Abstract
Objectives
To characterize the relationship between 25-hydroxyvitamin D [25(OH)D] status with pelvic floor symptom distress and impact on quality of life.
Methods
A retrospective chart review was performed in women with a 25(OH)D level drawn within one year of their Gynecology/Urogynecology visit. Validated questionnaires including the Colorectal Anal Distress Inventory (CRADI)-8 and Incontinence Impact Questionnaire (IIQ-7) were used. Multivariate analyses characterized PFD symptom differences among women by vitamin D status.
Results
394 women were included. Mean±SD 25(OH)D levels were higher in women without PFD symptoms [35.0±14.1 and 29.3±11.5 (ng/ml), respectively (p<0.001)]. The prevalence of vitamin D insufficiency was 51% (136/268). CRADI-8 scores and IIQ-7 scores were higher among women with vitamin D insufficiency (p=0.03 and p=0.001, respectively). Higher IIQ-7 scores were independently associated with vitamin D insufficiency (p<0.001).
Conclusions
Insufficient vitamin D is associated with increased colorectal symptom distress and greater impact of urinary incontinence on quality of life.
Keywords: vitamin D, urinary incontinence, women, pelvic floor disorders
INTRODUCTION
Vitamin D is a fat-soluble vitamin whose active metabolite [1,25(OH)2D3] plays a vital role in calcium homeostasis and thus is important in overall health. Vitamin D insufficiency is very common among the population of the United States. Seventy-percent of US youth (ages 1–21 years) have vitamin D deficiency or insufficiency. Additionally, 73% of US adults and up to 78% of older US adults (age 65 years or older) have insufficient vitamin D levels.[1–2] This epidemic also affects up to 80% of reproductive age women.[3] Factors that affect vitamin D status include ethnicity (especially among individuals with darker skin and African-Americans) [4–5], intake of vitamin D supplementation, obesity, seasonality (lower levels are found after winter months) [6], and regional location (lower levels found in areas of higher latitudes and lower sunlight).[4]
Insufficient vitamin D status has received increased attention and has been shown to be associated with various extra-skeletal medical conditions including cardiovascular disease [7], diabetes [7], asthma [8], and pre-eclampsia [9]. However, the most notable effect of insufficient vitamin D has been on musculoskeletal health. Observational and randomized studies have confirmed that lower levels of serum 25-hydroxyvitamin D [25(OH)D] are associated with decreased postural stability [10] and increased risk of falls.[11] In addition, human and animal in vitro studies consistently demonstrate a relationship between vitamin D and muscle growth and function [4–5,12] though the exact mechanism is yet to be clearly elucidated. Level I studies exploring the role of vitamin D in skeletal muscle strength and functional efficiency are inconclusive. [12–14] Despite discrepant conclusions, there is significant plausibility behind the hypothesis that vitamin D has an important role in skeletal muscle function and efficiency.
The pelvic floor is composed of the levator ani and coccygeus skeletal muscles. Pelvic floor muscle weakness is clinically observed in women with pelvic floor disorder (PFD) symptoms and thus may be impacted by insufficient serum vitamin D. The vitamin D receptor (VDR) has also been identified in the detrusor wall, thus insufficient vitamin D may impact bladder function. [15] While it is conceivable that vitamin D levels could significantly affect pelvic floor disorder symptoms, there is a paucity of information investigating this relationship and thus further studies are warranted. In order to further characterize the relationship between vitamin D and pelvic floor disorder symptoms, we conducted a retrospective electronic health record (EHR) review of women presenting for benign gynecologic and urogynecologic care to evaluate differences in vitamin D levels between these groups and to determine the impact that vitamin D insufficiency has on PFD symptoms and quality of life (QoL) using validated questionnaires.
MATERIALS AND METHODS
This was a retrospective EHR review of women who had an initial visit to the Urogynecology Care Clinic at the University of Alabama at Birmingham (UAB) between January 1, 2008 through December 31, 2010 and were identified as having had a total serum 25(OH)D drawn within one year of this visit by CPT code. This study was approved by the UAB Institutional Review Board.
The serum 25(OH)D panel was processed in an outpatient laboratory using liquid chromatography.[4–5,16] The 25(OH)D panel included total 25(OH)D (primary outcome measure) as well as 25(OH)D2 and D3 fractions. Vitamin D deficiency has been variably defined as serum 25(OH)D between 10–20 ng/ml,[17] therefore we defined deficient status as 25(OH)D <15 ng/ml. Insufficient vitamin D status was defined as serum 25(OH)D between 15 ng/ml – 29 mg/ml.[18] Vitamin D levels >30ng/ml were considered sufficient.
Two reviewers screened the EHR of women with vitamin D levels for inclusion and exclusion criteria. Inclusion criteria included women with at least one visit to the UAB Urogynecology Care Clinic who were older than 19 years of age. Exclusion criteria included: (1) women with medical disorders that could impair absorption or metabolism of vitamin D such as those with inflammatory bowel disease, history of gastric bypass surgery, chronic renal and/or liver disease, (2) women with neurologic or other conditions known to affect UI or FI such as multiple sclerosis, degenerative muscular diseases, history of cerebral vascular accident, or spinal cord injury, and/or diabetes mellitus with end stage disease (retinopathy, neuropathy, ophthalmic complications, or amputations) (3) incomplete/missing clinical data in the EHR (questionnaires, no vitamin D level within 12 months of visit). Women included were categorized based on the presence of a PFD diagnosis as defined by the International Urogynecological Association and the International Continence Society. [19] The presence of a PFD diagnosis was defined as having at least one PFD diagnosis (SUI, UUI, FI, or POP) as their primary diagnosis listed in the assessment portion of the medical note of the initial visit (PFD group). Those being seen for routine gynecologic care or another benign gynecologic condition at their initial visit were placed into the general gynecology group (GYN group). The PFD group was further dichotomized based on vitamin D status as sufficient and insufficient. Vitamin D deficient women were included in the insufficient group because of the low total number with this classification.
Using a standard data form, the EHR was abstracted to obtain additional demographic, medical, and laboratory data. Demographic and medical characteristics were obtained from the initial history and physical examination documentation. Demographic characteristics included: age, height, weight, and ethnicity (documented in clinic note as White, Black, Asian, Latino, and Indian). Body mass index (BMI) was calculated and reported as kg/m2. Medical characteristics abstracted for the PFD group included: (1) number of major medical conditions (obtained from past medical history), (2) number of prescription/over-the-counter medications to include calcium and/or vitamin D supplementation taken regularly (obtained from the medication reconciliation documentation), and (3) smoking history (obtained from the social history). In addition to the serum vitamin D levels, laboratory data also included a single value for the glomerular filtration rate (GFR) reported as ml/min/1.732. If more than one GFR value was present, we used the value closest to the date of the initial visit.
Validated questionnaires completed at the initial clinic visit and were abstracted from the EHR to measure PFD symptom severity and impact on quality of life (QoL). The Pelvic Floor Distress Inventory-Short Form 20 (PFDI-SF 20) is a 20 item valid and reliable questionnaire widely used for condition-specific symptom distress assessment of women with lower urinary and gastrointestinal tract dysfunction, and pelvic organ prolapse symptoms. The PFDI-SF 20 consists of 3 subscales: the Urinary Distress Inventory (UDI - 6), Pelvic Organ Prolapse Distress Inventory (POPDI - 6), and Colorectal-Anal Distress Inventory (CRADI - 8). Of the 20 questions, each question response has ‘yes’ or ‘no’ as potential answers. ‘No’ corresponds to a score of “0”. If the patient answers ‘yes’, then the response is based on an ordinal range from “1” to “4” in terms of the bother and severity of the symptoms: “1”=not at all, “2”=somewhat, “3”=moderately, and “4”=quite a bit. Each subscale score was obtained by calculating the mean value of all answered items within the subscale (0 – 4) and multiplying it by 25 to obtain the scale score (range 0 – 100). The total PFDI-SF 20 summary score was the sum of all 3 subscale scores (range 0 – 300). Higher scores on the summary and subscales scores reflect greater distress from PFD symptoms.[20]
The Medical, Epidemiologic, and Social Aspects of Aging (MESA) questionnaire is a self-reported questionnaire that includes 9 questions assessing stress incontinence and 6 questions assessing urgency urinary incontinence symptoms. The MESA questionnaire has response categories that correspond to a 4-point Likert scale: “never” (0 points), “rarely” (1 point), “sometimes” (2 points), and “always” (3 points). The subscale scores were summed; stress subscale scores ranged from 0 – 27, urgency subscale scores ranged from 0 – 18. The total score was the sum of the two subscale scores (range 0 – 45) where higher scores represent more symptoms.[21]
The Incontinence Impact questionnaire (IIQ) -7 is the short form of a validated instrument developed to measure the impact of UI on activities of daily life in women. This questionnaire includes 4 life impact domains to include physical activity, travel, emotional health, and social activities. Item responses are assigned values of 0 for “not at all”; 1 for “slightly”; 2 for “moderately”; and 3 for “greatly”. The summed score is a sum of all 7 questions and range from 0 to 21. This mean sum score divided by 7 and multiplied by 33.3 to convert to a 0–100 scale, where higher scores represent greater impact.[22]
Demographic, medical characteristics and laboratory values are presented as means and standard deviations for continuous and frequencies for categorical variables. Chi-square and Student’s t-test analysis compared the demographic characteristics and laboratory data between women in the GYN group and PFD group. After women in the PFD group were dichotomized according to their vitamin D status [sufficient and insufficient (which includes women with a deficient vitamin D level)], subgroup differences were compared using chi-square and Student’s t-test analysis for categorical and continuous variables. Validated questionnaire data were analyzed as continuous variables and were compared based on their vitamin D status using the Student’s t-test. Multivariable logistic regression models were constructed with vitamin D as the dependent variable (sufficient compared to insufficient/deficient subgroups) to evaluate differences in pelvic floor disorder symptoms in women with sufficient and insufficient/deficient vitamin D levels after adjustment for age, BMI, race/ethnicity, and the use of vitamin D supplementation. Analyses were performed using STATA 8.2 (Stata Corp, College Station, TX)
RESULTS
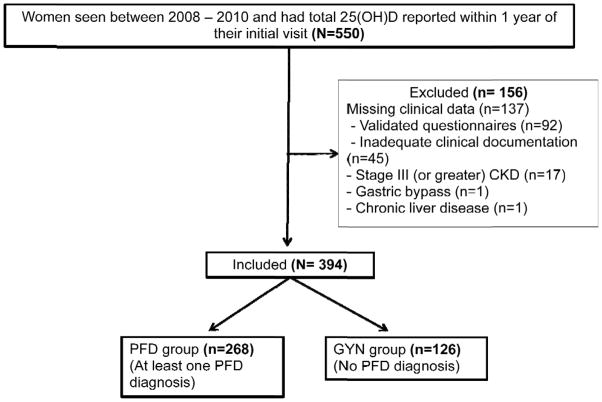
Over the 2 year period, 550 women were identified as new patients evaluated in the Urogynecology Care Clinic and had a serum 25(OH)D level drawn within one year of their initial visit. Of those women, 28% (n=156) were excluded due to a coexisting medical condition or because of missing clinical and questionnaire data. The remaining 394 women comprised the PFD and GYN groups. (Figure)
Figure 1.
Diagram of study population
Serum 25(OH)D levels were lower in the PFD group compared to the GYN group [25(OH)D = 29.3 ± 11.5 ng/ml vs. 25(OH)D = 35.0 ± 14.1 ng/ml, respectively] p < 0.001, (Table 1). Women in the PFD group were slightly older than the GYN group (64.3 ± 12.6 years vs. 60.2 ± 12.5 years, respectively, p = 0.005), but had no other significant differences in any other demographic or medical characteristics.
Table 1.
Comparison of the demographic characteristics and vitamin D levels of women with and without a PFD diagnosis
| No PFD Symptoms (Gyn Group) (n=126) | PFD Group (n=268) | P value | |
|---|---|---|---|
| Total 25(OH)D (ng/ml) | 35.0 ± 14.1 | 29.3±11.5 | 0.001 |
| Age (years) | 60.2±12.5 | 64.3±12.6 | 0.005 |
| BMI (kg/m2) | 26.8±7.8 | 27.8±7.6 | 0.97 |
| Non-Hispanic White n (%) | 104 (82%) | 225 (84%) | 0.79 |
| African American n (%) | 20 (16%) | 40 (15%) | 0.05 |
| GFR (ml/min/1.732) | 59.5±2.7 | 58.1±5.2 | 0.99 |
All values represent n (%) or mean ± standard deviation
GYN: women seeking care for benign gynecologic conditions
PFD: women seeking care for urinary incontinence, fecal incontinence, or pelvic organ prolapse
Among women in the PFD group, a total of 130 (48%) had sufficient vitamin D levels and 138 (52%) were vitamin D insufficient (including women with vitamin D deficiency n = 35, 13%). The mean serum 25(OH)D level in the sufficient women (38.4 ± 7.6 ng/ml) was significantly higher than that of vitamin D insufficient/deficient women (18.6±7.1 ng/ml) p=0.001. (Table 2) Women with insufficient/deficient vitamin D levels were significantly (p≤0.05) younger, had a higher mean BMI, and were more likely to be of African-American ethnicity than women with sufficient vitamin D levels. Women with insufficient vitamin D levels were less likely (p<0.01) to have vitamin D or calcium supplementation documented in the medical record than women with sufficient vitamin D levels. There were no statistically significant differences (p>0.05) in parity, number of medical conditions, number of medications, smoking status, or GFR in women with a PFD diagnosis according to vitamin D status.
Table 2.
Comparison of demographic and medical characteristics of women with pelvic floor disorder symptoms dichotomized based on their vitamin D status
| Total (n=268) | Vitamin D sufficient (n=130) | Vitamin D insufficient/deficient (n=138) | p | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 64.3±12.6 | 65.9±10.9 | 62.8±13.9 | 0.02 |
| BMI (kg/m2) | 27.8±7.6 | 25.8±6.4 | 29.7±8.2 | <0.001 |
| Parity (SVD) | 2.4±1.6 | 2.22±1.5 | 2.6±1.8 | 0.09 |
| Non-Hispanic White | 225 (84%) | 115 (51%) | 110 (49%) | 0.30 |
| African-American | 40 (15%) | 13 (32%) | 27 (68%) | 0.05 |
| Medical characteristics | ||||
| Medical problems (count) | 6±4.58 | 7±5.06 | 6±4.07 | 0.52 |
| Medications (count) | 8.9±5.3 | 9.4±4.9 | 8.5±5.6 | 0.15 |
| Significant smoking history | 30 (11%) | 12 (9%) | 18 (13%) | 0.32 |
| Vitamin D supplement | 132 (49%) | 77 (58%) | 55 (42%) | 0.002 |
| Calcium supplement | 126 (47%) | 77 (61%) | 49 (39%) | <0.001 |
| Laboratory data | ||||
| GFR (ml/min/1.732) | 58.1±5.2 | 58.0±5.4 | 58.3±5.0 | 0.66 |
| Vitamin D level (ng/ml) | 29.3±11.5 | 38.4±7.6 | 18.6±7.1 | 0.001 |
All values represent n (%) or mean ± standard deviation
Univariate analyses of validated questionnaire data (Table 3) reflected higher IIQ-7 scores (42.44±30.22 vs. 28.84±26.77, p=0.001) and CRADI – 8 subscale scores (33.87±26.45 vs. 27.26±23.80, p=0.03) among vitamin D insufficient/deficient women compared to vitamin D sufficient women. After controlling for age, BMI, ethnicity, and documented use of vitamin D supplementation in the multivariable logistic regression models, higher total IIQ-7 total scores, but not CRADI-8 scores, remained significantly associated with having vitamin D insufficiency/deficiency, p<0.001.
Table 3.
Validated questionnaire data among women with pelvic floor disorder symptoms compared based on vitamin D status
| Questionnaire (range) | Total group | Vitamin D sufficient | Vitamin D insufficient/deficient | p-value |
|---|---|---|---|---|
| POPDI-6 (0–100) | 31.9 ± 25.9 N=211 |
30.9 ± 24.4 N= 103 |
32.9 ± 27.3 N=108 |
0.56 |
| CRADI-8 (0–100) | 30.6 ± 25.4 N=208 |
27.3 ± 23.8 N=102 |
33.9 ±26.5 N=106 |
0.03 |
| UDI-6 (0–100) | 42.5 ± 26.6 N=211 |
43.3 ± 23.7 N=104 |
41.7 ± 29.2 N=107 |
0.65 |
| PFDI-20 Total (0–300) | 105.4 ± 65.8 N = 205 |
102.0 ± 61.3 N = 99 |
108.5 ± 61.3 N = 106 |
0.48 |
| MESA-Stress (0–27) | 11.0 ± 8.0 N=233 |
10.5 ± 7.5 N=114 |
11.5 ± 8.4 N= 119 |
0.33 |
| MESA-Urge (0–18) | 5.7 ± 4.7 N=232 |
5.4 ± 4.0 N=114 |
5.9 ± 4.7 N=118 |
0.40 |
| MESA total (0–45) | 16.6 ± 10.9 N = 231 |
15.9 ± 10.1 N = 114 |
17.3 ± 11.5 N = 117 |
0.15 |
| IIQ-7 (0–100) | 35.7 ± 29.3 N=176 |
28.8 ± 26.8 N=86 |
42.4 ± 30.2 N=90 |
0.001 |
All values represent mean ± standard deviation
DISCUSSION
In this retrospective EHR review, women presenting for gynecologic clinical care had a high prevalence of vitamin D insufficiency. Among women with a PFD, 51% had vitamin D insufficiency/deficiency as defined by a vitamin D level <30 ng/ml. In this study, women with vitamin D insufficiency were more likely to report distress from colorectal symptoms and had a greater impact from UI symptoms on quality of life. This association between vitamin D insufficiency and greater impact of UI on quality of life remained significant after controlling for potential confounding factors known to influence serum 25(OH)D levels. However, the type of UI symptoms and symptom distress related to UI did not differ based on vitamin D status.
To date, few studies investigating the relationship between vitamin D status and pelvic floor disorders exist in the literature. There have been two epidemiologic studies exploring the relevance of vitamin D to pelvic floor disorder symptoms in community dwelling women. The Leicesterishire MRC Incontinence Study Group (a longitudinal cohort study) reported that higher vitamin D intake was significantly associated with reduced risk of OAB onset (p=0.008).[23] In the NHANES cycle from 2005–2006, Badalian and Rosenbaum reported a prevalence of vitamin D insufficiency of 82% among non-pregnant women over 20 years of age. Additionally, the prevalence of urinary incontinence symptoms, but not fecal incontinence symptoms, was lower in the vitamin D sufficient group even after controlling for demographic factors shown to be associated with both pelvic floor disorders and vitamin D levels in the literature.[3] Our retrospective study supports the data from these epidemiologic studies by showing that vitamin D insufficiency is also prevalent in women seeking care for pelvic floor symptoms. We have further characterized the relationship between pelvic floor symptoms and insufficient vitamin D status by controlling for co-morbidities and other conditions related to vitamin D insufficiency. Despite adjustment for these factors, significant differences were still observed in the impact of urinary incontinence on quality of life in women with this condition.
We hypothesize that vitamin D is important in skeletal muscle efficiency and potentially in detrusor muscle/urothelial function thus explaining our observation that vitamin D insufficient women are more impacted by urinary incontinence when compared to vitamin D sufficient women. Vitamin D has been shown to increase skeletal muscle cell proliferation and muscle fiber size in vitro. [24–25] In addition, the vitamin D receptor (VDR) has been identified in human skeletal muscle nuclei, although inconsistently. [24–26] The role of vitamin D in the efficiency of skeletal muscle function is thought to be through the binding of the active metabolite to the VDR resulting in muscle growth. [24–25] While clinical observational and randomized studies have inconsistently demonstrated a relationship between skeletal muscle strength and vitamin D insufficiency or vitamin D supplementation, level I and II studies have consistently associated increased postural stability and decreased risk of falls with insufficient vitamin D. [10–11] The improvement in balance and prevention of falls observed may be secondary to more efficient muscle function rather than improved muscle strength. This hypothesis may be extrapolated to explain our observation in vitamin D insufficient women with urinary incontinence. The levator ani muscles of the pelvic floor are vital in the voluntary control of continence both mechanically and through neuronal pathways. Pelvic floor muscle weakness may prevent incontinent women from efficiently closing the urethra during times of increased intraabdominal pressure resulting in stress urinary incontinence.
Vitamin D insufficiency may also affect the detrusor wall contributing to symptoms of overactive bladder and urgency urinary incontinence (UUI). Gau reported two cases of resolution of urgency urinary incontinence with high dose vitamin D supplementation. [27] The VDR has been identified in both the urothelium and the smooth muscle of the detrusor wall. [15] In vitro studies of the human bladder have demonstrated that detrusor muscle relaxation resulted from inhibition of a calcium-sensitized pathway triggered by a vitamin D receptor agonist. [28] In addition, an epidemiologic study implicated a higher dietary intake of vitamin D to be associated with decreased risk of OAB. [23] This suggests that vitamin D insufficient women may have abnormal calcium homeostasis in the detrusor wall contributing to urinary urgency and UUI symptoms.
We also observed more severe bowel symptoms in women with vitamin D insufficiency. In one case series, Alkhatib and colleagues also observed a higher rate of vitamin D insufficiency and deficiency in their patients with fecal incontinence. [29] Fecal incontinence may result from a disruption or weakness in the puborectalis or external anal sphincter muscles, and is also affected by alterations in stool consistency and colonic transit time. Often women with FI have loose stool and diarrhea which may also impact the absorption of vitamin D. However, weak/disrupted external anal sphincter muscles may have an important impact on the severity of FI. [30] Although we observed that women with vitamin D insufficiency had more colorectal symptom distress on the CRADI-8, further studies are needed to explain the relationship of vitamin D with the severity of bowel symptoms.
Limitations of this study include its retrospective cohort design that may explain why we observed no differences in validated questionnaire scores with regard to the severity or type of urinary incontinence and prolapse symptoms among women with sufficient and insufficient vitamin D. Vitamin D levels measured are impacted by sunlight exposure, skin pigmentation, obesity, and oral supplementation. Our retrospective design prohibited the collection of important clinical details specifically the season at the time of serum 25(OH)D collection and the duration of vitamin D supplementation. However, our regression model accounted for the use of vitamin D supplementation, BMI, and ethnicity in order to eliminate these factors as potential confounders to the significant relationship observed.
To our knowledge, there were no other identified clinical cohort studies that investigated the relationship between vitamin D status and PFD symptoms in women. While limited by the retrospective design, this study serves as a foundation for future studies investigating the impact of vitamin D on PFDs. Our study is strengthened by the use of the gold standard technique of liquid chromatography to measure serum 25(OH)D. Further, the use of validated questionnaire measures for symptom severity and impact on quality of life allows for more valid and generalizable interpretation of the data. We also controlled for other co-morbid disorders that may affect vitamin D serum levels.
In conclusion, vitamin D insufficiency is prevalent in a population of women seeking care for pelvic floor symptoms. Our finding of a greater impact of UI symptoms on QoL in women with insufficient vitamin D was important. Vitamin D insufficiency may result in the reduced ability to voluntarily control stress leakage and a decreased effectiveness for urge-suppression strategies. Sufficient vitamin D serum levels may facilitate an increase pelvic floor muscle efficiency and a decrease in detrusor contractibility allowing for a more effective response to behavioral therapy by decreasing UI episodes. This potential relationship needs to be explored more robustly. Prior to advocating vitamin D supplementation for the treatment of PFD symptoms, more data are needed. Future research into the relationship between total 25(OH)D and pelvic floor disorders should be studied prospectively in order to determine the potential role of vitamin D supplementation as a part of a multi-component strategy for their treatment.
Acknowledgments
FUNDING: Partially funded by 2K24-DK068389 to Holly E. Richter from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Manuscript contribution:
C Parker-Autry: Protocol/project development, data collection, data analysis, manuscript writing/editing
A Markland: data analysis, manuscript writing/editing
A Ballard: data collection
D Downs-Gunn: data collection
H Richter: protocol/project development, manuscript writing/editing
FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE
References
- 1.Kumar J, Muntner P, Kaskel FJ, Hailper SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Haff Fuleihan G, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 3.Badalian SS, Rosenbaum PF. Vitamin D and pelvic Floor Disorders in Women: Results from the National Health and Nutrition Examination Survey. Obstet Gyneco. 2010;115:795–803. doi: 10.1097/AOG.0b013e3181d34806. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and Skin Physiology: A D-Lightful Story. J Bone Miner Re. 2007;22:S2, V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 5.Wang S. Epidemiology of vitamin D in health and disease. Nutr Res Rev. 2009:188–20. doi: 10.1017/S0954422409990151. [DOI] [PubMed] [Google Scholar]
- 6.Hanley DA, Davison KS. Vitamin D Insufficiency in North America. J Nutr. 2005;135:332–337. doi: 10.1093/jn/135.2.332. [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic Review: Vitamin D and Cardiometabolic Outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc. 2011;111(2):258–68. doi: 10.1016/j.jada.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and advese pregnancy outcomes in a group of high risk for pre-eclampsia. BJOG. 2010;117(13):1593–8. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff HA, Stahelin HB, Dick W, Akos R, Kneht M, Salis C, Nebiker M, Cheiler R, Pfeifer M, Beqerow B, Lew RA, Conzelmann M. Effects of vitamin D and Calcium supplementation on falls: A randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 12.Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, Mughal MZ. A Randomized, Controlled Trial of Vitamin D Supplementation upon Musculoskeletal Health in Postmenarchal Females. J Clin Endocrin Metab. 2010;95(10):0000–0000. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 13.Lips P, Binkley N, Pfeifer M, Recker R, Samanta S, Cohn DA, Chandler J, Rosenberg E, Papanicolaou DA. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr. 2010 Apr;91(4):985–91. doi: 10.3945/ajcn.2009.28113. [DOI] [PubMed] [Google Scholar]
- 14.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, Allain TJ. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33(6):589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 15.Crescioli C, Morelli A, Adorini L, Ferruzzi P, Luconi M, Vannelli GB, Marini M, Gelmini S, Fibbi B, Donati S, Villari D, Forti G, Colli E, Andersson KE, Maggi M. Human Bladder as a Novel Target for Vitamin D Receptor Ligands. J Clin Endocrinol Metab. 2005;90:962–972. doi: 10.1210/jc.2004-1496. [DOI] [PubMed] [Google Scholar]
- 16.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nut. 2008;87:1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer Mm. Vitamin D insufficiency: disease or no disease? J Bone Miner Res. 2008;23:1052–60. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Neurourology and Urodynamics. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 20.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193:103–13. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol Med Sc. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 23.Dallosso HM, McGrother CW, Matthes RU, Donaldson MMK. Nutrient Composition of the Diet and the Development of Overactive Bladder: A Longitudinal Study in Women. Neurourol Urodynam. 2004;23:204–210. doi: 10.1002/nau.20028. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. The Histochemical Journal. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff-Ferrari HA, Borchers M, Guddat F, Durmuller U, Stahelin HB, Dick W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases with Age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, DeLuca HF. Is the Vitamin D Receptor Found in Muscle? Endocrinology. 2011;152:354–363. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 27.Gau JT. Urinary Incontinence resolved after adequate vitamin D supplementation: a report of two cases. JAGS. 2010;58 (12):2438–2439. doi: 10.1111/j.1532-5415.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 28.Penna G, Fibbi B, Susana Amuchastegui, Corsiero E, LAverny G, Silvestrini E, Chavalmane A, Morelli A, Sarchielli E, Vannelli GB, Gacci M, Colli E, Maggi M, Adorini The Vitamin D Receptor Agonist Elocalcitol Inhibits IL-8-Dependent Benign Prostatic Hyperplasia Stromal Cell Proliferation and Inflammatory Response by Targeting the RhoA/Rho Kinase and NF-kB Pathways. The Prostate. 2009;69:480–493. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 29.Alkhatib AA, Tuteja AK. High Prevalence of Vitamin D Deficiency Among patients with Fecal Incontinence. Dig Dis Sci. 2010 doi: 10.1007/s10620-010-1173-x. [DOI] [PubMed] [Google Scholar]
- 30.Gleason JL, Markland A, Greer WJ, Szychowski JM, Gerten KA, Richter HE. Anal sphincter repair for fecal incontinence: effect on symptom severity, quality of life, and anal sphincter squeeze pressures. Int Urogynecol J. 2011;22(12):1587–92. doi: 10.1007/s00192-011-1551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]