Abstract
Background:
Ciclesonide is an inhaled corticosteroid administered by a metered dose inhaler (MDI) to treat bronchial asthma. After inhalation, the inactive ciclesonide is converted by esterases in the airways to active metabolite desisobutyryl-ciclesonide (des-CIC).
Aim:
To compare the pharmacokinetic (PK) parameters of des-CIC in children after administration of therapeutic dose of ciclesonide with and without spacer (AeroChamber Plus™).
Methods:
Open-label, 3 period, cross over, repeated dose, PK study in 37 children with mild to moderate stable asthma (age: 6–11 y; body weight: 20–53 kg). During each 7-day treatment period, ciclesonide was inhaled once in the morning: A) 160 μg MDI with spacer, B) 80 μg MDI with spacer, and C) 160 μg MDI without spacer. Serum PK parameters of ciclesonide and des-CIC were determined on Day 7 of each period. The primary PK parameters were the AUCτ and Cmax for des-CIC.
Results:
Inhaling ciclesonide with spacer led to a dose proportional systemic exposure (AUCτ) of des-CIC (0.316 μg*h/L for 80 μg and 0.663 μg*h/L for 160 μg). The dose-normalized systemic exposure for des-CIC (based on AUCτ) was 27% higher after inhalation of ciclesonide 80 μg or 160 μg with spacer than without spacer; the corresponding Cmax values for des-CIC were, respectively, 63% and 55% higher with spacer. No clinically relevant abnormalities or adverse drug reactions were observed.
Conclusions:
Inhalation of therapeutic ciclesonide dose with spacer led to a slight increase in the systemic exposure of des-CIC, which does not warrant dose adjustment.
Keywords: asthma, children, ciclesonide, pharmacokinetics, spacer
Introduction
Asthma is a chronic inflammatory disease of the airways, manifested by airway hyper-responsiveness, acute bronchoconstriction, airway edema, and mucous plugging. Inhaled corticosteroids (ICS) are the most effective drugs available to treat chronic asthma and are recommended by current international guidelines as first line therapy for patients of all ages with persistent asthma of all severities.1–3
Ciclesonide is a single isomer corticosteroid that was developed for the treatment of bronchial asthma, using a metered dose inhaler (MDI) with a propellant hydrofluoroalkane (HFA)-134a. The lung deposition of ciclesonide is more than 50% in healthy subjects and patients with asthma4,5 and this finding is consistent with the pharmacokinetic investigations in both groups.6 Following inhalation of ciclesonide, the inactive parent compound ciclesonide is converted by esterases in the airways tissue to the pharmacologically active metabolite desisobutyryl-ciclesonide (des-CIC),which has a relative glucocorticoid receptor affinity of 1200 (receptor affinity of dexamethasone = 100 ). The oral bioavailability of ciclesonide is negligible (<1%); thus, the swallowed drug does not contribute to systemic bioavailability. About 99% of ciclesonide and des-CIC bind to plasma protein and both are rapidly cleared from the systemic circulation. These properties ensure high local anti-inflammatory activity of des-CIC in the lung associated with low systemic activity.7–11 The efficacy and safety of ciclesonide with the dose 80 μg/day or 160 μg/day in treatment of asthma has been demonstrated in several studies with adults,7,12–14 adolescent,15 and children.16–20
Efficacy of any drug delivered via MDI depends not only on the therapeutic compound, its galenic formulation, and particle size distribution but also on the user’s good ‘hand-lung coordination’ with simultaneous release of the actuator on the MDI and breathing in of the released drug. Spacer devices can reduce coordination difficulties and accommodate delivery of the drug to the lung. Spacers may not be required for patients with good inhalation technique. However, especially in young children, elderly, or disabled persons, spacers can reduce difficulties that may occur during actuation-inhalation coordination.21–24 Furthermore, spacers can reduce the possibility of local adverse effects, because less of the released drug deposits in the oropharyngeal cavity and substantially improve dose delivery to target organ—the lung.21,25 Indeed, recent guidelines clearly recommend the use of spacers with MDI for young children.1
The aim of the present study was to compare the pharmacokinetic (PK) characteristics of the active metabolite des-CIC of ciclesonide (des-CIC), when ciclesonide was administered to children with asthma via MDI-HFA with and without spacer (AeroChamber Plus™).
Materials and Methods
Ethics
The study was approved by the responsible ethics committee (Ethics Committee for the Institute for Health Protection of Mother and Child Dr. Vukan Cupic, Novi Beograd, Serbia) and was performed as a single center study in the above Institute in Serbia. The study conformed to the Declaration of Helsinki (Somerset West Amendment, 1996), Note for Guidance on Good Clinical Practice (CGMP/ICH/135/95). Before the study start, each parent, or legal guardian gave written informed consent to participate in the study.
Patients and study design
A total of 37 pediatric patients with mild to moderate stable persistent asthma (24 boys and 13 girls, age range 6 to 11 years) were enrolled into the study (Table 1). The study had the following design: open label, randomized, three-period cross over, and repeated dose.
Table 1.
Demographic characteristics at baseline.#
| Variable | (N = 37) |
|---|---|
| Age [years] | |
| Mean ± SD | 9 ± 2 |
| Median (min, max) | 8 (6, 11) |
| Age group n (%) | |
| 6 to ≤8 years | 20 (54.1%) |
| >8 to ≤11 years | 17 (45.9%) |
| Gender, n (%) | |
| Male | 24 (64.9%) |
| Female | 13 (35.1%) |
| Height [cm] | |
| Mean ± SD | 138 ± 13 |
| Median (min, max) | 140 (116, 173) |
| Weight [kg] | |
| Mean ± SD | 33 ± 9 |
| Median (min, max) | 34 (20, 53) |
| Body mass index [kg/m2] | |
| Mean ± SD | 17 ± 2 |
| Median (min, max) | 17 (14, 21) |
| FEV1 [L] | |
| Mean ± SD | 1.8 ± 0.4 |
| Median (min, max) | 2.0 (1.1, 2.9) |
| FEV1 [% predicted] | |
| Mean ± SD | 87 ± 10.4 |
| Median (min, max) | 87 (64, 109) |
#Patients were using their usual asthma medication including ICS.
Abbreviations: ICS, inhaled corticosteroids; N, number of patients in analysis set; SD, standard deviation; min, minimum; max, maximum.
The study consisted of a screening examination, three 7-day treatment periods, and an end-of-study examination. Ciclesonide was administered once daily in the morning during all treatments. Treatment A: inhalation dose of ciclesonide 160 μg by MDI with spacer; Treatment B: ciclesonide 80 μg by MDI with spacer; and Treatment C: ciclesonide 160 μg by MDI without spacer. There was no washout period between the treatments, because on Day 1 of each treatment period no pharmacokinetics were performed and after each treatment period a steady state conditions could be assumed. Ciclesonide with an HFA-MDI (Alvesco®) was supplied by Nycomed GmbH (formerly Altana Pharma AG); the AeroChamber Plus™ spacer was purchased from Trudell Medical International (London, ON, Canada).
Patients were screened within 2 weeks of study start. The investigator instructed each patient and parent/guardian on how to correctly use the inhaler/spacer by using a placebo MDI. Throughout the entire study, the patients used their usual asthma medication in addition to the study medication. The usual medication was ICS (not more than 200 μg/d fluticasone propionate or 400 μg/d beclomethasone dipropionate or 400 μg/d budesonide), and/or leukotriene modifiers and/or short acting β2-agonists (such as salbutamol). The usual medications (except short acting β2-agonists) should have been taken at a constant dose for at least 4 weeks prior to start of study and during the study; daily time and mode of administration remained the same also when study medication was used.
For the present study, patients were allocated to a treatment sequence according to a random list and inhaled the first dose of the study drug with the guidance of a study nurse. After 6 days of using the treatment at home, the patients returned to the study centre at −13 h before the next drug inhalation and pharmacokinetic evaluation on Day 7. The study was completed with an end-of-study examination that was performed within 1 to 7 days after the last inhalation of the study drug.
Main inclusion criteria
The main inclusion criteria were: patients (male or female), aged between 6 and 11 years (inclusive), with a history or current stable persistent mild to moderate bronchial asthma consistent with the GINA 2004 classification, requiring regular treatment with ICS, and/or leukotriene modifiers and/or short acting β2-agonists (salbutamol) as needed (FEV1 > 60% of predicted in children not treated with inhaled corticosteroids and >80% of predicted in children treated with inhaled glucocorticosteroids at least 4 h after use of short acting β2-agonist.2
Main exclusion criteria
Patients were not eligible for the study if, according to the investigator/sponsor, they were diagnosed with any disease (with the exception of asthma) that might have interfered with study-related procedures or interpretation of the study results; had history of upper and/or lower respiratory tract infection or asthma exacerbation within 4 weeks prior to screening visit; had history or current evidence of clinically relevant hypersensitivity to drugs or food (except allergic asthma and rhinitis).
Study assessments
On the days before the pharmacokinetic profiling, patients reported to the clinical ward in the evening and were confined until completion of the 24 h blood sampling. In the morning of the study days for pharmacokinetic profiling, an indwelling cannula was inserted into a forearm vein to allow repeated blood sampling.
Treatment compliance
On Day 1 and Day 7 of each study period, patients inhaled ciclesonide under the supervision of the study nurse. During the ambulant treatment period, compliance was confirmed by a daily telephone call and additionally the patients documented intake of study drug in the patient’s diary. Starting at the screening visit, the patients recorded into the dairy, the daily PEF measurements (using the Mini Wright Peak Flow Meter® provided by the sponsor), number of puffs of rescue medication, and asthma score.
Blood sampling for determination of pharmacokinetic parameters
For the determination of serum concentration of ciclesonide and des-CIC on Day 7 of each treatment period, blood samples (2 mL) were taken via an intravenous catheter, at pre-dose, 0.5 h, 1 h, 1.5 h, 3 h, 6 h, 9 h, 12 h, and 24 h after inhalation. Blood samples were taken into tubes with serum clot activator (e.g. Vacuette®, Greiner). Immediately after blood collection, the blood samples were placed at 5 ± 3 °C for up to 90 min to allow clotting and centrifuged at 4 °C at 2,200 g for 10 min, to obtain serum. The serum samples were transferred into polypropylene tubes, transported on dry ice, and stored at −70 °C until analysis described below.
Bioanalysis of ciclesonide and desisobutyryl-ciclesonide (des-CIC)
Serum concentrations of ciclesonide (the parent compound) and des-CIC (the pharmacologically active metabolite of ciclesonide) were determined on 300 μL of serum, using a validated high-performance liquid chromatography method with mass-spectrometric detection (HPLC-MS/MS), at the Department of Bio-analytics (Nycomed GmbH, Konstanz, Germany). The assay was linear in the range of 0.01 μg/L and 2 μg/L. The lower limit of quantification (LLOQ) for both compounds was 0.01 μg/L. For ciclesonide, the inter-day precision (between-day coefficient of variation) ranged between 2.7% and 6.0%. Inter-day accuracy ranged between 97.6% and 100.5%. For des-CIC, the inter-day precision ranged between 2.5% and 5.4% and inter-day accuracy ranged between 97.2% and 101.7%.
Safety investigations
Safety investigations included: monitoring of adverse events (AEs) throughout the study. Clinical laboratory evaluation (blood chemistry, hematology, urine analysis), physical examination, and vital signs (blood pressure, pulse rate, and body temperature), were monitored at the screening examinations, Day 1, Day 7, and end-of-study.
Pulmonary function tests (FEV1, FVC, and PEF) were performed at screening, Day 1 and Day 7 (predose) of each treatment period, and at the end-of-study visit, according to the standards set by the American Thoracic Society (ATS) and European Respiratory Society (ERS) Task Force.26,27
Primary and secondary objectives
The primary objective of the study was to characterize on Day 7, the steady-state PK of the active metabolite of ciclesonide (des-CIC). The primary PK parameters are the area under the curve over the dosage interval τ (AUCτ, where τ is 24 h ) and the maximum serum concentration (Cmax) of des-CIC, after inhalation of ciclesonide at two dose strengths via MDI, with the spacer (AeroChamber Plus™) compared with inhalation of the reference dose without a spacer.
The secondary objectives of the study were for des-CIC: AUC from time zero to last observed concentration (AUC0–last), time to reach maximum serum concentration (tmax), and elimination half-life (t1/2); for ciclesonide (parent compound): AUCτ, AUC0–last, Cmax, tmax, and t1/2.
Data analysis
After inhalation of the study drug, the PK parameter estimates of ciclesonide and des-CIC were obtained by a non-compartmental analysis approach, using WinNonLin, version 4.0.1 (Pharsight, Mountain View, CA, USA). The observed serum Cmax, and tmax were obtained directly from the source data.
Estimates of AUC0–last were obtained using linear trapezoidal integration up to the last sampling point. Estimates of AUCτ were derived by AUC0–last + Clast/λz denotes the last up to the 24 h time point (where Clast quantifiable serum concentration).
Based on previous experience from PK studies, it was anticipated that only a limited number of samples would contain des-CIC at concentrations above the LLOQ of 10 ng/L, an aspect particularly relevant during Treatment B when ciclesonide 80 μg/d was used. To avoid the possibility that a single intermittent measurement above the LLOQ was declared as the maximum concentration, the Cmax was determined only if after the inhalation at least three neighboring serum concentrations exceeded the LLOQ. For a reliable calculation of AUCτ, the extrapolated areas were less than 20% of the total AUC.
The focus of the PK analysis was on descriptive statistics. If at least 15 patients had paired values for Treatments A, B, and C for either of the variables AUCτ and Cmax, an analysis of variance (ANOVA) was performed using the 90% confidence interval (CI) for the ratios of the least-squares means [(LSM) with the WinNonLin (version 4.0.1) linear mixed effects modeling]. Test/Reference ratios of geometric means for AUCτ, and Cmax of des-CIC were calculated for Treatment A (Test) and Treatment C (Reference) as well as for Treatment B (Test) and Treatment C (Reference).
For the ANOVA, sequence and period were considered as fixed and patient nested in a sequence as random effect, resulting in a 90% CI for the ratio of the expected means. Each PK characteristic was log-transformed prior to analysis and for each log-transformed variable, a 90% CI was computed for the difference of means between the treatments under comparison. The limits of the 90% CI on the log-scale were log-transformed, whereby the confidence limits on the original scale for the ratio of geometric means were expressed as percentages.
Results
Patients
A total of 37 pediatric patients (24 boys and 13 girls; median age 8 years age range 6 to 11 years; median weight 34 kg, range 20 to 53 kg) with mild to moderate stable persistent asthma were enrolled into this single centre study. The demographic and baseline characteristics are summarized in Table 1.
Of the 37 patients who enrolled into the study, 36 completed the study according to the study protocol; 1 patient discontinued the study prematurely due to an adverse event (asthma exacerbation after an overall 5 days in the study, receiving Treatment B during the first administration period), described below in the section ‘Safety and Tolerability’. Three patients were classified as protocol violators (1 patient due to premature study discontinuation, 2 patients for inhaling the study medication at the incorrect time on kinetic day and due to missing serum samples following inhalation). For pharmacokinetic evaluation, these patients were excluded from the per-protocol analysis. For des-CIC, the data for the per-protocol and intention-to-treat population were very similar.
Pharmacokinetics
The inactive parent compound ciclesonide
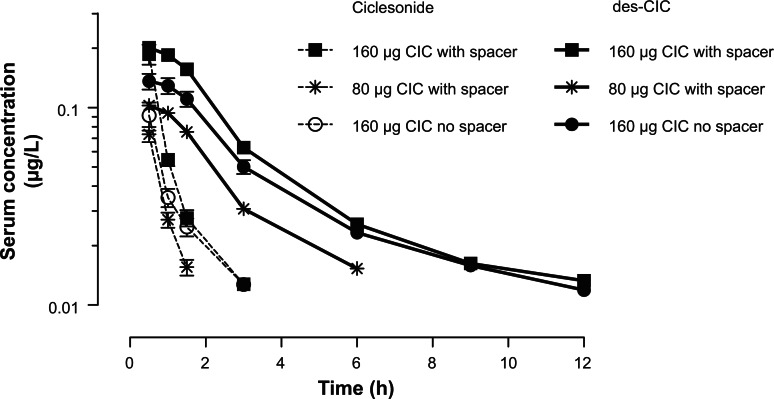
The serum concentration time profile for the parent compound ciclesonide after a 7-day repeated once daily dose of ciclesonide 160 μg with spacer (Test Treatment A), ciclesonide 80 μg with spacer (Test Treatment B), and ciclesonide 160 μg without spacer (Reference Treatment C) is shown in (Fig. 1).
Figure 1.
Serum concentration-time profiles of ciclesonide and des-CIC of ciclesonide measured on Day 7 at steady-state (per-protocol population). Serum concentration-time profiles of ciclesonide (broken line) and des-CIC of ciclesonide (solid line) measured on Day 7 at steady-state of each treatment period in 6 to 11-year-old pediatric patients with stable asthma (per-protocol population). Patients were allocated to a randomized treatment sequence. After using the treatment at home for 6 days, the patients returned to the study centre, before the next drug inhalation and pharmacokinetic evaluation on Day 7. The lower limit of quantification (LLOQ) for both compounds was 0.01 μg/L. Shown are Mean and Standard Error of the Mean.
Due to the low systemic absorption of ciclesonide and consequently limited number of time points for serum concentration of ciclesonide, the concentration-time profile curves could be described only for the first few hours following inhalation. For Treatment A and C, the serum concentrations of ciclesonide were detectable for up to 3 h. For Treatment B, the serum concentrations were measurable for only up to 1.5 h (Fig. 1). The values for mean ± SEM for Cmax were 0.192 ± 0.022 μg/L (Treatment A), 0.096 ± 0.007 μg/L (Treatment B), and 0.117 ± 0.019 μg/L (Treatment C) (Fig. 1). For all three treatments, the tmax values were observed at the first available time point after inhalation (0.5 h).
The active metabolite des-CIC
The serum concentration—time profile curves for the active metabolite des-CIC are shown in Figure 1. After inhalation of 160 μg ciclesonide (Treatment A and Treatment C), the serum concentration-time profiles curves were obtained up to 12 h. After inhalation of 80 μg ciclesonide (Treatment B), the serum concentrations of des-CIC were low and detectable for up to 6 h. After inhalation of ciclesonide without a spacer (Treatment C), the mean serum concentration-time profile curve for des-CIC was in between the two curves obtained for Treatment A and B (Fig. 1).
The evaluated PK parameters for des-CIC are summarized in Table 2. After inhalation of ciclesonide with spacer (Treatment A and B), both the Cmax, and AUCτ showed a linear dose proportional increase. Furthermore, the variability (reflected as the standard error of the mean [SEM]) for the primary pharmacokinetic parameter Cmax and AUCτ des-CIC was similar when the inhalation was performed with spacer (Treatment A) or without spacer (Treatment B) (Table 2).
Table 2.
Pharmacokinetic parameter estimates of the active metabolite des-CIC in serum of pediatric patients with stable asthma.
| Treatment variable mean ± SEM | Treatment A 160 μg CIC with spacer | Treatment B 80 μg CIC with spacer | Treatment C 160 μg CIC no spacer |
|---|---|---|---|
| N | 31 | 33 | 33 |
| Cmax (μg/L) | 0.208 ± 0.012 | 0.106 ± 0.006 | 0.141 ± 0.013 |
| AUCτ (μg*h/L) | 0.663 ± 0.042 | 0.316 ± 0.022## | 0.542 ± 0.042## |
| AUC0–last | 0.610 ± 0.040 | 0.261 ± 0.019 | 0.443 ± 0.043 |
| T1/2 (h) | 3.19 ± 0.22 | 1.78 ± 0.11 | 3.02 ± 0.21# |
| tmax (h) | 0.69 ± 0.044 | 0.70 ± 0.048 | 0.73 ± 0.054 |
N, 32 for this value;
N, 29 for this value; AUCτ, 100%; AUClast, includes more than 80% of AUCτ
Abbreviations: Cmax, Maximum serum concentration; AUC, Area under the AUCτ; AUC over the dosage interval τ (at steady-state); AUC0-last, AUC from time zero to the last observed concentration.
The results of ANOVA for the ratios of treatments (Test/Reference) and the 90% confidence intervals are shown in Table 3. Based on the dose normalized values for AUCτ, inhaling either 160 μg or 80 μg ciclesonide with spacer led to an exposure of des-CIC that was respectively, 27% and 26% higher than after 160 μg inhaled without the spacer; the dose normalized Cmax values were slightly higher with spacer (55% to 63%) than without the spacer (Table 3).
Table 3.
Ratios and 90% confidence limits of des-CIC pharmacokinetic parameter estimates on Day 7 (per-protocol population).
| Geometric least squares means for des-CIC | ||||
|
| ||||
| Variable | Treatment A 160 μg CIC with spacer (Test) | Treatment C 160 μg CIC no spacer (Reference) | Ratio [% Ref] | 90% Confidence interval# |
|
| ||||
| AUCτ (μg*h/L) | 0.601 | 0.475 | 127 | 107–149 |
| Cmax (μg/L) | 0.190 | 0.123 | 155 | 132–181 |
|
| ||||
| Variable | Treatment B 80 μg CIC with spacer (Test) | Treatment C 160 μg CIC no spacer (Reference) | Ratio [% Ref] | 90% Confidence interval# |
|
| ||||
| AUCτ (μg*h/L) | 0.599 | 0.475 | 126 | 106–149 |
| Cmax (μg/L) | 0.201 | 0.123 | 163 | 140–190 |
90% Confidence Interval for difference between the treatments. AUC, Area under the AUCτ, AUC over the dosage interval τ (at steady-state).
For the ANOVA, sequence and period were considered as fixed and patient nested in a sequence as random effect, resulting in a 90% CI for the ratio of the expected means. Each PK characteristic was log-transformed prior to analysis and for each log-transformed variable, a 90% CI was computed for the difference of means between the treatments under comparison. The limits of the 90% CI on the log-scale were log-transformed resulting in confidence limits on the original scale for the ratio of geometric means expressed as percentages.
As shown in Table 2, the tmax of des-CIC was similar for all treatments (0.7 h). The mean half-life (t1/2) of des-CIC was shorter for Treatment B (1.78 h) compared with Treatment A (3.19 h), and Treatment C (3.02 h). This is likely to be due to the lower dose of ciclesonide used during Treatment B and the subsequent incomplete serum concentration time profile, whereby the t1/2 represents a mixed distribution/elimination phase.
Lung Function
The lung function did not change to any relevant extent between screening, Day 7 of each treatment period, and study end. The values (mean ± SD) at screening and study end for FEV1 [L] were 1.81 ± 0.44 and 1.76 ± 0.42, respectively. Values for FVC and PEF were similar at study end and study start.
Safety and Tolerability
Safety of ciclesonide was monitored during the entire study as the occurrence of adverse events (AEs). During the three treatment periods, 24 patients reported a total of 31 AEs. The intensity of all but one of the AEs was mild or moderate. All AEs resolved completely and all were assessed as ‘not related’ to the study medication (by investigator and sponsor). No clinically relevant abnormalities in laboratory values were observed. The administration of ciclesonide with or without spacer was well tolerated.
One patient experienced a serious AE (asthma exacerbation) after receiving ciclesonide 80 μg with spacer for 5 days (Treatment B). The event was assessed as ‘not related’ to the study medication (investigator and sponsor). The patient was withdrawn from the study and after treatment (with intravenous methylprednisolone, inhaled salbutamol, and oxygen), recovered by the 6th day without sequelae.
Discussion
Ciclesonide, is a new ICS delivered via HFA-MDI, which has been shown to be effective and safe in clinical studies with adults and children.7,12,13,15–20,28 The study presented here is part of a drug characterization program for ciclesonide to evaluate the effect of a spacer (AeroChamber Plus™) on the PK parameters in children with asthma (6 to 11 years old).
According to the current GINA guidelines, monotherapy with ICS is the first-line treatment for adult and children with persistent asthma.1–3 If the inhalation technique is not optimal, the inhaled drug either does not reach the lung or only in variable quantity and the drug is likely deposited in the oropharyngeal cavity. The use of a spacer, fitted to an MDI, is recommended for patients (such as young children or elderly) who may have poor coordination with the actuation-inhalation technique.1,2,21–24 Results of systematic studies on the effect of spacer attached to the MDI have shown that for some drugs the use of a spacer leads to an increase in pulmonary deposition, which in turn may translate into a better clinical effect of the drug and to a decrease in the oropharyngeal deposition which may lead to an improved safety with fewer adverse reactions.29,30 These aspects have implications on the possibility to adjust (reduce) the administered dose of the inhaled drug, which is particularly relevant for patients that require maintenance therapy.
In the present study, ciclesonide was inhaled once in the morning, using therapeutic doses with and without spacer, attached to the MDI. The study consisted of 3 treatment period of 7 days each (Treatment A, B, C), with PK parameters measured on Day 7 of each treatment period (at steady-state). The focus of the study was on the steady-state PK parameters of des-CIC (the active metabolite), rather than on the parent compound ciclesonide. This is because ciclesonide has only about 1% of glucocorticoid-binding affinity and does not possess any relevant glucocorticoid activity.11
For the PK parameters of des-CIC, it was shown in the present study that inhaling ciclesonide with spacer led to a dose proportional systemic exposure (AUCτ) of des-CIC (0.316 ng*h/mL for 80 μg dose and 0.663 ng*h/mL for 160 μg dose). The dose-normalized systemic exposure (AUCτ) of des-CIC after the inhalation of ciclesonide (80 μg or 160 μg) with spacer was 27% higher than without spacer. The corresponding dose-normalized results of des-CIC for Cmax showed an increase of 55% to 63% with the spacer than without the spacer (Table 3). Such an increase in the AUCτ or Cmax of des-CIC is minimal in comparison with other drugs29,30 including fluticasone propionate (Advair HFA), which had a reported increase of 406% for the AUC0–last and of 210% for Cmax with spacer.31 Therefore, based on the results of the present study with ciclesonide and in comparison with recommendations used for other ICS, the observed increase in the AUCτ and Cmax for des-CIC with spacer is slight and does not warrant dose adjustment of ciclesonide HFA-MDI in children.
Results of studies in adults with asthma who have good inhalation technique have shown that lung deposition of ciclesonide inhaled via HFA-MDI without a spacer have a median lung deposition of 53.2%.4,5 Compared with other therapeutically used ICS, this is a very high proportion of drug reaching the lungs, including the small airways and alveoli; little of the inhaled ICS is deposited in the oropharyngeal region, reducing the chance for oropharyngeal side effects.4,21,22,24,32,33 For ciclesonide, unlike for other ICS, oropharyngeal deposition of the active drug is low because ciclesonide is an inactive parent compound9,34,35 with little activation occurring in the oropharynx.10,35,36 Ciclesonide is activated predominately in the airways upon conversion by esterases to its major pharmacologically active metabolite des-CIC.9,34,35 Once deposited in the oropharynx, it will be swallowed and may enter to the systemic circulation. However, because the oral bioavailability of ciclesonide and des-CIC is less than 1%, swallowed drug is not likely to contribute to any kind of systemic adverse events.
In the present study, the serum concentration of ciclesonide was detectable for only about 3 h after inhalation, despite the use of a highly sensitive detection assay. For des-CIC, the serum concentrations were observed for up to 12 h after administration, at which time the concentration values were near the lower limit of quantification. These data indicate that a once daily administration of ciclesonide to children does not lead to systemic accumulation of either ciclesonide or des-CIC, whether inhaled with or without a spacer. Furthermore, based on these data from once daily administration, it could be postulated that twice daily administration of ciclesonide would lead to similar PK profiles without any accumulation of the parent compound or the active metabolite. The AUCτ could be expected to be similar for a dosing interval tau (τ) of 12 or 24 h.
No clinically relevant abnormalities were observed during the study; one serious adverse (asthma exacerbation) was reported, which resolved after treatment. The event was assessed as ‘not related’ to the study medication; the patient recovered after 6 days without sequelae. A few patients reported adverse events that were mild or moderate in intensity; all events resolved completely and all were assessed by the investigator as ‘not related’ to ciclesonide. The safety results are consistent with those of other clinical studies with ciclesonide which confirmed good overall safety profile in adults and children treated for asthma.7,16–20
Results of the present study with children regarding the effect of a spacer are in agreement with an earlier study with adults.37 In that study, inhalation of ciclesonide via HFA-MDI with spacer (AeroChamber Plus™) did not influence the PK of the pharmacologically active metabolitedes-CIC.37 Moreover, the present data are supported by the population pharmacokinetic studies which have shown no age-related differences for the PK characteristics of des-CIC.38 Collectively, the PK results of the study with adults and the present study with children could be extrapolated to elderly patients with asthma who may require a spacer, suggesting that a dose adjustment of ciclesonide HFA-MDI is not required when using a spacer.
In conclusion, the results of the present study showed that in young children, inhalation of a therapeutic dose of ciclesonide with or without spacer attached to the HFA-MDI had no effect on the safety of ciclesonide. The use of spacer led to a slight increase in the systemic exposure of des-CIC, which does not warrant dose adjustment.
Acknowledgments
The authors thank Mr. Rolf Herzog (Nycomed GmbH, Konstanz, Germany) for performing the bioanalytical measurements and to Dr. Kathy B. Thomas (formerly of Nycomed GmbH, Konstanz, Germany) for valuable suggestions during the preparation of the manuscript.
Footnotes
Financial Support
This study was sponsored by Nycomed GmbH Konstanz, Germany (formerly ALTANA Pharma AG).
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Levy ML, Thomas M, Small I, Pearce L, Pinnock H, Stephenson P. Summary of the 2008 BTS/SIGN British Guideline on the management of asthma. Prim Care Respir J. 2009;18(Suppl 1):S1–16. doi: 10.3132/pcrj.2008.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health . National Heart, Lung and Blood Institute/WHO Report. NIH Publication; 2006. Global initiative for asthma (GINA) Global strategy for asthma management and prevention; revised. 02–3659. [Google Scholar]
- 3.Wechsler ME. Managing asthma in primary care: putting new guideline recommendations into context. Mayo Clin Proc. 2009;84:707–17. doi: 10.4065/84.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach C, Bethke TD, Boudreau RJ, et al. Two-dimensional and three- dimensional imaging show ciclesonide has high lung-deposition and peripheral distribution: a nonrandomized study in healthy volunteers. J Aerosol Med. 2006;19:117–26. doi: 10.1089/jam.2006.19.117. [DOI] [PubMed] [Google Scholar]
- 5.Newman S, Salmon A, Nave R, Drollmann A. High lung deposition of 99 mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir Med. 2006;100:375–84. doi: 10.1016/j.rmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Nave R, Gunawardena KA, Zech K, Bethke TD. Pharmacokinetic disposition of inhaled ciclesonide and its metabolite desisobutyryl-ciclesonide in healthy subjects and patients with asthma are similar. Int J Clin Pharmacol Ther. 2006;44:1–7. doi: 10.5414/cpp44001. [DOI] [PubMed] [Google Scholar]
- 7.Deeks ED, Perry CM. Ciclesonide: a review of its use in the management of asthma. Drugs. 2008;68:1741–70. doi: 10.2165/00003495-200868120-00010. [DOI] [PubMed] [Google Scholar]
- 8.Nave R, Bethke TD, van Marle SP, Zech K. Pharmacokinetics of [14C] ciclesonide after oral and intravenous administration to healthy subjects. Clin Pharmacokinet. 2004;43:479–86. doi: 10.2165/00003088-200443070-00004. [DOI] [PubMed] [Google Scholar]
- 9.Nave R, Fisher R, Zech K. In vitro metabolism of ciclesonide in human lung and liver precision-cut tissue slices. Biopharm Drug Dispos. 2006;27:197–207. doi: 10.1002/bdd.500. [DOI] [PubMed] [Google Scholar]
- 10.Nave R. Clinical pharmacokinetic and pharmacodynamic profile of inhaled ciclesonide. Clin Pharmacokinet. 2009;48:243–52. doi: 10.2165/00003088-200948040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Stoeck M, Riedel R, Hochhaus G, et al. In vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonide. J Pharmacol Exp Ther. 2004;309:249–58. doi: 10.1124/jpet.103.059592. [DOI] [PubMed] [Google Scholar]
- 12.Buhl R, Vinkler I, Magyar P, et al. Comparable efficacy of ciclesonide once daily versus fluticasone propionate twice daily in asthma. Pulm Pharmacol Ther. 2006;19:404–12. doi: 10.1016/j.pupt.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Niphadkar P, Jagannath K, Joshi JM, et al. Comparison of the efficacy of ciclesonide 160 microg QD and budesonide 200 microg BID in adults with persistent asthma: a phase III, randomized, double-dummy, open-label study. Clin Ther. 2005;27:1752–63. doi: 10.1016/j.clinthera.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Magnussen H, Hofman J, Staneta P, Lawo JP, Hellwig M, Engelstatter R. Similar efficacy of ciclesonide once daily versus fluticasone propionate twice daily in patients with persistent asthma. J Asthma. 2007;44:555–63. doi: 10.1080/02770900701537081. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen JH, Gyurkovits K, Rauer H, Engelstatter R. Randomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthma. Respir Med. 2007;101:2182–91. doi: 10.1016/j.rmed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once-daily ciclesonide in children: efficacy and safety in asthma. J Pediatr. 2006;148:377–83. doi: 10.1016/j.jpeds.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen S, Garcia Garcia MM, Manjra A, Theron I, Engelstaetter R. A comparative study of inhaled ciclesonide 160/microg/day and fluticasone propionate 176 microg/day in children with asthma. Pediatr Pulmonol. 2006;41:954–61. doi: 10.1002/ppul.20474. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen S, Engelstätter R, Weber HJ, et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulm Pharmacol Ther. 2009;22:214–20. doi: 10.1016/j.pupt.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Skoner DP, Maspero J, Banerji D. Assessment of the long-term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics. 2008;121:e1–14. doi: 10.1542/peds.2006-2206. [DOI] [PubMed] [Google Scholar]
- 20.von Berg A, Engelstätter R, Minic P, et al. Comparison of the efficacy and safety of ciclesonide 160 microg once daily vs. budesonide 400 microg once daily in children with asthma. Pediatr Allergy Immunol. 2007;18:391–400. doi: 10.1111/j.1399-3038.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 21.Bell J, Newman S. The rejuvenated pressurised metered dose inhaler. Expert Opin Drug Deliv. 2007;4:215–34. doi: 10.1517/17425247.4.3.215. [DOI] [PubMed] [Google Scholar]
- 22.Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944–8. doi: 10.1034/j.1398-9995.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen S, Frost L, Arnfred T. Errors in inhalation technique and efficiency in inhaler use in asthmatic children. Allergy. 1986;41:118–24. doi: 10.1111/j.1398-9995.1986.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 24.Vella C, Grech V. Assessment of use of spacer devices for inhaled drug delivery to asthmatic children. Pediatr Allergy Immunol. 2005;16:258–61. doi: 10.1111/j.1399-3038.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 25.Roller CM, Zhang G, Troedson RG, Leach CL, Le Souef PN, Devadason SG. Spacer inhalation technique and deposition of extrafine aerosol in asthmatic children. Eur Respir J. 2007;29:299–306. doi: 10.1183/09031936.00051106. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Hansel TT, Benezet O, Kafe H, et al. A multinational, 12-week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthma. Clin Ther. 2006;28:906–20. doi: 10.1016/j.clinthera.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Newman SP. Spacer devices for metered dose inhalers. Clin Pharmacokinet. 2004;43:349–60. doi: 10.2165/00003088-200443060-00001. [DOI] [PubMed] [Google Scholar]
- 30.Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50:1177–90. [PubMed] [Google Scholar]
- 31.Qaqundah P, Kervin E, Mehta R, et al. American Thoracic Society. Toronto, OT, Canada: 2008. Pharmacodynamic and pharmacokinetic comparisons after Advair Discus and Advair HFA administration in pediatric subjects; p. A1084. [Google Scholar]
- 32.Pickering H, Pitcairn GR, Hirst PH, et al. Regional lung deposition of a technetium 99 m-labeled formulation of mometasone furoate administered by hydrofluoroalkane 227 metered-dose inhaler. Clin Ther. 2000;22:1483–93. doi: 10.1016/s0149-2918(00)83046-5. [DOI] [PubMed] [Google Scholar]
- 33.Thorsson L, Edsbacker S, Conradson TB. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 34.Gulliver T, Morton R, Eid N. Inhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerations. Paediatr Drugs. 2007;9:185–94. doi: 10.2165/00148581-200709030-00007. [DOI] [PubMed] [Google Scholar]
- 35.Richter K, Kanniess F, Biberger C, Nave R, Magnussen H. Comparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthma. J Clin Pharmacol. 2005;45:146–52. doi: 10.1177/0091270004271094. [DOI] [PubMed] [Google Scholar]
- 36.Nave R, Zech K, Bethke TD. Lower oropharyngeal deposition of inhaled ciclesonide via hydrofluoroalkane metered-dose inhaler compared with budesonide via chlorofluorocarbon metered-dose inhaler in healthy subjects. Eur J Clin Pharmacol. 2005;61:203–8. doi: 10.1007/s00228-005-0910-0. [DOI] [PubMed] [Google Scholar]
- 37.Drollmann A, Nave R, Steinijans VW, Baumgaertner E, Bethke TD. Equivalent pharmacokinetics of the active metabolite of ciclesonide with and without use of the AeroChamber Plus™ spacer for inhalation. Clin Pharmacokinet. 2006;45:729–36. doi: 10.2165/00003088-200645070-00007. [DOI] [PubMed] [Google Scholar]
- 38.Rohatagi S, Arya V, Zech K, et al. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol. 2003;43:365–78. doi: 10.1177/0091270002250998. [DOI] [PubMed] [Google Scholar]