Abstract
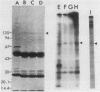
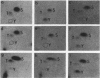
The level of phosphotyrosine in vinculin was determined in chicken embryo fibroblasts transformed by various strains of avian sarcoma virus. As previously reported (Sefton et al., Cell 24:165-174, 1981), vinculin was phosphorylated at tyrosine residues in most cultures examined, but the level varied greatly and no detectable change was found in cultures infected with Fujinami sarcoma virus or UR2 sarcoma virus. Regardless of the level of vinculin phosphorylation, the number of organized microfilament bundles was found to be decreased in all transformed cells. These results strongly suggest that tyrosine phosphorylation of vinculin is not an obligatory step in cell transformation by this class of oncogenes, nor is it correlated with the associated cytoskeletal disarray.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduzzi P. C., Notter M. F., Morgan H. R., Shibuya M. Some biological properties of two new avian sarcoma viruses. J Virol. 1981 Oct;40(1):268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Collett M. S., Siddiqui A., Marczynska B., Deinhardt F., Erikson R. L. Detection of the viral sarcoma gene product in cells infected with various strains of avian sarcoma virus and of a related protein in uninfected chicken cells. J Virol. 1979 Mar;29(3):1196–1203. doi: 10.1128/jvi.29.3.1196-1203.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T., Singer S. J. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6687–6691. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Chuong C. M. Embryonic to adult conversion of neural cell adhesion molecules in normal and staggerer mice. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7036–7040. doi: 10.1073/pnas.79.22.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Cook R., Miller G. J., Erikson R. L. The same normal cell protein is phosphorylated after transformation by avian sarcoma viruses with unrelated transforming genes. Mol Cell Biol. 1981 Jan;1(1):43–50. doi: 10.1128/mcb.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang E., Hanafusa H. Cytoplasmic localization of the transforming protein of Fujinami sarcoma virus: salt-sensitive association with subcellular components. J Virol. 1983 Feb;45(2):782–791. doi: 10.1128/jvi.45.2.782-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Iwashita S., Kitamura N., Yoshida M. Molecular events leading to fusiform morphological transformation by partial src deletion mutant of Rous sarcoma virus. Virology. 1983 Mar;125(2):419–431. doi: 10.1016/0042-6822(83)90213-1. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Neel B. G., Wang L. H., Mathey-Prevot B., Hanafusa T., Hanafusa H., Hayward W. S. Isolation of 16L virus: a rapidly transforming sarcoma virus from an avian leukosis virus-induced sarcoma. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5088–5092. doi: 10.1073/pnas.79.16.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3514–3518. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L., Rosok M. J. Transformation parameters and pp60src localization in cells infected with partial transformation mutants of Rous sarcoma virus. Mol Cell Biol. 1983 Apr;3(4):731–746. doi: 10.1128/mcb.3.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosok M. J., Rohrschneider L. R. Increased phosphorylation of vinculin on tyrosine does not occur during the release of stress fibers before mitosis in normal cells. Mol Cell Biol. 1983 Mar;3(3):475–479. doi: 10.1128/mcb.3.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Nigg E. A., Singer S. J., Walter G. Cytoskeletal targets for viral transforming proteins with tyrosine protein kinase activity. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):939–951. doi: 10.1101/sqb.1982.046.01.088. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]