Abstract
Due to the possibility of underlying hepatobiliaryor bone diseases, the diagnostic work up of a child with elevated alkaline phosphatase (AP) levels can be quite costly. In a significant proportion of these patients, elevated AP is benign, requiring no intervention: hence, known as transient hyperphosphatasemia (THP) of infants and children. A 27-month old previously healthy Caucasian female was found to have isolated elevation of AP four weeks after the initial symptoms of acute gastroenteritis. One month later, when seen in hepatobiliary clinic, signs and symptoms of gastrointestinal, hepatobiliary, or bone disease were absent and physical examination was normal. The diagnosis of THP was made, and, as anticipated, AP levels normalized after four months. Using this case as an example, we suggest an algorithm that can be utilized as a guide in a primary care setting to arrive at the diagnosis of THP and avoid further tests or referrals.
Keywords: hyperphosphatasemia, abnormal liver enzymes, gamma-glutamyl transferase, 25-OH-vitamin-D
Introduction
It is relatively common for primary care physicians to encounter children with elevated alkaline phosphatase (AP) levels. Given the possibility of underlying asymptomatic hepatobiliary diseases, these patients are usually referred to either a pediatric gastroenterologist or a hepatologist for evaluation and management. Similarly, to determine the cause, apart from referral to a specialist, the diagnostic work-up can be quite costly, reflecting the broad scope of hepatobiliary and skeletal diseases known to be associated with AP elevation. Importantly, in a significant proportion of these patients, elevated AP is benign, and gradually resolves without intervention, questioning the rationale for an exhaustive evaluation of every patient with this biochemical abnormality. The benign elevation of AP is referred to as transient hyperphosphatasemia (THP), a condition most commonly observed in infants and children younger than 5 years of age without evidence of bone, gastrointestinal or liver disease on history, physical examination or laboratory investigations. Furthermore, this condition has no long-term adverse consequences.1,2 Because the epidemiology of THP is not well understood and clear guidelines for evaluating this problem are lacking, THP is usually diagnosed by excluding other diseases, which, may require extensive biochemical and radiographic studies, all adding significant costs for the health care system. Herein, with the help of an illustrative example, we suggest a clinical algorithm outlining minimum tests for the diagnosis and management of a patient with THP avoiding unnecessary investigations and referral to a specialist. Although the discussion is relevant to pediatric gastroenterologists, the main objective of the paper is to help the primary care physician determine when a “wait and see” approach is optimal, and when a referral to a specialist becomes necessary in the setting of a child with elevated AP.
Illustrative Case
A 27-month old previously healthy Caucasian female presented to the Pediatric Hepatobiliary Clinic for evaluation and management of elevated serum AP concentration. Two months before her gastrointestinal clinic visit, she had vomiting and diarrhea consistent with acute viral gastroenteritis which gradually resolved spontaneously without any specific therapy or sequelae. Although most of the symptoms of acute gastroenteritis resolved in one week, mild loose stools persisted for a month prompting blood tests performed by the primary care physician. These indicated WBC 6.9 × 109 cells per liter, hemoglobin 11.9 g/dl, platelet count 372 × 109 per liter, neutrophil count 34.1%, lymphocytes 50.7%, monocytes 13.4%, eosinophils 1.4%, basophils 0.4%, and erythrocyte sedimentation rate 4 mm/h. Biochemical investigations revealed sodium 142 mmol/L, potassium 4.9 mmol/L, bicarbonate 23 mmol/L, chloride 103 mmol/L, magnesium 1.9 mg/dl, phosphate 4.4 mg/dl, calcium 9.9 mg/dl, creatinine 0.37 mg/dl. The hepatic biochemical tests were normal with a total protein 6.4 g/dl, albumin 4.4 g/dl, aspartate transaminase 37 U/L (0–37), alanine transaminase 17 U/L (0–41), and gamma-glutamyl transferase 9 U/L (5–61). Alkaline phosphatase was abnormal at 1510 U/L (61–320); the isoenzyme fractionation revealed liver-specific-AP-activity of 374 U/L and bone-specific-AP-activity of 456 U/L. Calcidiol (25-OH-vitamin-D) level was 49.7 ng/ml (30–100).
When the patient was seen at the hepatobiliary clinic, signs and symptoms of liver or bone disease such as conjunctival icterus, dermal jaundice, pale stools, dark urine, pruritus, easy bruising or bleeding, limping, bone pain, or weight loss were not encountered during or after this acute illness. Past medical, family and social histories were negative. On physical examination, she was a well nourished female with normal vital signs and both weight and height between the 25–50th percentile. There were no dysmorphic features, skeletal abnormalities, bony tenderness, conjunctivalicterus, hepatosplenomegaly or any other stigmata of chronic liver or bone disease.
A month after the first determination, AP level had declined to 830 U/L. After the careful history, examination and review of the above studies, the diagnosis of THP was made and no further investigations were deemed necessary. As expected AP level normalized at four months (243 U/L; normal less than 282).
Discussion
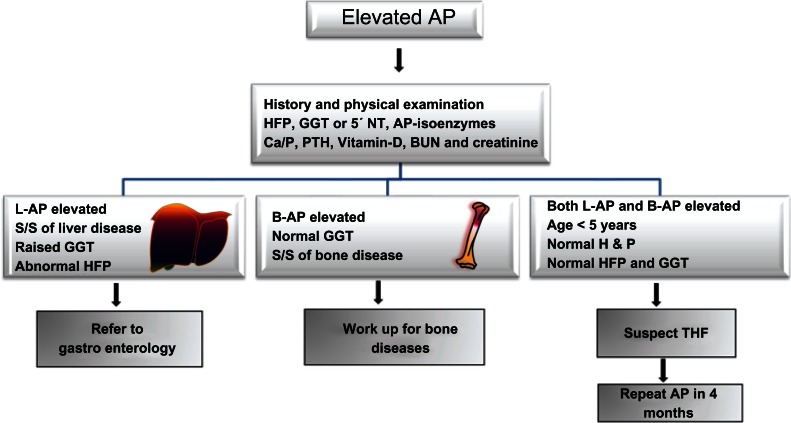
Due to the typical age, normal physical examination, and elevation of both liver and bone-specific AP-isoenzymes the diagnosis of THP was made according to the commonly used criteria.3 Because no tests were done at the onset of gastroenteritis coupled with relatively short half life of AP (seven days) we were unable to establish a definitive link between gastroenteritis and elevated AP levels. Interestingly, apart from well known elevation of liver and bone-specific AP-isoenzymes, a rise in intestinal AP-isoenzyme has also been documented albeit in a minority of patients with THP.4 However, because intestinal isoenzyme fraction was not assessed, we can only postulate that elevation of total AP activity was partly due to intestinal isoenzyme. Indeed acute gastroenteritis has been suggested to be a risk factor for THP.5 The family was reassured and further investigations were not carried out. Normalization of AP in four months supported our initial impression of THP. We suggest an easy to use clinical algorithm (Fig. 1) for primary care physicians as a guide to determine when further work-up or referral to a gastroenterologist can be deferred.
Figure 1.
Algorithm for the assessment and management of a child with isolated elevated serum alkaline phosphatase in the primary care setting.
Abbreviations: GGT, γ-glutamyltranspeptidase; L-AP, liver-specific alkaline phosphatase; B-AP, bone-specific alkaline phosphatase; 5′NT, 5′-nucleotidase; HFP, hepatic function panel; THP, transient hyper-alkaline phosphatemia; Ca/P, calcium/phosphorus; PTH, parathyroid hormone.
AP is present in many tissues throughout the body: therefore, serum AP level represents the collective activity of a group of enzymes which catalyze the hydrolysis of organic phosphate esters. Most of the circulating AP is, however, derived from liver; bone; intestinal tract; and kidneys. When investigating the cause of abnormal values of AP, several physiological factors pertinent to infants and children are important to understand. First, the reference AP levels gradually rise during the first decade and the peak values (in early teens) are three to four times higher than the normal adult values. Secondly, although the prevalence and risk factors remain ill defined, THP is relatively common condition in children younger than five years of age. For example, up to 1.5% of healthy Finnish infants and toddlers were reported to have THP in a prospective study.6 Finally, where as the biochemical mechanisms of pathologic elevations of AP can be delineated in most cases, the underlying pathophysiological mechanisms of THP remain elusive: therefore, even with extensive investigations, establishment of the underlying etiology is generally not possible. Therefore, when the criteria utilized in this and earlier reports are met, additional investigations are neither evidence based nor necessary, given the spontaneous and uneventful resolution of this condition in a few months.7 In the primary care setting, the emphasis should be on clinical assessment supplemented by limited laboratory investigations as outlined in the algorithm (Fig. 1).
The history should focus on symptoms of liver, intestinal, renal, and bone diseases with careful evaluations of any drug administrations─prescribed as well as over the counter. Specific inquiries should be made about the use of anticonvulsants such as Phenobarbital, Phenytoin or Carbamazepine.8–10 Symptoms of anorexia, fatigue, fever, and weight loss should prompt further evaluation of possible underlying pathological conditions. For example, specific enquiries should be made about risk factors for rickets such as prematurity, exposure to sunlight, duration of breast feeding and whether or not the child has been consuming adequate quantities of vitamin-D fortified formula. Symptoms indicative of hepatobiliary disease such as dark colored urine, pruritis, bruising and steatorrhea should be carefully elicited. A thorough physical examination should be completed─searching for signs of underlying hepatobiliary dysfunction such as icterus, clubbing, hepatosplenomegaly, ascites, palmar erythema, and spider telangiectasia. Similarly signs of rickets (swelling at the costo-chondral junction and long bones) should be carefully looked for. Apart from the hepatic function panel, laboratory testing should include gamma-glutamyltranspeptidase or 5’-nucleotidase to determine if AP elevation is the result of underlying hepatobiliary disease. Likewise, calcium, phosphorus, parathyroid hormone, vitamin D level, blood urea nitrogen and creatinine should be assessed. If these tests yield normal results, then further evaluation can be withheld for three to four months, by which time AP concentration should be repeated. Given the benign nature of this entity, rechecking AP earlier than four months is unnecessary. Although the suggested four month interval for the follow up determination of AP is arbitrary, we recommend waiting this long to be consistent with widely accepted definition of THP.3 In conclusion, THP is a relatively common condition among healthy infants and toddlers which resolves without any intervention. Extensive work up or referral to a specialist can be avoided by following an easy use algorithm with emphasis on history and physical examination. It is understood that based on the unique local expertise and access to follow up care, primary care physicians can utilize this algorithm to custom design the care of an individual patient.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. This work was exempt from approval by the Institutional Review Boards of the University of Florida. Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund.
References
- 1.Huh SY, Feldman HA, Cox JE, Gordon CM. Prevalence of transient hyperphosphatasemia among healthy infants and toddlers. Pediatrics. 2009;124:703–9. doi: 10.1542/peds.2008-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt PA, Steel AE, Armstrong AM. Transient hyperphosphatasaemia of infancy following rotavirus infection. J Infect. 1984;9:283–5. doi: 10.1016/s0163-4453(84)90640-6. [DOI] [PubMed] [Google Scholar]
- 3.Kraut JR, Metrick M, Maxwell NR, Kaplan MM. Isoenzyme studies in transient hyperphosphatasemia of infancy. Ten new cases and a review of the literature. Am J Dis Child. 1985;139:736–40. doi: 10.1001/archpedi.1985.02140090098042. [DOI] [PubMed] [Google Scholar]
- 4.Behulova D, Bzduch V, Holesova D, Vasilenkova A, Ponec J. Transient hyperphosphatasemia of infancy and childhood: study of 194 cases. Clin Chem. 2000;46:1868–9. [PubMed] [Google Scholar]
- 5.Stein P, Rosalki SB, Foo AY, Hjelm M. Transient hyperphosphatasemia of infancy and early childhood: clinical and biochemical features of 21 cases and literature review. Clin Chem. 1987;33:313–8. [PubMed] [Google Scholar]
- 6.Asanti R, Hultin H, Visakorpi JK. Serum alkaline, phosphatase in healthy infants. Occurrence of abnormally high values without known cause. Ann Paediatr Fenn. 1966;12:139–42. [PubMed] [Google Scholar]
- 7.Kutilek S, Bayer M, Markova D. Prospective follow-up of children with transient hyperphosphatasemia. Clin Pediatr (Phila) 1997;36:491–2. doi: 10.1177/000992289703600815. [DOI] [PubMed] [Google Scholar]
- 8.Morijiri Y, Sato T. Factors causing rickets in institutionalised handicapped children on anticonvulsant therapy. Archives of Disease in Childhood. 1981;56:446–9. doi: 10.1136/adc.56.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valmadrid C, Voorhees C, Litt B, Schneyer CR. Practice Patterns of Neurologists Regarding Bone and Mineral Effects of Antiepileptic Drug Therapy. Archives of Neurology. 2001;58:1369–74. doi: 10.1001/archneur.58.9.1369. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaidou P, Georgouli H, Kotsalis H, Matsinos Y, Papadopoulou A, Fretzayas A, et al. Effects of Anticonvulsant Therapy on Vitamin D Status in Children: Prospective Monitoring Study. Journal of Child Neurology. 2006;21:205–210. doi: 10.2310/7010.2006.00050. [DOI] [PubMed] [Google Scholar]