549.9240 ([M+Na]+ calcd 549.9224).
4-Chloro-N-(4-chlorobenzoyl)-N-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-benzamide (IMDNQ 7)
Yellow solid (27%); m.p. 212–213°C; IR (cm−1) 1737.38, 1714.75, 1696.91, 1673.62, 1589.20, 1571.10; 1H-NMR (CDCl3) 7.33–7.36 (m, 4H), 7.68–7.72 (m, 4H), 7.79–7.85 (m, 2H), 8 09–8.11 (m, 1H), 8.20–8.22 (m, 1H); 13C-NMR (CDCl3) 127.72, 127.80, 129.18, 130.34, 130.50, 131.32, 132.52, 134.92, 134.95, 139.67, 142.46, 143.87, 170.40, 177.05, 178.59; ESI MS m/z 505.975 ([M+Na]+ calcd 505.973).
2-Chloro-N-(2-chlorobenzoyl)-N-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-benzamide (IMDNQ 8)
Yellow solid (49%); m.p. 217–218°C; IR (cm−1) 1720.19, 1681.22, 1618.00, 1588.02; 1H-NMR (CDCl3) 7.10–7.16 (m, 2H), 7.21–7.31 (m, 4H), 7.79–7.89 (m, 4H), 8.17–8.20 (m, 1H), 8.20–8.26 (m, 1H); 13C-NMR (CDCl3) 126.76, 126.93, 127.60, 127.80, 129.69, 130.54, 130.85, 131.37, 132.36, 132.52, 132.56, 132.75, 134.14, 134.75, 134.91, 142.65, 144.43, 177.13, 178.22; ESI MS m/z 505.9741 ([M+Na]+ calcd 505.9730).
N-(3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-4-fluoro-N-(4-fluorobenzoyl)-benzamide (IMDNQ 9)
Yellow solid (56%); m.p. 284–286°C; IR (cm−1) 3074.94, 1719.61, 1689.97, 1672.75, 1591.10; 1H-NMR (CDCl3) 7.00–7.06 (m, 1H), 7.75–7.85 (m, 1H), 8.10–8.12 (m, 6H), 8.20–8.22 (m, 4H); 13C-NMR (CDCl3) 115.97, 116.19, 116.41, 126.65, 126.96, 127.67, 127.77, 130.53, 130.57, 130.58, 131.35, 131.62, 131.71, 132.75, 134.88, 142.42, 144.13, 164.14, 166.68, 170.33, 177.13, 178.63; ESI MS m/z 474.034 ([M+Na]+ calcd 474.032).
3-Chloro-N-(3-chlorobenzoyl)-N-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)-benzamide (IMDNQ 10)
Yellow solid (31%); m.p. 258–260°C; IR (cm−1) 3075.36, 1713.64, 1698.11, 1672.50, 1591.48, 1571.31; 1H-NMR (CDCl3) 7.27–7.31 (t, J = 7.85 Hz, 2H), 7.42–7.45 (ddd, J = 1.03, 2.09, 8.07 Hz, 2H), 7.60–7.63 (td, J = 1.07, 7.68 Hz, 2H), 7.70–7.71 (t, J = 1.82 Hz, 2H), 7.80–7.85 (m, 2H), 8.11–8.14 (m, 1H), 8.21–8.23 (m, 1H); 13C-NMR (CDCl3) 127.02, 127.93, 128.03, 129.34, 130.21, 130.75, 131.55, 133.29, 135.14, 135.21, 136.06, 143.00, 143.84, 170.20, 177.25, 178.66; ESI MS m/z 505.9741 ([M+Na]+ calcd 505.9730).
RESULTS AND DISCUSSION
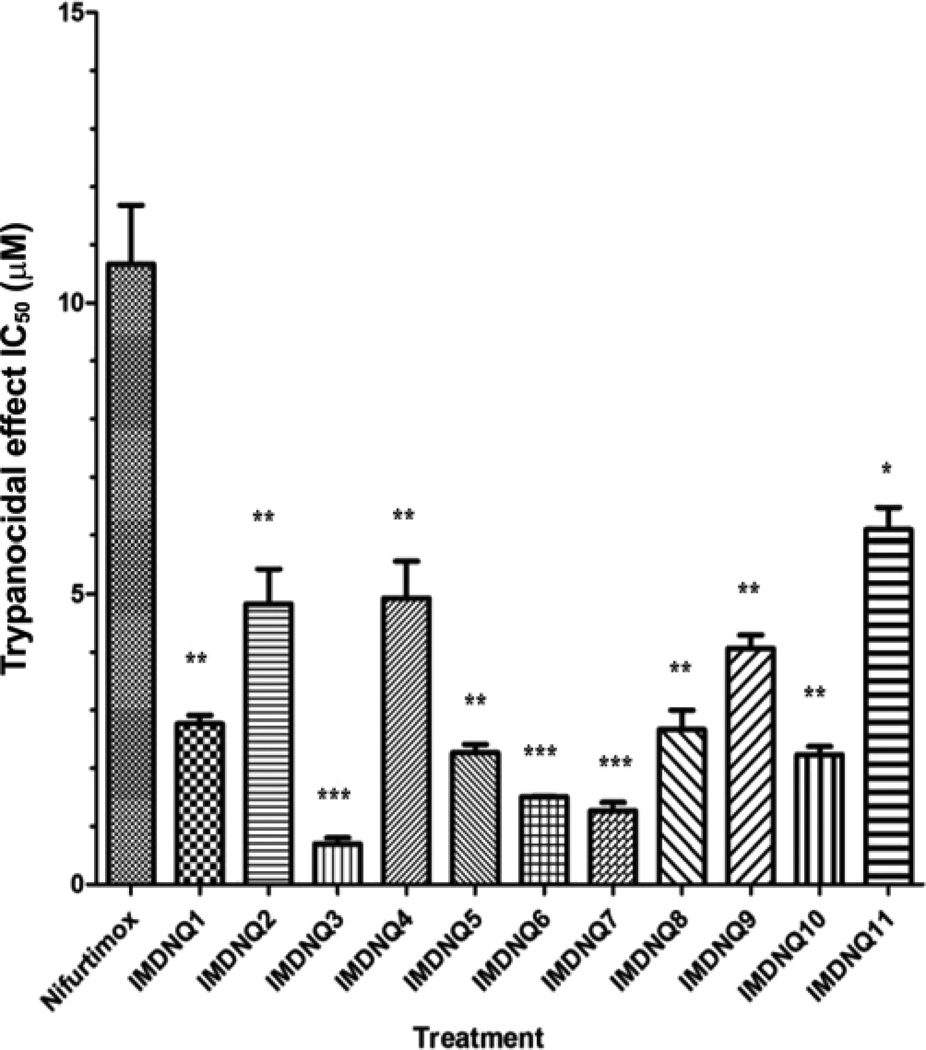
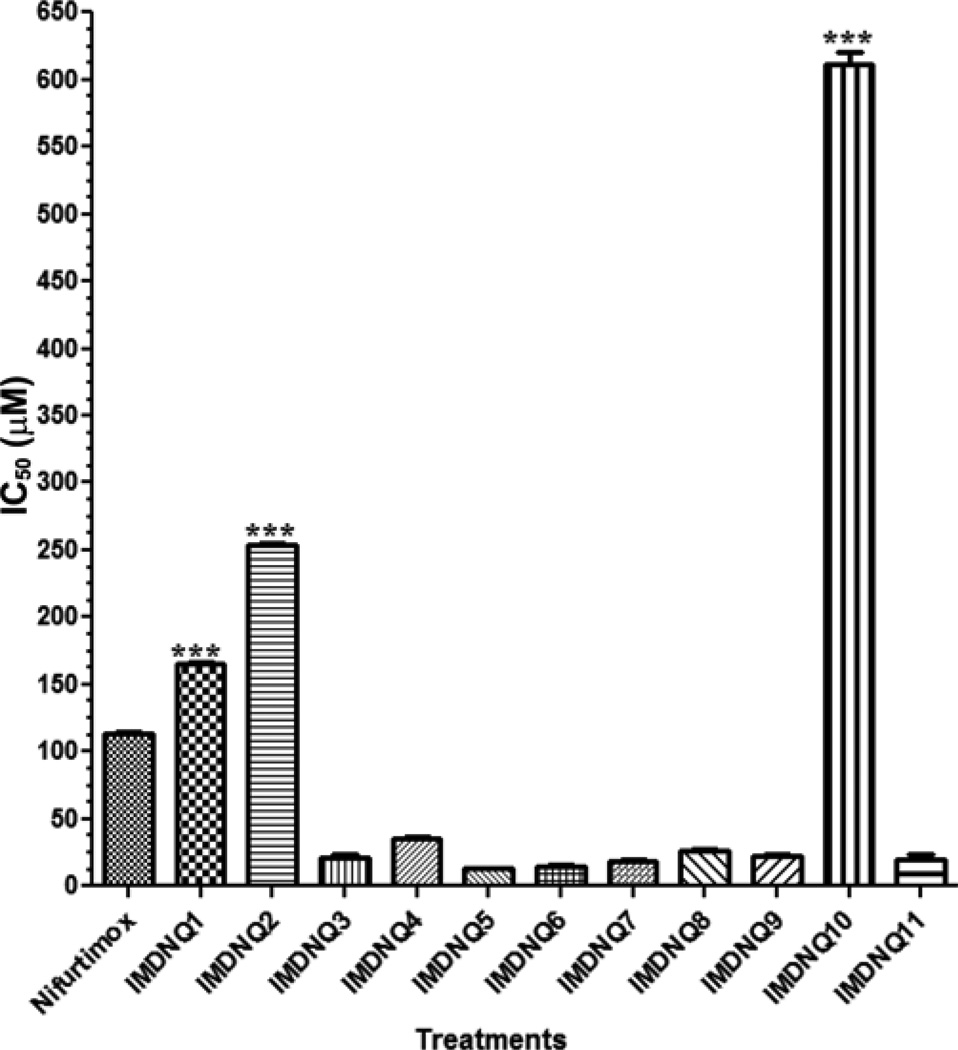
The antitrypanocidal activities, cytotoxicity, and selectivity indices of the eleven imido-substituted 1,4-naphthoquinones and nifurtimox are summarized in Table I. Compared to nifurtimox (IC50 = 10.67 µM), all the imido-naphthoquinone analogs (IMDNQ1 – IMDNQ11) studied are significantly more potent on T. cruzi with IC50 values ranging from 0.7 µM to 6.1 µM (p < 0.05, Fig. 3). A cytotoxicity study on a Balb/C 3T3 mouse fibroblast cell line showed that three of the compounds, IMDNQ1, IMDNQ2, IMDNQ10, are considerably less cytotoxic than nifurtimox (Fig. 4). As shown in Fig. 5, these three analogs (IMDNQ1, IMDNQ2, IMDNQ10) and the more cytotoxic IMDNQ3 displayed selectivity indices of 60.25, 53.97, 275.30, and 31.83, respectively, rendering them significantly more selective in inhibiting the parasite’s growth than nifurtimox (selectivity index = 10.86).
Table I.
Trypanocidal activity, cytotoxicity, and selectivity indices of the chemical compounds
| Antitrypanocidal activity IC50 (µM) |
Cytotoxicity IC50 (µM) |
Selectivity index (Cytotoxicity/ Activity) |
|
|---|---|---|---|
| Nifurtimox | 10.67 ± 1.01 | 114.0 ± 1.03 | 10.86 ± 0.93 |
| IMDNQ1 | 2.77 ± 0.15 | 165.9 ± 1.24 | 60.25 ± 2.79 |
| IMDNQ2 | 4.83 ± 0.60 | 253.7 ± 1.87 | 53.97 ± 5.95 |
| IMDNQ3 | 0.70 ± 0.10 | 21.67 ± 1.45 | 31.83 ± 3.17 |
| IMDNQ4 | 4.93 ± 0.64 | 35.50 ± 1.76 | 7.43 ± 0.97 |
| IMDNQ5 | 2.27 ± 0.15 | 12.83 ± 0.73 | 5.70 ± 0.06 |
| IMDNQ6 | 1.51 ± 0.01 | 14.17 ± 1.42 | 9.43 ± 0.94 |
| IMDNQ7 | 1.27 ± 0.15 | 17.83 ± 2.21 | 14.23 ± 1.62 |
| IMDNQ8 | 2.67 ± 0.33 | 26.50 ± 1.61 | 10.17 ± 0.97 |
| IMDNQ9 | 4.07 ± 0.23 | 21.83 ± 1.88 | 5.43 ± 0.69 |
| IMDNQ10 | 2.23 ± 0.15 | 610.9 ± 10.27 | 275.3 ± 13.73 |
| IMDNQ11 | 6.10 ± 0.38 | 19.67 ± 3.71 | 3.20 ± 0.44 |
Each value represents the mean ± S.E. of three experiments.
Fig. 3.
Trypanocidal effects of the imido-substituted 1, 4-naphthoquinones on T. cruzi. Results are expressed as the means ± S.E. of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with Nifurtimox
Fig. 4.
Cytotoxicity of the imido-substituted 1, 4-na-phth-oquinones on Balb/C 3T3 mouse fibroblasts. Results are expressed as the means ± S.E. of three experiments. ***p < 0.0001 compared with Nifurtimox
Fig. 5.
Selectivity indices of the imido-substituted 1, 4-na-phthoquinones. Results are expressed as the means ± S.E. of three experiments. **p < 0.01, ***p < 0.0001 compared with Nifurtimox
In the pursuit of novel selective antitrypanosomal agents with little or no toxicity to mammalian cells, eleven imido-substituted 1,4-naphthoquione analogs were developed as a new class of antitrypanosomal agent. The study of these cyclic and acyclic imido-sub-stituted 1,4-naphthoquinone derivatives on T. cruzi revealed potent antitrypanosomal properties against T. cruzi by inhibiting their proliferation in vitro. All the eleven imido-substituted 1,4-naphthoquinone displayed more potent antitrypanosomal activities than the clinically used nifurtimox. Further, comparison of the antitryponosomal activities with cytotoxicity on a Balb/C 3T3 mouse fibroblast cell line revealed that four of these compounds, IMDNQ1, IMDNQ2, IMDNQ3, and IMDNQ10 show considerably better selectivity than nifurtimox as seen from the selectivity indices values (Table I and Fig. 5). Of the compounds studied, the structure-activity relationship revealed that dibu-tyrylamino derivative (IMDNQ3) is the most potent with an IC50 value of 0.7 µM against T. cruzi. However, this compound is among the most cytotoxic to the mouse fibroblast cells (IC50 = 21.67 µM) in this study, and as shown in previous reports of the cytotoxicity of compound IMDNQ3 on different human cancer cell lines (Bakare et al., 2003; Berhe et al., 2008). The potent activity in the antitrypanosomal study nevertheless provided IMDNQ3 with a good selectivity index of 31.83. On the other hand, two of the cyclic imido-na-phthoquinone analogs, IMDNQ1 and IMDNQ2, while showing potent antitrypanosomal activities (IC50 = 2.77 µM for IMDNQ1 and 4.89 µM for IMDNQ2), are relatively non-toxic to the mouse fibroblast cell (IC50 values of 165.9 µM for IMDNQ1 and 253.7 µM for IMDNQ2). This selectivity means that the two compounds are of greater value and interest than the most potent antitrypanosomal compound, IMDNQ3. Of greater interest is the selectivity of IMDNQ10 (selectivity index of 275.3), which showed potent antitrypanosomal activity with an IC50 value of 2.23 µM against T. cruzi, while being non-cytotoxic to the mouse fibroblast cell (IC50 value of 610.9 µM). Compound IMDNQ10 is a diaryl imido analog with meta-chloro-substituent on each of the imide aryl groups and thus significantly differs from the two cyclic imido-derivatives IMDNQ1 and IMDNQ2. The other cyclic imido-analog IMDNQ4 is also potent against T. cruzi with an IC50 value of 4.93 µM, but is more cytotoxic in the mouse fibroblast cell (IC50 = 35.50 µM). This greater cytotoxicity could be due to the presence of the oxygen atom in the cyclic imide moiety which could facilitate further hydrogen-bonding to molecular targets in the mouse fibroblast cell, rendering it more susceptible to the cytotoxic activity of compound IMDNQ4. Comparison of all the diaryl imido derivatives (IMDNQ5 to IMDNQ10) showed that, with the exception of compound IMDNQ10, all the other diarylimido compounds (IMDNQ5 to IMDNQ9) are reasonably cytotoxic to the mouse fibroblast cells and consequently show poorer selectivity indices (Table I, Fig. 5). The non-cytotoxic diarylimido naphthoquin-one IMDNQ10 possess a chloro group in the meta-position of each aryl group in the diarylimido-moiety, while the other analogs possess a chloro or fluoro group in either a para- or ortho-position. The reason for the observed difference in cytotoxicity between a chloro group in a meta- (IMDNQ10), para- (IMDNQ7) or ortho- (IMDNQ8) position in these compounds is not immediately apparent; however, it could be suggested that the presence of the chloro group in a meta-position could have distorted the interaction of compound IMDNQ10 with a potential binding site necessary for cytotoxicity in the mouse fibroblast cells. The replacement of the 3-chloro group on the 1,4-naphtho-quinone ring with a 3-bromo group did not drastically affect the activities and selectivity indices of these compounds, as can be seen in the activity and selectivity of compound IMDNQ6 versus IMDNQ8 and also compound IMDNQ5 versus IMDNQ9 (Table I, Fig. 2). There appears to be a slight increase in antitrypano-somal activity when the 3-chloro group is substituted with a 3-bromo group as seen in compounds IMDNQ9 (IC50 = 4.07 µM) and IMDNQ5 (IC50 = 2.27 µM). This can also be seen when the activity of compound IMDNQ8 (IC50 = 2.67 µM) is compared with that of IMDNQ6 (IC50 = 1.51 µM). Similarly, there is a corresponding slight increase in cytotoxicity on the Balb/ C 3T3 mouse fibroblast cell line when the 3-chloro group is substituted with a 3-bromo group, as again can be seen in compound IMDNQ9 (IC50 = 21.83 µM) vs IMDNQ5 (IC50 = 12.83 µM) and in compound IMDNQ8 (IC50 = 26.50 µM) vs IMDNQ6 (IC50 = 14.17 µM). Comparison of compounds IMDNQ7 and IMDNQ9 revealed that substituting the para-chloro group in the diarylimido moiety of IMDNQ7 with the smaller and more electronegative fluoro group in IMDNQ9 slightly reduced both the antitrypanosomal and cytotoxic activities (Table I). The bis-chloroacetylamino derivative IMDNQ11 is the least potent antitrypanosomal imido naphthoquinone in this study with an IC50 value of 6.10 µM. This compound is also the least selective analog with a selectivity index of 3.20 (Table I, Fig. 5).
All the imido-substituted naphthoquinones studied are more potent antitrypanosomal agents than the clinically used nifurtimox; however, only four compounds, IMDNQ1, IMDNQ2, IMDNQ3 and IMDNQ10 with selectivity indices of 60.25, 53.97, 31.83, and 275.3, respectively, are significantly more selective than nifur-timox (selectivity index of 10.86). Further, three of these compounds are relatively non-cytotoxic to the Balb/C 3T3 mouse fibroblast cell line with IC50 values of significantly more than 100 µM. These compounds thus represent a novel class of selective antitrypano-somal agents for further drug development. We are currently studying the mechanism of antitrypano-somal activity of this imidonaphthoquinone class of compounds.
ACKNOWLEDGEMENTS
This work was supported in part by the WBHR-LSAMP Program, NSF#0401723 and by an NIH grant from the Research Centers in Minority Institutions (RCMI) Program of the Division of Research Infrastructure, National Center for Research Resources (RCMI-NIH 2G12RR003048).
REFERENCES
- Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, Covas DT, Silva LS, Andrade JG, Travassos LR, Almeida IC. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am. J. Trop. Med. Hyg. 2004;71:594–597. [PubMed] [Google Scholar]
- Bakare O, Ashendel CL, Peng H, Zalkow LH, Burgess EM. Synthesis and MEK1 inhibitory activities of imido-substituted 2-chloro-1,4-naphthoquinones. Bioorg. Med. Chem. 2003;11:3165–3170. doi: 10.1016/s0968-0896(03)00267-0. [DOI] [PubMed] [Google Scholar]
- Berhe S, Kanaan Y, Copeland RL, Jr., Wright DA, Zalkow L, Bakare O. Microwave-assisted synthesis of imido-substituted 2-chloro-1,4-naphthoquinone derivatives and their cytotoxic activities on three human prostate cancer cell lines. Lett. Drug Des. Discov. 2008;5:485–488. [Google Scholar]
- Copeland RL, Jr., Das JR, Bakare O, Enwerem NM, Berhe S, Hillaire K, White D, Beyene D, Kassim OO, Kanaan YM. Cytotoxicity of 2,3-dichloro-5,8-dimethoxy-1,4-naphthoquinone in androgen-dependent and-independent prostate cancer cell lines. Anticancer Res. 2007;27:1537–1546. [PubMed] [Google Scholar]
- Coura R, De Castro SLA. critical review on Chagas’ disease chemotherapy. Mem. do Inst. Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, Ferreira LC. Mutagenicity of nifurtimox and benznidazole in the Salmonella/microsome assay. Braz. J. Med. Biol. Res. 1986;19:19–25. [PubMed] [Google Scholar]
- Hanford EJ, Zhan FB, Lu Y, Giordano A. Chagas disease in Texas: recognizing the significance and implications of evidence in the literature. Soc. Sci. Med. 2007;65:60–79. doi: 10.1016/j.socscimed.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl. Trop. Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LJ, Chang FC, Lee KH, Wang JP, Teng CM, Kuo SC. Synthesis antiplatelet, antiinflammatory and antiallergic activities of substituted 3-chloro-5,8-dimethoxy-1,4-naphthoquinone and related compounds. Bioorg. Med. Chem. 1998;6:2261–2269. doi: 10.1016/s0968-0896(98)80006-0. [DOI] [PubMed] [Google Scholar]
- Kartoflitskaya AP, Stepanyuk GI, Yushkova VV, Marintsova NG, Novikov VP. Synthesis and the anti hypoxic and antiischemic activity of some 2-chloro-1,4-naphthoquinone derivatives. Pharm. Chem. J. 1997;31:291–292. [Google Scholar]
- Melo ME, Ferreira LC. Screening the mutagenic activities of commonly used antiparasite drugs by the Simultest a simplified Salmonella/microsome plate incorporation assay. Rev. Inst. Med. Trop. Sao Paulo. 1990;32:269–274. doi: 10.1590/s0036-46651990000400006. [DOI] [PubMed] [Google Scholar]
- Nagel R, Nepomnaschy I. Mutagenicity of 2 anti-chagasic drugs and their metabolic deactivation. Mutat. Res. 1983;117:237–242. doi: 10.1016/0165-1218(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Ramos EI, Garza KM, Krauth-Siegel RL, Bader J, Martinez LE, Maldonado RA. 2,3-diphenyl-1,4-naphthoquinone: a potential chemotherapeutic agent against Trypanosoma cruzi. J. Parasitol. 2009;95:461–466. doi: 10.1645/GE-1686.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas C, Tapia RA, Ciudad K, Armstrong V, Orellana M, Kemmerling U, Ferreira J, Maya JD, Morello A. Trypanosoma cruzi: activities of lapachol and alpha-and beta-lapachone derivatives against epimastigote and trypomastigote forms. Bioorg. Med. Chem. 2008;16:668–674. doi: 10.1016/j.bmc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Kabayo JP. Trypanosomiasis vector control in Africa and Latin America. Parasites Vectors. 2008;1:24. doi: 10.1186/1756-3305-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Costa E, Trindade U, Teixeira D, Pinto M, Santos G, Malta V, De Simone C, Pinto A, De Castro S. Synthesis of naphthofuranquinones with activity against Trypanosoma cruzi. Eur. J. Med. Chem. 2006;41:526–530. doi: 10.1016/j.ejmech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Tandon VK, Singh RV, Yadav DB. Synthesis evaluation of novel 1,4-naphthoquinone derivatives as antiviral antifungal and anticancer agents. Bioorg. Med. Chem. Lett. 2004;14:2901–2904. doi: 10.1016/j.bmcl.2004.03.047. [DOI] [PubMed] [Google Scholar]