Abstract
The thermal limit for metazoan life, expected to be around 50°C, has been debated since the discovery of the Pompeii worm Alvinella pompejana, which colonizes black smoker chimney walls at deep-sea vents. While indirect evidence predicts body temperatures lower than 50°C, repeated in situ temperature measurements depict an animal thriving at temperatures of 60°C and more. This controversy was to remain as long as this species escaped in vivo investigations, due to irremediable mortalities upon non-isobaric sampling. Here we report from the first heat-exposure experiments with live A. pompejana, following isobaric sampling and subsequent transfer in a laboratory pressurized aquarium. A prolonged (2 hours) exposure in the 50–55°C range was lethal, inducing severe tissue damages, cell mortalities and triggering a heat stress response, therefore showing that Alvinella’s upper thermal limit clearly is below 55°C. A comparison with hsp70 stress gene expressions of individuals analysed directly after sampling in situ confirms that Alvinella pompejana does not experience long-term exposures to temperature above 50°C in its natural environment. The thermal optimum is nevertheless beyond 42°C, which confirms that the Pompeii worm ranks among the most thermotolerant metazoans.
Introduction
Deep-sea hydrothermal vents are believed to host the most thermophilic microorganisms, and the actual upper thermal limit (UTL) for life was indeed recorded in hydrothermal Archae, which grow at temperatures up to 122°C [1]. Some vent animals also thrive close to the hydrothermal fluids and live at the edge of the UTL for metazoan life (50°C, [2]), like some of the alvinellid polychaetes, chimney dwellers found exclusively in association to high temperature venting. Discrete measurements have reported temperatures around 100°C [3], [4] in the close surrounding of the emblematic species Alvinella pompejana Desbruyères and Laubier 1980 [5]. Furthermore, continuous recordings inside the worm tubes witnessed of sustained temperature of 60°C, well beyond the metazoan UTL, with regular spikes above 80°C [6], [7]. Several studies on A. pompejana thence focused on both the thermostability and the optimal efficiency of its macromolecules, and while proving molecular performance similar or greater than for homeotherms, nevertheless suggested body temperatures below 50°C [8]. However, recent in vitro studies continuously present in situ thermal limit inference above 50°C as a start for molecular investigations while simultaneously emphasizing this in vitro/in situ discrepancy [9], [10], [11], [12], [13], [14]. Only the in vivo approach can solve this contentious issue, but so far this species hardly survives recovery from 2,500 meters depth, precluding the empirical determination of thermal limit on live specimens [8], [15], [16], [17], [18]. To minimize the collection and depressurization trauma, we developed a system of isobaric sampling and transfer of A. pompejana colonies towards a receiving high-pressure aquarium named BALIST, according to the project’s acronym (Biology of ALvinella, Isobaric Sampling and Transfer). This system allowed in vivo experimentation to be carried out under controlled temperature, at in situ pressure of 25 MPa, which subsequently provided the first empirical demonstration of A. pompejana’s thermal limit.
Materials and Methods
Animal Collection and Experimentation
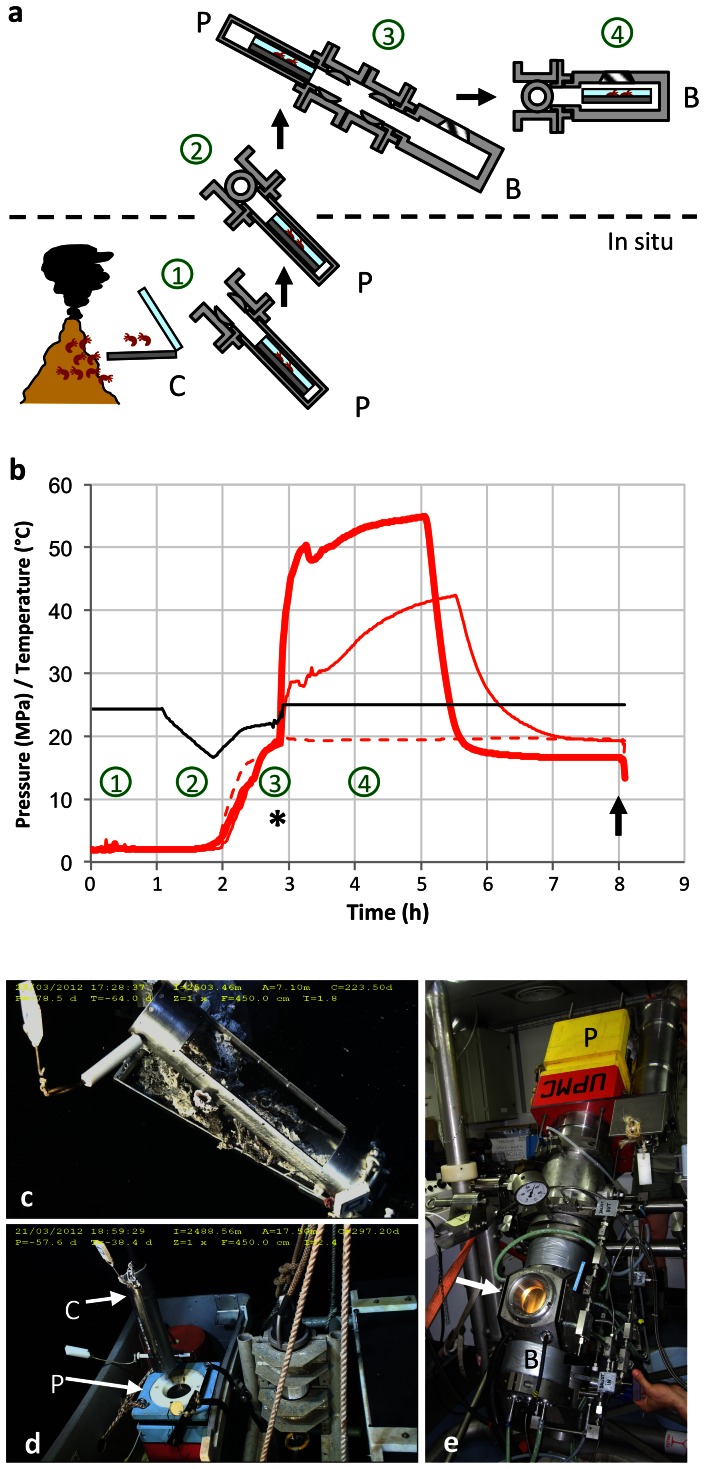
Alvinella pompejana were collected using the DSV Nautile (Bio9 and P vent sites, East Pacific Rise, 9°50′N, 2,500 m depth, Mescal 2012 cruise). During 4 different dives, 70 worms were recovered under pressure by using the PERISCOP system [19], composed of an in situ sampling cell and an isobaric recovery device. Pompeii worm colonies were sampled as such, and placed inside the sampling cell, using the submersible’s hydraulic arm (Figure 1c). Two autonomous recorders placed inside the sampling cell in direct contact with the samples, provided temperature history during sampling (NKE instruments). Another autonomous recorder provided pressure history, and was checked upon recovery on board of the ship, prior to transfer towards the BALIST aquarium (Text S1, Figure S1). Reproducible pressure profiles remained within 68% (mean value, s.d. = 0.5%, n = 3) to 91% (mean value, s.d. = 2.4%, n = 3) of in situ pressure throughout 3 of the recovery processes, and pressure was set to 25 MPa after successful transfer in the BALIST aquarium, in order to proceed to in vivo experimental heat-exposures (Figure 1b). In one case however, the pressure profile in PERISCOP was different (reaching a minimum of 52% of in situ value during the ascent, before increasing again to 63% upon reaching the surface), and the samples (referred to as ‘sampling’) were processed directly after recovery. Survival of all animals was ascertained through observations of movements, just before sampling their coelomic fluid. The trypan blue exclusion test was employed to determine the percentage of viable coelomocytes (free circulating cells) per individual [20]. Cell counting was performed on board using a Malassez chamber. The animals were further dissected and stored in liquid nitrogen pending analyses. Although not subjected to specific property regulations (international water areas), authors have obtained permission to use samples for any analysis from both chief-scientists. This study did not involve endangered or protected species.
Figure 1. Isobaric sampling and transfer of the Pompeii worms (step = circled number in a and b).
a, 1: in situ sampling of Alvinellas inside a “crocodile” cylinder (C), further closed and inserted in the recovery cell PERISCOP (P). 2: PERISCOP (closed) ascends through the water column. 3: On the ship, samples are transferred from PERISCOP (P) to BALIST (B). 4: thermal exposures. b, Pressure (black line) and temperature (red lines) recordings inside the "crocodile" (arbitrary time units). The asterisk and arrow indicate the beginning and end of the 3 thermal exposures (20, 42 and 55°C, dotted, plain and bold lines respectively). Numbers circled in green refer to the steps described in fig. 1a. c, d, see a, step 1 (Photographic credit Ifremer/MESCAL 2012). e, see a, step 3. A viewport (arrow) allows sample observation inside BALIST.
Microscopy
A portion of the dorsal and ventral regions were fixed in 2.5% glutaraldehyde–seawater solution and post-fixed in 1% osmium tetroxide. Samples were embedded in epoxy resin, semi-thin sections were stained with toluidine blue and observed with a BX61 microscope (Olympus).
RNA Extraction and Real-time Quantitative RT-PCR (qPCR)
Total RNA was extracted from grounded tissues [21] and controlled for their quality with the Experion® automated electrophoresis system (BioRad) and quantified by spectrophotometry. qPCR analyses were performed as previously described [22] with specific primers designed for hsp70 gene (Hsp70 sequence has been deposited with GenBank database under accession number JX560964; sequenced as previously described [22] and assembled with clone Tera 04634 from Alvinella database [12]) and for reference RPS26 gene (Alvinella database [12]). The hsp70 expression was normalized to the RPS26 expression.
Results and Discussion
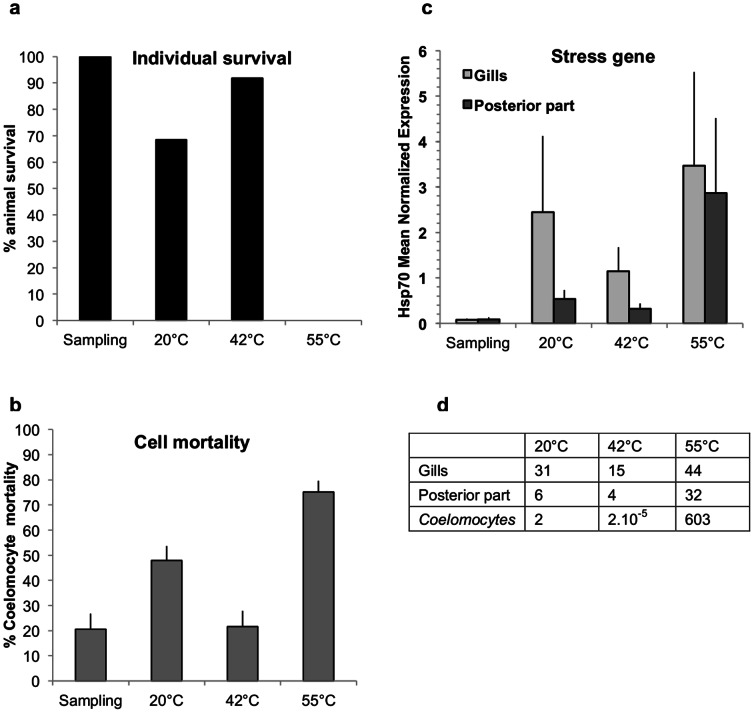
Worm colonies collected with the isobaric sampling device and further transferred towards the BALIST high-pressure aquarium, were subjected to three thermal regimes, a constant mild 20°C-exposure, and two heat-exposures followed by a 3 hour-recovery period at 20°C (Figure 1). The heat-exposures lasted about 2 hours, the first one ramped from 30°C to 42°C, and the second one from 50°C to 55°C (thereafter referred to as ‘42°C’ and ‘55°C’ experiments respectively). The behaviour of A. pompejana was observed through the aquarium viewport as far as possible, which proved feasible only during the 55°C-exposure because the animals remained inside their tubes during the 20°C-experiment and were not in front of the viewport during the 42°C-experiment. During the first 10 minutes of the 55°C-experiment, individuals of A. pompejana, after a short period of ‘normal’ behaviour during which they ventilated their tube by moving up and down, were observed leaving their tubes (Figure 2) and crawling on the surface of the colony. Since A. pompejana is extremely sedentary and rarely leaves its tube [3], this unnatural behaviour likely reveals a disturbance. At the end of the experiment at 55°C, all worms (n = 18) were dead (Figure 3a, Figure 4b). They all showed very serious damage of their tissues and cells, with a detachment of the tegument and a disorganization of internal tissues (Figure 4), as well as a high mortality of circulating cells (75±4%, Figure 3b). The mRNA extracted from the tissues (gills and posterior part) and circulating cells of these animals contained a significantly higher quantity of hsp70 stress gene transcripts in comparison with animals directly processed after sampling (Kruskal-Wallis test, ‘gills’ H = 7.536 p = 0.056, ‘posterior part’ H = 12.40 p = 0.006; post-hoc Mann-Whitney two-sided test for ‘55°C gills’ p = 0.0119 and ‘55°C posterior part’ p = 0.0079; t-test following a mean resampling by bootstrap (n = 100) using the R library ‘stats’, and Bonferroni correction, pairwise t-test p-value<0.0001; Figure 3c and 3d). Since HSP70 proteins are known to be mobilized in response to environmental stresses, among which temperature [23], this confirms that a thermal exposure up to 55°C is harmful for A. pompejana. The results of this study provide the first direct empirical evidence that A. pompejana cannot withstand prolonged exposure to temperatures in the 50–55°C range, and that its thermal optimum lies below 50°C. Since a higher thermal tolerance was expected from in situ measurements, this emphasizes the difficulty of assessing the species thermal tolerances through in situ probing in such a stochastic environment.
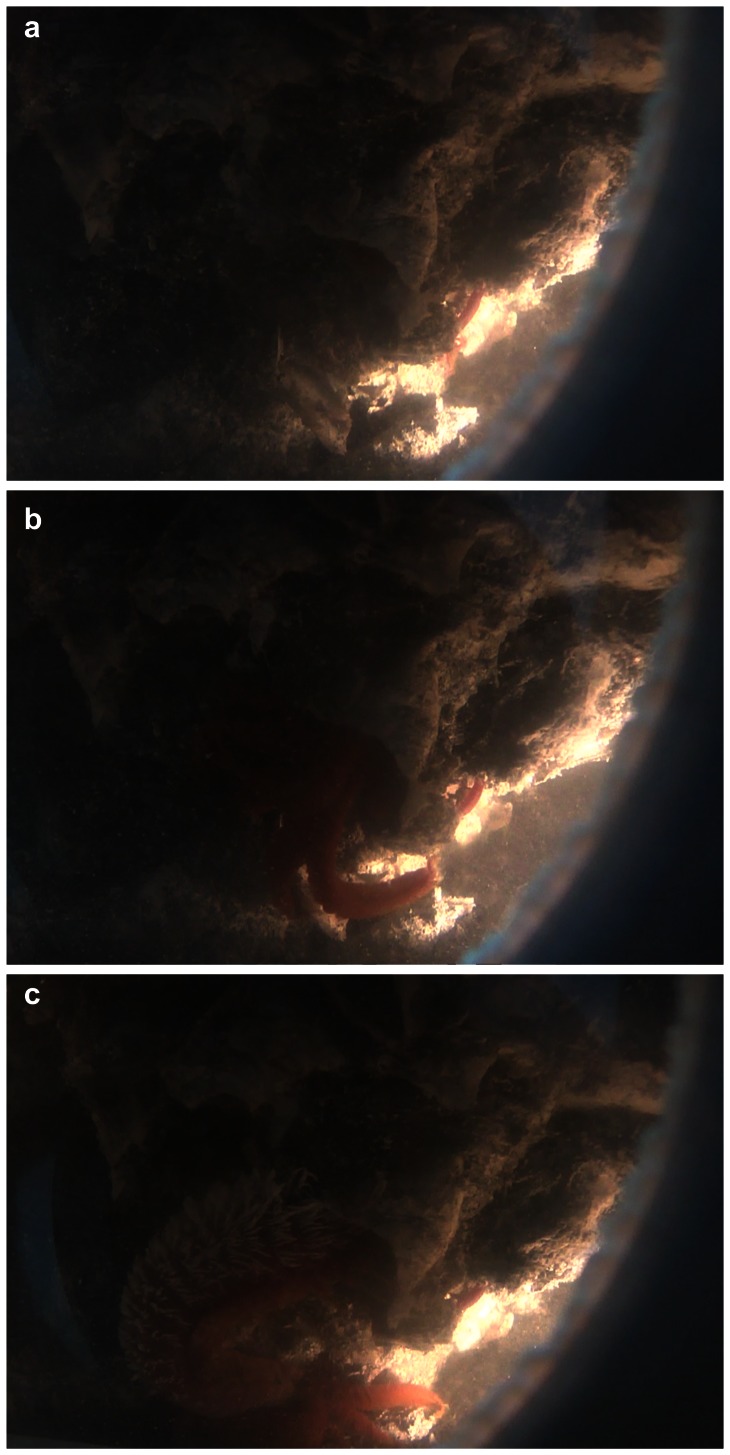
Figure 2. In vivo experiments on Alvinella pompejana.
Views of a portion of A. pompejana tubes inside the BALIST aquarium during the 10 first minutes of the 55°C-exposure experiment. In the middle of the figure, an individual is observed leaving its tube (approximate diameter of tube opening = 1 cm).
Figure 3. Temperature effect on survival, cell mortality and hsp70 gene expression in A. pompejana.
a, Survival of A. pompejana specimens after the recovery from the PERISCOP sampling device (‘sampling’, n = 9) and the subsequent in vivo experiments in the BALIST aquarium (‘20°C’, n = 19; ‘42°C’, n = 24; ‘55°C’, n = 18). b, Coelomocytes death in animals from Fig. 3a. c, Hsp70 gene expression (mean for n = 5 individuals ± s.e.m.). Among the four A. pompejana’s hsp70 genes (Alvinella database [12]), two forms showed significant changes according to temperature, and the form that showed the most significant changes is presented here. d, Normalized fold hsp70 expression in gills, posterior part and coelomocytes of experimented animals with respect to ‘sampling’ specimens.
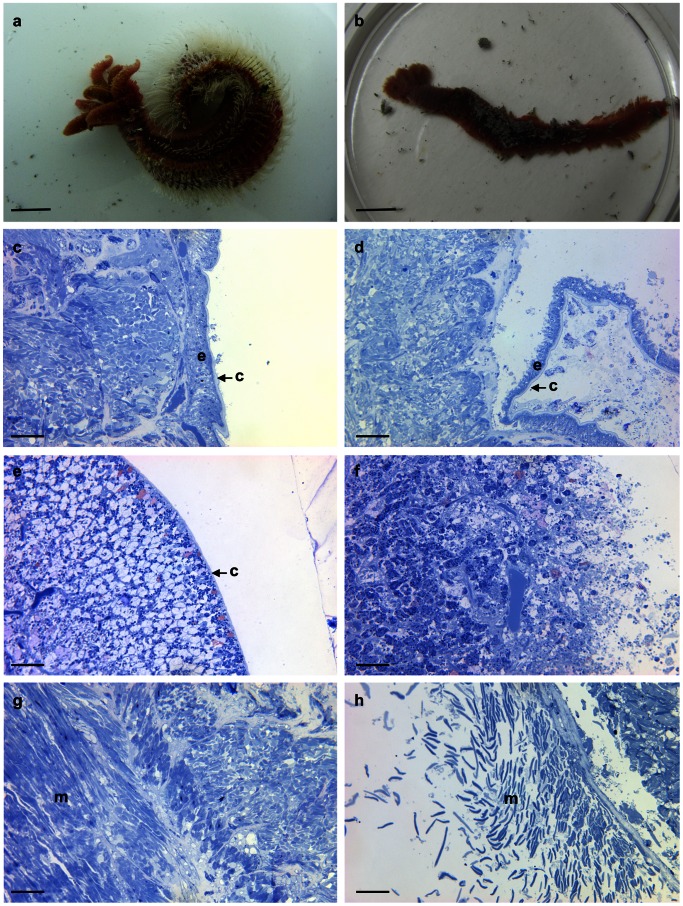
Figure 4. Morphological consequences of a 55°C-exposure in A.
pompejana .°C-exposed (left column) versus 55°C-exposed (right column) specimens. a, b, General view of specimens after the in vivo experiments; c, d, Sections of the dorsal region showing the delamination of the epidermis (e) and cuticle (c) at 55°C; e, f, Sections of the ventral tube secreting region showing the disappearance of the tegument and disorganization of the underlying tissues at 55°C; g, h, Sections of muscles (m) showing the disorganization of cells at 55°C. Bar represents approximately 1 cm in a, b and 50 µm in c-h.
In contrast with the severe 55°C-heat shock, the 42°C-experiment displayed the highest survival rates at both specimen and cellular levels (92% and 78% respectively; Figure 3a), with no observable structural damage in the tissues (data not shown). The animals did trigger a mild heat stress response with a level of hsp70 gene expression significantly lower than for the ‘55°C’ specimens (t-test: see previous resampling procedure, pairwise t-test p-value <0.0001; Figure 3c and 3d). Taken together, these data suggest that A. pompejana is able to cope with temperatures greater than 40°C, at least for 1 hour, without a high thermal stress response. Regarding the constant 20°C experiment, no obvious structural damage was evidenced (Figure 4). However, specimen survival (13 alive/19 total) was lower than for ‘sampling’ (9/9) or for ‘42°C’ (22/24) individuals (Figure 3a). Besides, cell mortalities were significantly higher in specimens from the 20°C exposure (48%) vs. sampling (20.6%) and 42°C (21.6%) animals (t-test: see previous resampling procedure, pairwise t-test p-value<0.0001; Figure 3b). And finally, stress gene expression followed the same trend with values significantly higher in animals subjected to the ‘20°C’ vs. ‘sampling’ and ‘42°C’ treatments (t-test: see previous resampling procedure, pairwise t-test p-value<0.0001; Figure 3c and 3d). Specimen survival, cell mortality and stress gene expression data therefore show that A. pompejana endured more damages subsequent to the 20°C exposure when compared to the 42°C experiment (1h20 above 30°C followed by 1 hour above 40°C). This finding reinforces the idea that A. pompejana is a thermophilic species with the lower boundary of its thermal optimum being above 20°C.
With an optimal thermal range expanding from above 20°C to beyond 40°C, A. pompejana ranks among the most thermotolerant metazoan species. Interspecies comparisons for thermal limit/tolerance remain however a difficult issue, due to the variety of indexes and protocols used to evaluate the animals’ thermal scope. The highest thermal tolerance limits estimated for any animal were previously reported in the hot springs ostracod Potamocypris sp., which survived prolonged exposures to 49°C [24], and in the desert ants Cataglyphis bombycina and Cataglyphis bicolor based on their critical maximum temperature (CTmax 55°C and 54°C [25]). More recently, the alvinellid Paralvinella sulfincola was proven highly thermotolerant based on its preference for temperatures in the 40°–50°C zone and its ability to withstand short exposures at 55°C (thermal limit 50–55°C [17], [18]). A. pompejana can be suggested to share a similar thermal preference with the alvinellid P. sulfincola, and clearly has a shifted stress response to higher temperatures when compared to the desert ants. The ant response reached its highest point at 37°C and ended around 45°C [25] while A. pompejana should yield its highest expression in the 42°C–55°C range. This means that A. pompejana only triggers this molecular response upon temperature extremes, and would not have a constantly up-regulated heat shock response, in order to counter-balance the rapid protein denaturation that might have been expected from an animal at the edge of its thermal preference. This was confirmed by the low levels of hsp70 expression measured in the freshly collected worms (‘sampling’, Figure 3c and 3d) in comparison with the thermal stress response induced during the in vivo experiments, and especially the 55°C-experiment. Moreover, the low natural vs. in vivo stimulated levels of hsp70 expression mean that these ‘sampling’ worms had not experienced heat stress during the hours that preceded the collection. A comparable result was previously obtained in another vent chimney dweller, the Atlantic shrimp Rimicaris exoculata [21]. As proposed for other vent species, these animals may prefer temperatures well within their tolerated range, thereby maintaining a ‘safety margin’ against rapid temperature fluctuation that could expose them to thermal extremes [26].
The extreme temperature variability in the close surrounding of A. pompejana, and notably the sharp thermal gradient the animal is believed to endure in its tube (up to 60°C from the opening to the bottom) [3], [6], have led to propose this animal as one of the most eurythermal on earth. The hsp70 gene analyses revealed a differential expression in the anterior and posterior parts of the worms (‘20°C’ and ‘42°C’ specimens; Figure 2c), the posterior part responding less to thermal variations than the gills (One-way ANOVA across treatments following bootstrap resampling (n = 100) performed on gills (F = 27.84, p-value = 2 e−7) and posterior part (F = 5.42, p-value = 0.02)). Added to previous results on differential cell membrane compositions [27], this tends to confirm the existence of such a thermal gradient inside the tube and consequently the exceptional eurythermy of A. pompejana.
In conclusion, while A. pompejana is not as thermophilic as previously suspected, it nevertheless remains among the most thermotolerant and eurythermal metazoans. The accurate definition of the metazoan UTL will further require standardized indexes, like the CTmax (i.e. a behavioural response [28]), to be provided for alvinellids. Since these tubicolous animals may retract inside their tubes, or move behind the tube masses, behavioural studies are challenging, and future isobaric sampling processes could aim at collecting Alvinella worms without their tubes. Finally, isobaric sampling and transfer should allow many more deep-sea species to be studied alive. Such studies are urgently needed to better understand the biological responses of deep fauna to environmental changes, in times when evidence for anthropogenic impact on the world's largest ecosystem is accumulating [29].
Supporting Information
Description of the BALIST aquarium.
(DOCX)
Acknowledgments
We thank the captain and crew of the RV Atalante, the DSV Nautile group (IFREMER), along with N. Le Bris and F. Lallier chief scientists of the Mescal 2010 and 2012 cruises. We also acknowledge the help of S. Bornens (IFR 83 Biologie Intégrative, UPMC), J. Frelat (UMR 7190, UPMC) and S. Hourdez (UMR 7144, UPMC).
Funding Statement
This research was supported by the programs BALIST ANR-08-BLAN-0252 (http://www.agence-nationale-recherche.fr/) and BQR UPMC 2008 (http://www.upmc.fr/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, et al.. (2008) Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA 105: 10949–10954 doi : 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed]
- 2.Pörtner H (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol Part A 132: 739–761 doi :10.1016/S1095-6433(02)00045-4. [DOI] [PubMed]
- 3. Le Bris N, Gaill F (2007) How does the annelid Alvinella pompejana deal with an extreme hydrothermal environment ? Rev Environ Sci Biotechnol 6 197–221 doi: 10.1007/s11157-006-9112-1 [Google Scholar]
- 4. Chevaldonné P, Desbruyères D, Childress JJ (1992) Some like it hot…and some even hotter. Nature 359: 593–594. [Google Scholar]
- 5. Desbruyères D, Chevaldonné P, Alayse-Danet AM, Caprais JC, Cosson R, et al. (1998) Biology and ecology of the “Pompeii worm” (Alvinella pompejana Desbruyères and Laubier), a normal dweller of an extreme deep-sea environment: A synthesis of current knowledge and recent developments. Deep-Sea Res Pt II 45 Issues 1–3: 383–422. [Google Scholar]
- 6. Cary SC, Shank T, Stein J (1998) Worms bask in extreme temperatures. Nature 391: 545–546. [Google Scholar]
- 7. Le Bris N, Zbinden M, Gaill F (2005) Processes controlling the physico-chemical micro-environments associated with Pompeii worms. Deep Sea Res Part I 52: 1071–1083. [Google Scholar]
- 8. Chevaldonné P, Fisher CR, Childress JJ, Desbruyères D, Jollivet D, et al. (2000) Thermotolerance and the “Pompeii worms”. Mar Ecol Prog Ser 208: 293–295. [Google Scholar]
- 9. Grzymski JJ, Murray AE, Campbell BJ, Kaplarevic M, Gao GR, et al. (2008) Metagenome analysis of an extreme microbial symbiosis reveals eurythermal adaptation and metabolic flexibility. P Natl Acad Sci USA 105 (45): 17516–17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee CK, Cary SC, Murray AE, Daniel RM (2008) Enzymatic approach to eurythermalism of Alvinella pompejana and its episymbionts. Appl Environ Microbiol 74(3): 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin DS, DiDonato M, Barondeau DP, Hura GL, Hitomi C, et al. (2009) Superoxide dismutase from the eukaryotic thermophile Alvinella pompejana: structures, stability, mechanism, and insights into amyotrophic lateral sclerosis. J Mol Biol 385: 1534–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagnière N, Jollivet D, Boutet I, Brélivet Y, Busso D, et al. (2010) Insights into metazoan evolution from Alvinella pompejana cDNAs. BMC Genomics 11: 634–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashiwagi S, Kuraoka I, Fujiwara Y, Hitomi K, Cheng QJ, et al. (2010) Characterization of a Y-Family DNA polymerase eta from the eukaryotic thermophile Alvinella pompejana. J Nucleic Acids: doi : 10.4061/2010/701472. [DOI] [PMC free article] [PubMed]
- 14.Jollivet D, Mary J, Gagnière N, Tanguy A, Fontanillas E, et al.. (2012) Proteome adaptation to high temperatures in the ectothermic hydrothermal vent Pompeii worm. Plos One: doi: 10.1371/journal.pone.0031150. [DOI] [PMC free article] [PubMed]
- 15.Shillito B, Le Bris N, Gaill F, Rees JF, Zal F (2004) First access to live Alvinellas. High Pressure Res 24, 169–172.
- 16. Van Dover CL, Lutz RA (2004) Experimental ecology at deep-sea hydrothermal vents: a perspective. J Exp Mar Biol Ecol 300: 273–307. [Google Scholar]
- 17. Lee RW (2003) Thermal tolerances of deep-sea hydrothermal vent animals from the Northeast Pacific. Biol Bull 205: 98–101. [DOI] [PubMed] [Google Scholar]
- 18. Girguis PR, Lee RW (2006) Thermal preference and tolerance of Alvinellids. Science 312: 231. [DOI] [PubMed] [Google Scholar]
- 19. Shillito B, Hamel G, Duchi C, Cottin D, Sarrazin J, et al. (2008) Live capture of megafauna from 2300 m depth, using a newly designed Pressurized Recovery Device. Deep-Sea Res I 55: 881–889. [Google Scholar]
- 20.Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3B. [DOI] [PubMed]
- 21. Ravaux J, Cottin D, Chertemps T, Hamel G, Shillito B (2009) Hydrothermal vent shrimps display low expression of heat-inducible hsp70 gene in nature. Mar Ecol Prog Ser 396: 153–156 doi: 10.3354/meps08293 [Google Scholar]
- 22. Cottin D, Shillito B, Chertemps T, Thatje S, Léger N, et al. (2010) Comparison of heat shock responses between the hydrothermal vent shrimp Rimicaris exoculata and the related coastal shrimp Palaemonetes varians . J Exp Mar Biol Ecol 393: 9–16. [Google Scholar]
- 23. Feder ME, Hofmann GE (1999) Heat shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282. [DOI] [PubMed] [Google Scholar]
- 24. Wickstrom CE, Castenholz RW (1973) Thermophilic ostracod: aquatic metazoan with the highest known temperature tolerance. Science 181: 1063–1064. [DOI] [PubMed] [Google Scholar]
- 25. Gehring WJ, Wehner R (1995) Heat-shock proteins synthesis and thermotolerance in Cataglyphis, an ant from the sahara desert. Proc Natl Acad Sci USA 92: 2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bates AE, Lee RW, Tunnicliffe V, Lamare MD (2010) Deep-sea hydrothermal vent animals seek cool fluids in a highly variable thermal environment. Nat Commun 1 14 doi: 10.1038/ncomms1014 [DOI] [PubMed] [Google Scholar]
- 27. Phleger CF, Nelson MM, Groce AK, Cary SC, Coyne KJ, et al. (2005) Lipid biomarkers of deep-sea hydrothermal vent polychaetes- Alvinella pompejana, A. caudata, Paralvinella grasslei and Hesiolyra bergii . Deep-sea Res 52: 2333–2352. [Google Scholar]
- 28. Lutterschmidt WI, Hutchinson VH (1997) The critical thermal maximum: history and critique. Can J Zool 75: 1561–1574. [Google Scholar]
- 29. Ramirez-Llodra E, Tyler PA, Baker MC, Bergstad OA, Clark MR, et al. (2011) Man and the last great wilderness: human impact on the deep sea. PloS ONE 6(7): e22588 Doi:10.1317/journal.pone.0022588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of the BALIST aquarium.
(DOCX)