Abstract
Background
Child undernutrition affects millions of children globally. We investigated associations between suboptimal growth and mortality by pooling large studies.
Methods
Pooled analysis involving children 1 week to 59 months old in 10 prospective studies in Africa, Asia and South America. Utilizing most recent measurements, we calculated weight-for-age, height/length-for-age and weight-for-height/length Z scores, applying 2006 WHO Standards and the 1977 NCHS/WHO Reference. We estimated all-cause and cause-specific mortality hazard ratios (HR) using proportional hazards models comparing children with mild (−2≤Z<−1), moderate (−3≤Z<−2), or severe (Z<−3) anthropometric deficits with the reference category (Z≥−1).
Results
53 809 children were eligible for this re-analysis and contributed a total of 55 359 person-years, during which 1315 deaths were observed. All degrees of underweight, stunting and wasting were associated with significantly higher mortality. The strength of association increased monotonically as Z scores decreased. Pooled mortality HR was 1.52 (95% Confidence Interval 1.28, 1.81) for mild underweight; 2.63 (2.20, 3.14) for moderate underweight; and 9.40 (8.02, 11.03) for severe underweight. Wasting was a stronger determinant of mortality than stunting or underweight. Mortality HR for severe wasting was 11.63 (9.84, 13.76) compared with 5.48 (4.62, 6.50) for severe stunting. Using older NCHS standards resulted in larger HRs compared with WHO standards. In cause-specific analyses, all degrees of anthropometric deficits increased the hazards of dying from respiratory tract infections and diarrheal diseases. The study had insufficient power to precisely estimate effects of undernutrition on malaria mortality.
Conclusions
All degrees of anthropometric deficits are associated with increased risk of under-five mortality using the 2006 WHO Standards. Even mild deficits substantially increase mortality, especially from infectious diseases.
Introduction
Restricted growth as a result of inadequate nutrition and infections is an important cause of morbidity and mortality in infants and children worldwide [1]–[3]. It has been estimated that 30% of children under five (170 million) in the world are moderately or severely stunted, and 19% (110 million) are moderately or severely underweight [4]. In addition to these, 144 million have mild stunting and 148 million mild underweight [4]. Several prospective studies have shown associations of undernutrition with increased risk of various disease outcomes, and reduced survival, in children [5]–[14].
A 1994 meta-analysis of 8 studies in 6 countries [15] reported mortality rate ratios ranging from 2.5 to 8.4 for mild and moderate underweight children, relative to a ‘normal’ weight-for-age. The 2004 World Health Organization (WHO) Comparative Risk Assessment (CRA) [16] Study was the first global pooling project that analyzed the association of weight-for-age Z score (WAZ) with mortality in four categories: WAZ <−3, WAZ −3 to <−2, WAZ −2 to <−1 and WAZ≥−1. The pooled analysis was updated in the 2008 Lancet Series on Maternal and Child Undernutrition [1]. This analysis further investigated height/length-for-age Z score (HAZ, a measure of stunting) and weight-for-length/height Z score (WHZ, a measure of wasting) as other anthropometric measurements. Virtually all mortality effect estimates in the 2008 Lancet Series were smaller than those reported in the CRA Study, by as much as 83%. At the extreme, the CRA Study found that all levels of underweight significantly increased malaria mortality rates with rate ratios ranging from 2.1 (1.48, 3.02) for mild underweight to 9.5 (3.25, 27.66) for severe underweight, while the 2008 Lancet Series results found no significant association of underweight with malaria mortality, with odds ratios ranging from 0.8 (0.2, 3.2) to 1.6 (1.0, 2.7) for different degrees of underweight.
The two studies differed in several aspects which makes it difficult to assess why the associations changed and what effect sizes should be used for assessing the health consequences of suboptimal growth. First, the cohorts used in the two pooling analyses differed, although 7 common cohorts were included in both analyses. Second, the CRA Study used the 1977 U.S. National Center for Health Statistics/WHO International Growth Reference (NCHS/WHO 1977) [17] to define WAZ, while the 2008 Lancet Series defined WAZ, HAZ and WHZ using the 2006 World Health Organization (WHO) Child Growth Standards (WHO 2006) [18]. Third, the CRA Study estimated mortality rate ratios while the 2008 Lancet Series estimated mortality odds ratios. Fourth, the CRA Study extracted and pooled results of existing studies for computing summary effect estimates whereas the Lancet Series ran Generalized Linear Mixed models using individual-level data. Fifth, the CRA Study could not adjust for confounding because the authors did not have access to individual-level data; the 2008 Lancet Series applied a 15% attenuation to the estimated odds ratios to adjust for confounding by some socioeconomic factors [1].
The renewed policy and programmatic attention to child nutrition and growth means that there is a need for definitive estimates of the association between children’s anthropometric status and total and cause-specific mortality. Examples of such policies and programs include the United Nations (UN) Secretary-General’s “Every Woman Every Child” [19] global movement with the accompanying “Global Strategy for Women’s and Children’s Health” [19], [20] and the more recent United Nations Children’s Fund (UNICEF) “Committing to Child Survival: A Promise Renewed” [21] initiative for ending preventable child deaths, as well as the “Scaling Up Nutrition” movement [22]. It is also important for clinicians, program designers and policy makers to understand how the change in growth standards has affected the mortality effect estimates among children classified as stunted, wasted, or underweight, using comparable data and methods. Finally, the availability of data on the full distributions of anthropometric indicators [4] makes it desirable to assess the association with mortality in narrower Z score bands.
To address these issues, we collated and analyzed data from 10 large prospective studies in low- and middle-income countries. We used these cohorts to investigate the association between different indicators of suboptimal growth and mortality in children 1 week to 59 months old, using both the more recent WHO 2006 standards and the older NCHS/WHO 1977 reference and employing identical methods. We also assessed how effect sizes are affected by adjustment for potential confounding and used narrower Z score bands to estimate a more detailed dose-response relationship between suboptimal growth and mortality.
Methods
Ethics Statement
Pooled analysis of anonymized data was assessed as exempt by the Harvard School of Public Health’s Institutional Review Board.
Study Selection
The principal investigators of all studies that were included in the analysis of child undernutrition and mortality in the CRA Study [16] and the 2008 Lancet Series [1] were contacted to request individual-level data. We included prospective cohort studies or randomized trials that measured height and weight and assessed vital status of children during follow-up. We successfully obtained data from nine of the ten studies included in the CRA Study [23]–[30] and seven of the eight studies in the 2008 Lancet Series [23]–[25], [27], [29], [30] (both earlier projects included studies in Pakistan which are not part of the current analysis). Anonymized data from ten studies in Bangladesh [25], Ghana [23], Guinea-Bissau [29], India [23], Indonesia [28], Nepal [30], Peru [23], the Philippines [24], Senegal [27], and Sudan [26] were included in this analysis. Five of the selected studies were randomized controlled trials of vitamin A supplementation [23], [26], [30].
Eligibility and Anthropometric Assessment
Eligible participants were children who were one week to 59 months old and had at least one recorded visit in which weight or height (or length) was measured. We computed WAZ, HAZ and WHZ using the WHO growth standards of 2006 [18] as well as the NCHS/WHO 1977 reference [17]. We truncated extreme length/height values (defined as <45 cm or >110 cm for children <2 years of age, and <65 cm or >120 cm for children between 2 and 5 years of age) and set them to the limits of the defined plausible range to prevent undue influence of extreme values. In the main analysis, categories of WAZ, HAZ and WHZ were defined as mild (−2≤Z<−1), moderate (−3≤Z<−2), or severe (Z<−3) anthropometric deficits with the reference category being no deficit (Z≥−1). For analyses involving finer categories, we used decrements of 0.5 Z scores down to <−4, with the same reference category (i.e., Z≥−1).
Weight and height/length were measured multiple times during follow-up in all cohorts. Each child’s Z scores and related anthropometric status category at the most recent measurement were carried forward until the next visit. We used the most updated anthropometric status at each time point. The median interval between the last anthropometric measurements and death was 11 weeks and the interquartile range was 4 to 16 weeks. Each eligible child contributed person-time until age 59 months, death, or the administrative end of follow-up in the child’s particular cohort, whichever occurred first. The 5-year age limit was used because the WHO 2006 standards were provided for children up to 60 months old [18].
Outcome Definition
Our primary outcome was death from any cause. The ascertainment procedures of vital status and causes of death for included studies have been reported elsewhere [23]–[30]. Briefly, in all studies vital status was ascertained at regular study visits. Causes of death were available for all cohorts except the study from Indonesia [28]. The other nine studies ascertained cause of death using verbal autopsy methods; four studies also used hospital records when available [23], [29]. In cause-specific analyses, we classified deaths as those due to diarrheal diseases, respiratory tract infections, measles, malaria, and ‘other infectious diseases’ (septicemia, unspecified febrile illness, tuberculosis, meningitis, hepatitis or cellulitis).
Statistical Analyses
All analyses were conducted separately for WAZ, HAZ, and WHZ. We pooled individual-level data from cohorts and estimated mortality hazard ratios (HR) for children in each anthropometric status category relative to those with Z≥−1, using the counting process formulation of Cox proportional hazards regression models [31]. Cox regression uses the partial likelihood method to estimate hazard ratios, removing the need to specify baseline hazard functions. It allows for use of censored data, i.e. when study participants' event times are unknown for reasons including loss to follow-up or study termination. Hazard ratios are valid when censoring is non-informative. Cox regression also allows for stratification of models to adjust for variables for which the proportional hazards assumption does not hold [32]. We used child’s age (in weeks) as the time scale and stratified models on cohort to allow separate baseline hazards. We further adjusted for child’s sex and the assigned treatment in the randomized trials in our minimally adjusted models. Some additional covariates were available in 6 cohorts (e.g., household assets, mother’s education, household water source, and sanitation. See Table S1) but the available covariates differed across cohorts. Therefore, the maximally-adjusted HRs were estimated separately for each of the 6 cohorts and the HRs were pooled using a random effects meta-analysis using the method of DerSimonian and Laird [33]. We repeated the minimally adjusted analysis in these 6 cohorts using the meta-analysis approach to provide a set of consistent results and evaluated the magnitude of confounding by the additional covariates.
Sensitivity Analyses
Childhood is a period of relatively rapid growth, and changes in nutrition or disease episodes can change a child’s Z score within weeks/months depending on the measure. While the included studies had measured anthropometric status in relatively short intervals, Z scores from the previous visit may quickly become outdated, leading to measurement error in exposure. To assess the sensitivity of our results to how long WAZ, HAZ and WHZ values were carried forward, we conducted a sensitivity analysis in which we carried the last observed anthropometric measurement only up to 4 months (after which the person-time from the child was censored). In this sensitivity analysis, the median time interval between anthropometric measurements and death was 8 weeks (interquartile range 3 to 12 weeks).
We also conducted a sensitivity analysis on cause of death classification. Specifically, 39 deaths in two studies were reported to be due to both respiratory tract infections and diarrheal diseases. In the main analysis we categorized these deaths under both analyses but in a sensitivity analysis, we alternatively considered these deaths as due to one or the other cause, but not both.
All analyses were performed using SAS version 9.3 (SAS Institute Inc. Cary NC USA) and R version 2.13.0 (2011-04-13).
Results
Characteristics of the selected cohorts are presented in Table 1. The cohorts included 53 809 children aged 1 week to 59 months old who participated in 10 studies in Asia, Africa and South America. Study recruitment periods ranged from 1977 (Indonesia) to 1997 (Ghana). Participants contributed a total of 55 359 person-years, during which 1315 deaths occurred. In the pooled sample, 63% of children were underweight (WAZ<−1), 67% were stunted (HAZ<−1), and 34% were wasted (WHZ<−1) at baseline. The prevalence proportions of severe baseline underweight, stunting and wasting were 12%, 20% and 3%, respectively. Table 2 presents the age distribution of children at study entry and exit. Approximately one third of children were 0–5 months old at entry; a fifth of the 1315 deaths also occurred in this age group. The 54–59 month group had the fewest deaths.
Table 1. Characteristics of included studies.
| Author, year | Country | Study design | Study recruitment dates | Age range at recruitment [months] | No. of participants recruited | No. of eligibleParticipants(current analysis) | Proportion female (%) | No. of deaths(current analysis) | Median (total) follow-up for current analysis [years, (person years)] |
| Arifeen, 2001 [25] | Bangladesh | Prospective cohort | 1993–1995 | 0–0.4 | 1677 | 1581 | 49.2 | 119 | 1.0 (1240) |
| WHO/CHD, 1998 [23] | Ghana | RCTa | 1995–1997 | 0.5–5 | 2882 | 2869 | 50.9 | 61 | 0.8 (1902) |
| Mølbak, 1992 [29] | Guinea-Bissau | Prospective cohort | 1987–1990 | 0–50 | 1165 | 1145 | 49 | 114 | 1.2 (1267) |
| WHO/CHD, 1998 [23] | India | RCTa | 1995–1996 | 1–5 | 3981 | 3926 | 48.3 | 88 | 0.9 (3074) |
| Katz, 1989 [28] | Indonesia | Prospective cohort | 1977 | 0–71 | 4696 | 3899 | 48.4 | 156 | 1.0 (4488) |
| West, 1991 [30] | Nepal | RCTa | 1989 | 0–60 | 6617 | 6418 | 48.4 | 137 | 1.6 (8051) |
| WHO/CHD, 1998 [23] | Peru | RCTa | 1995–1996 | 1–6 | 2437 | 2393 | 49.2 | 15 | 0.8 (1726) |
| Adair, 1993 [24] | Philippines | Prospective cohort | 1982–1983 | 0–3 | 3080 | 2948 | 47.1 | 110 | 1.8 (4924) |
| Garenne, 2000 [27] | Senegal | Prospective cohort | 1983 | 0–61 | 5781 | 5750 | 49.5 | 354 | 0.9 (4589) |
| Fawzi, 1997 [26] | Sudan | RCTa | 1988 | 0–89 | 29 615 | 22 880 | 48.9 | 161 | 1.5 (24 098) |
Randomized trial of vitamin A supplementation.
Table 2. Age distribution of children at study entry and exit.
| Study entry | Study exit | ||
| Age (months) | No. at baseline (% of all children) | No. censoreda (% of all children) | No. of deaths (% of all children) |
| 0–5 | 18480 (34.34) | 2704 (5.03) | 292 (0.54) |
| 6–11 | 3563 (6.62) | 9416 (17.50) | 232 (0.43) |
| 12–17 | 4250 (7.90) | 2753 (5.12) | 191 (0.35) |
| 18–23 | 4282 (7.96) | 4076 (7.57) | 182 (0.34) |
| 24–29 | 3954 (7.35) | 3844 (7.14) | 172 (0.32) |
| 30–35 | 4226 (7.85) | 4144 (7.70) | 100 (0.19) |
| 36–41 | 4014 (7.46) | 4230 (7.86) | 65 (0.12) |
| 42–47 | 3899 (7.25) | 3987 (7.41) | 40 (0.07) |
| 48–53 | 3818 (7.10) | 4115 (7.65) | 27 (0.05) |
| 54–59 | 3323 (6.18) | 13225 (24.58) | 14 (0.03) |
| Total | 53809 (100) | 52494 (97.56) | 1315 (2.44) |
Number censored includes children who were administratively censored and those lost to follow-up.
Undernutrition and All-cause Mortality
Even mild anthropometric deficit was associated with significantly higher hazard of dying in childhood, with the strength of association increasing as Z scores decreased (Table 3). For example, using the WHO 2006 standards, severely underweight children (WAZ<−3) died at a rate 9.40 times higher (95% Confidence Interval 8.02, 11.03) than children with WAZ of −1 or greater. Mortality hazards for children with mild and moderate underweight were also significantly higher than those with WAZ≥−1, with HRs of 1.52 (1.28, 1.81) and 2.63 (2.20, 3.14), respectively. Wasting was a stronger risk factor for mortality as compared with underweight or stunting. Children with severe wasting had 11.63 fold higher mortality rates (9.84, 13.76) than children with WHZ≥−1 compared with 5.48 (4.62, 6.50) for severe stunting. Similarly, mortality HRs for moderate and mild wasting were, respectively, 48% and 11% larger than HRs for moderate and mild stunting (Table 3). There was no significant heterogeneity in hazard ratios across studies (see Table S2).
Table 3. Minimally adjusteda hazard ratios (HR) using WHO 2006 standards and NCHS/WHO 1977 reference.
| WHO 2006 | NCHS/WHO 1977 | |||
| No. of deaths | HR (95% CI) | No. of deaths | HR (95% CI) | |
| Weight-for-Age Z score | ||||
| <−3 | 489 | 9.40 (8.02, 11.03) | 411 | 12.75 (10.48, 15.50) |
| −3 to<−2 | 284 | 2.63 (2.20, 3.14) | 366 | 3.84 (3.16, 4.67) |
| −2 to<−1 | 287 | 1.52 (1.28, 1.81) | 286 | 1.72 (1.43, 2.08) |
| ≥−1 | 254 | Ref | 251 | Ref |
| Height/Length-for-Age Z score | ||||
| <−3 | 477 | 5.48 (4.62, 6.50) | 381 | 6.58 (5.50, 7.87) |
| −3 to<−2 | 305 | 2.28 (1.91, 2.72) | 318 | 2.78 (2.33, 3.30) |
| −2 to<−1 | 283 | 1.46 (1.23, 1.74) | 328 | 1.62 (1.37, 1.91) |
| ≥−1 | 239 | Ref | 277 | Ref |
| Weight-for-Length/Height Z score | ||||
| < −3 | 220 | 11.63 (9.84, 13.76) | 120 | 17.71 (14.28, 21.97) |
| −3 to<−2 | 205 | 3.38 (2.86, 3.98) | 225 | 4.96 (4.17, 5.90) |
| −2 to<−1 | 308 | 1.62 (1.41, 1.87) | 394 | 1.87 (1.62, 2.15) |
| ≥ −1 | 571 | Ref | 565 | Ref |
Models were stratified on cohort and adjusted for age (as the time scale, in weeks), child’s sex and assigned treatment in randomized trials.
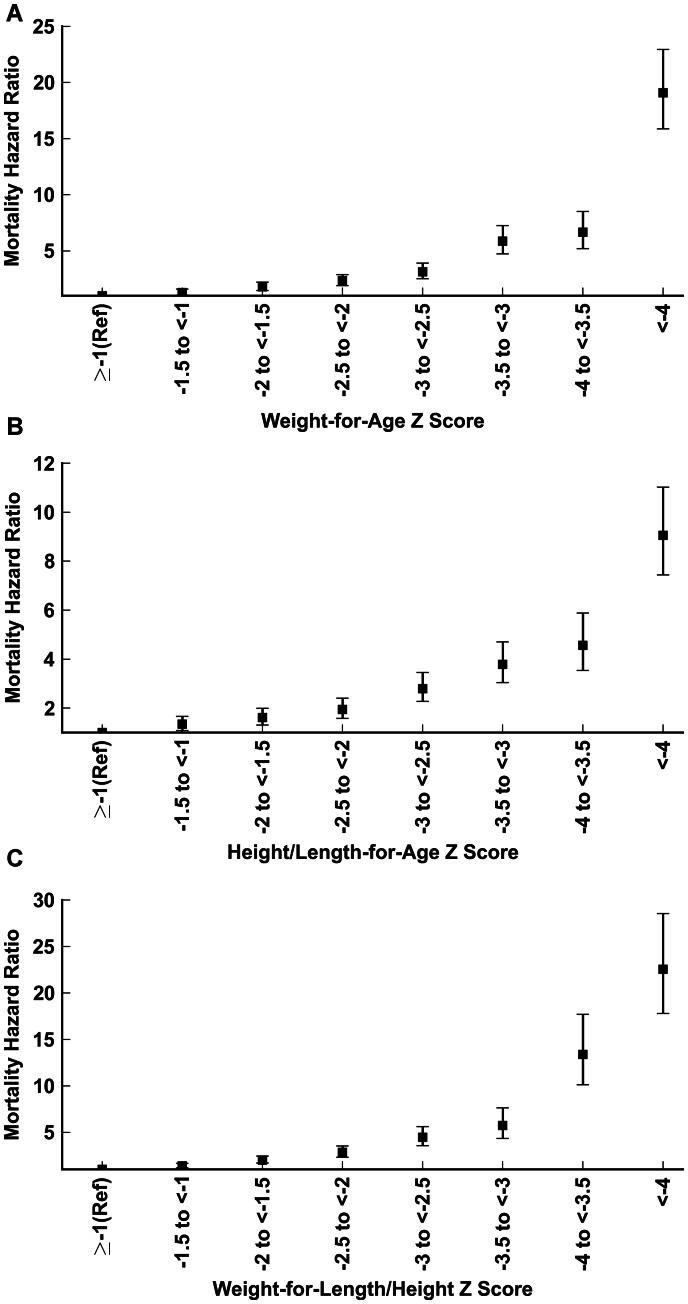
Mortality HRs using decrements of 0.5 Z scores are presented in Figure 1, which shows a clear dose-response curve for all 3 anthropometric indices. Mortality hazards were elevated by as much as 22 fold (17.80, 28.54) for very severely wasted children with WHZ<−4, and HRs reached 9.05 (7.44, 11.02) and 19.09 (15.88, 22.94) for very severely stunted and underweight children, respectively.
Figure 1. Associations of anthropometric measures (in increments of 0.5 Z scores) with all-cause mortality, WHO 2006 child growth standards.
(A) Weight-for-age; (B) Height/length-for-age; (C) Weight-for-Height/Length.
All-cause mortality HRs were larger when the NCHS/WHO 1977 reference was used compared with HRs estimated using the WHO 2006 standards. WAZ hazard ratios for all-cause mortality were 13% to 46% larger with the NCHS/WHO 1977 reference. HAZ hazard ratios were 11% to 22% larger, and WHZ hazard ratios were 15% to 52% larger (Table 3).
Table 4 shows HRs from minimally and maximally adjusted models in 6 cohorts that had data on some additional potential confounders. The pooled point estimates of the maximally adjusted HRs were generally lower than the minimally adjusted HRs (by 3–10% for WAZ, 8–14% for HAZ and 0.8–6% for WHZ). However, the 95% confidence intervals for these estimates overlapped substantially with those of the minimally adjusted models, suggesting that the magnitude of confounding by the measured variables in these cohorts was small.
Table 4. Minimally-adjusteda versus maximally-adjusted hazard ratios (HR) for 6 cohortsb that reported additional baseline covariates beyond sex and randomized treatment assignment, WHO 2006 standards.
| Minimally adjusted hazard ratios | Maximally adjusted hazard ratios | |||||
| Pooledc HR (95% CI) | I2 | p valued | Pooledc HR (95% CI) | I2 | p valued | |
| Weight-for-Age Z score | ||||||
| < −3 | 12.80 (6.97, 23.49) | 42% | 0.12 | 11.88 (6.03, 23.43) | 49% | 0.08 |
| −3 to<−2 | 3.39 (2.12, 5.41) | 0% | 0.57 | 3.06 (1.79, 5.22) | 15% | 0.32 |
| −2 to<−1 | 1.72 (1.08, 2.73) | 0% | 0.9 | 1.67 (1.04, 2.70) | 0% | 0.85 |
| ≥ −1 | Ref | Ref | ||||
| Height/Length-for-Age Z score | ||||||
| < −3 | 6.41 (3.77, 10.89) | 29% | 0.21 | 5.50 (3.04, 9.98) | 39% | 0.14 |
| −3 to<−2 | 2.45 (1.56, 3.87) | 0% | 0.61 | 2.12 (1.33, 3.38) | 0% | 0.63 |
| −2 to<−1 | 1.56 (0.98, 2.46) | 0% | 0.97 | 1.44 (0.90, 2.30) | 0% | 0.99 |
| ≥ −1 | Ref | Ref | ||||
| Weight-for-Length/Height Z score | ||||||
| < −3 | 14.32 (8.76, 23.41) | 28% | 0.22 | 14.20 (7.98, 25.27) | 42% | 0.13 |
| −3 to<−2 | 3.70 (2.43, 5.61) | 0% | 0.78 | 3.47 (2.24, 5.36) | 0% | 0.84 |
| −2 to<−1 | 1.67 (1.13, 2.46) | 0% | 0.95 | 1.61 (1.08, 2.42) | 0% | 0.81 |
| ≥ −1 | Ref | Ref | ||||
Adjusted for age (as the time scale, in weeks), child’s sex, cohort characteristics, and assigned treatment (in randomized trials).
Nepal, Indonesia, Philippines, Bangladesh, Sudan, Guinea-Bissau.
Hazard ratios were estimated in each cohort separately and then pooled using a random effects meta-analysis (see Methods).
p value for test of no heterogeneity.
Effects on Cause-specific Mortality
Of the 1315 deaths among eligible children, 371 were from diarrheal diseases, 187 from respiratory tract infections, 51 from malaria, 80 from measles, and 90 from ‘other infectious causes’. Having a mild anthropometric deficit (i.e. −2≤Z<−1) was associated with significantly higher mortality HRs ranging from 1.55 to 1.92 across different anthropometric indices for respiratory tract infections and from 1.60 to 1.73 for diarrheal diseases (Table 5). Moderate deficits (i.e. −3≤Z<−2) also increased the hazard of dying from measles with HRs ranging from 2.58 to 3.12. Severe anthropometric deficits (Z<−3) increased the hazard of dying from ‘other infectious causes’ (HRs ranging from 3.01 to 11.21) (Table 5). While the HRs for mild categories were not statistically significant for measles and other infectious diseases, there was a suggestion of a dose-response relationship. The largest effects were seen for deaths from diarrheal diseases, with HRs of 12.33 (9.18, 16.57) for severe wasting, and 11.56 (8.63, 15.48) for severe underweight. Three cohorts reported malaria deaths, but the number of deaths was not large enough to provide precise estimates of the effects of undernutrition.
Table 5. Minimally adjusteda hazard ratio (HR) estimates for specific causes of mortality, WHO 2006 standards.
| Mortality from respiratory tract infections | Mortality from diarrheal disease | Mortality from other infectious causesb | Mortality from malariac | Mortality from measlesd | ||||||
| No. ofdeaths | HR (95% CI) | No. ofdeaths | HR (95% CI) | No. ofdeaths | HR (95% CI) | No. ofdeaths | HR (95% CI) | No. ofdeaths | HR (95% CI) | |
| Weight-for-Age Z score | ||||||||||
| < −3 | 68 | 10.10 (6.53, 15.64) | 154 | 11.56 (8.63, 15.48) | 36 | 8.28 (4.32, 15.89) | 3 | 1.29 (0.39, 4.29) | 26 | 7.73 (4.15, 14.39) |
| −3 to<−2 | 41 | 3.11 (1.93, 5.02) | 74 | 2.86 (2.03, 4.03) | 13 | 1.58 (0.73, 3.45) | 10 | 1.65 (0.77, 3.53) | 23 | 3.12 (1.67, 5.80) |
| −2 to<−1 | 43 | 1.85 (1.17, 2.91) | 77 | 1.73 (1.24, 2.40) | 21 | 1.54 (0.78, 3.03) | 16 | 1.26 (0.66, 2.39) | 13 | 1.00 (0.49, 2.03) |
| ≥ −1 | 35 | Ref | 66 | Ref | 19 | Ref | 22 | Ref | 18 | Ref |
| Height/Length-for-Age Z score | ||||||||||
| < −3 | 61 | 6.39 (4.19, 9.75) | 136 | 6.33 (4.64, 8.65) | 29 | 3.01 (1.55, 5.82) | 10 | 1.92 (0.89, 4.11) | 29 | 6.01 (3.00, 12.07) |
| −3 to<−2 | 38 | 2.18 (1.39, 3.43) | 79 | 2.38 (1.71, 3.31) | 24 | 1.86 (0.97, 3.57) | 11 | 1.06 (0.48, 2.32) | 22 | 2.79 (1.40, 5.56) |
| −2 to<−1 | 46 | 1.55 (1.02, 2.37) | 85 | 1.67 (1.20, 2.30) | 17 | 0.95 (0.48, 1.87) | 12 | 0.74 (0.35, 1.56) | 15 | 1.25 (0.61, 2.58) |
| ≥ −1 | 41 | Ref | 66 | Ref | 20 | Ref | 18 | Ref | 14 | Ref |
| Weight-for-Length/Height Z score | ||||||||||
| < −3 | 28 | 9.68 (6.07, 15.43) | 73 | 12.33 (9.18, 16.57) | 14 | 11.21(5.91, 21.27) | 1 | 1.24 (0.17, 9.29) | 13 | 9.63 (5.15, 18.01) |
| −3 to<−2 | 41 | 4.66 (3.07, 7.09) | 61 | 3.41 (2.52, 4.63) | 11 | 2.73 (1.35, 5.54) | 4 | 1.43 (0.52, 3.94) | 12 | 2.58 (1.32, 5.06) |
| −2 to<−1 | 47 | 1.92 (1.31, 2.84) | 83 | 1.60 (1.23, 2.11) | 23 | 1.65 (0.98, 2.79) | 8 | 0.86 (0.39, 1.90) | 15 | 1.02 (0.56, 1.85) |
| ≥ −1 | 70 | Ref | 149 | Ref | 42 | Ref | 38 | Ref | 40 | Ref |
Adjusted for age (as the time scale, in weeks), child’s sex, cohort characteristics, and assigned treatment (in randomized trials).
Septicemia, unspecified febrile illness, tuberculosis, meningitis, hepatitis or cellulitis.
Sensitivity Analyses
Sensitivity analyses showed that our results were robust to the duration of time used to carry forward the last measurements of anthropometric indices (see Table S3). Similarly, results were robust to the assignment of 39 deaths to one or the other of the two reported causes of death. HRs changed by less than 14% in the latter sensitivity analyses, and the differences were not statistically significant.
Discussion
Although it has been known that undernutrition increases mortality in children, effect estimates from the 2008 Lancet Series re-analyses using the new WHO growth standards noticeably differed from results of the CRA analysis which used the NCHS/WHO 1977 reference, especially for cause-specific mortality. Using pooled data from 10 prospective cohorts, we found that childhood undernutrition is strongly associated with elevated all-cause mortality when either the currently recommended WHO 2006 child growth standards or the older NCHS/WHO 1977 reference were used. The associations remained significant after adjusting for additional confounders in 6 cohorts. We also observed a clear dose-response relationship between the three anthropometric indices and under-five mortality. Using finer categories revealed a smooth dose-response relationship with a 20 fold increased mortality hazard for very severely wasted children. Furthermore, undernutrition was found to increase the rate of dying from infections including those of the gastrointestinal and respiratory tracts.
Given the relatively small number of malaria deaths, we had less than 45% power to detect the observed HR estimates. Therefore, we were unable to make any statement about childhood undernutrition and malaria mortality, which would require a larger study in populations where malaria is a common cause of death. Some prior studies reported an increased risk of malaria morbidity and mortality as a result of undernutrition. These studies included clinic or hospital-based studies [34]–[38] and cross-sectional studies [39], [40] which did not distinguish between preexisting and recent undernutrition (a plausible consequence of the acute malaria illness which caused hospitalization and eventual death [41]). Some reports have also suggested protective effects of undernutrition on severe malaria. Some of these studies involved study populations that comprised entirely of cerebral malaria patients or deaths due to malaria [42], [43], others lacked an adequately nourished comparison group [44]–[46], and none adjusted for confounding by socioeconomic and other factors which may have been responsible for the observed association. Children from lower socioeconomic backgrounds may be more frequently exposed to mosquito bites and malaria parasitemia and therefore develop some immunity and protection against severe manifestations [47]. Such children are also more likely to be undernourished, leading to the paradoxical finding of lower malaria mortality in undernourished compared to adequately nourished children. A few other prospective cohort studies in malaria-endemic populations did not find a significant association between childhood undernutrition and malaria morbidity [48]–[50].
The results of the current analysis are consistent with a previous pooling analysis in 2008 [1] though the magnitudes of association from our analysis are larger. The odds ratios in the previous pooling study were obtained using a logistic regression model and after applying a 15% attenuation to adjust for confounding by socioeconomic factors, determined from two studies in Nepal and Honduras [1]. We used a proportional hazard model to incorporate censoring due to loss to follow-up and adjusted for confounding in each study separately because relationships between potential confounders and exposures or outcome may differ across cohorts. Another potential reason for the larger effect estimates reported here may be the use of updated anthropometric indices as opposed to baseline measures which were used in the previous study. Baseline measures are not prone to confounding by disease status during follow-up but may not reflect the nutritional status of the child close to the time of death, which is especially relevant for underweight and wasting that may have a short latency time for their effect on mortality.
Our results, based on the same cohort studies and identical analytical methods, resolve any questions about the role played by the change in growth standards in producing the differences in effect estimates reported by the CRA Study [16] and the 2008 Lancet Series [1]. The WHO 2006 standards tend to classify children in lower Z score categories than the NCHS/WHO 1977 reference and this reclassification leads to lower HRs in the deficient categories. However, the differences with HRs using the old reference were often not statistically significant.
A commonly accepted pathway through which poor growth may increase mortality risk is through secondary immune suppression and increased susceptibility to infections, with worsening undernutrition in a vicious cycle. Undernutrition impairs the immune response, specifically that of innate and cell-mediated immune response [2], [51]–[53]. Immunological studies have shown that severe undernutrition may decrease immunoglobulin A production [54], [55], and impair T lymphocyte function [56], [57] and cytokine production [58]. Undernourished individuals may also suffer from impaired inflammatory response due to reduced synthesis of modulating molecules such as Interlukine-1 and Interlukine-6 [58], reduction in chemokines needed for macrophage mobilization, and impaired phagocytosis and microbial killing due to reductions in complement protein C3 [2], [51]. Acting together, these impairments result in decreased resistance to infections. Other complications of undernutrition may include fluid and electrolyte imbalances, hypoglycemia, hypothermia, and cardiac and respiratory dysfunction [59], [60].
Strengths of our study include the use of individual-level data from 10 large cohort studies. We used the most recent measures of anthropometric status as opposed to baseline values because the updated measures reflect the more recent nutritional status which may be more relevant for acute outcomes. We used a standard definition of anthropometric status categories [1], [16], as well as finer categories to provide a better understanding of the dose-response relationship between undernutrition and mortality. Our analyses utilized the more up-to-date WHO standards of 2006 and provided a comparison with results when the NCHS/WHO 1977 reference is used.
Our results should be interpreted with some limitations in mind. As in any observational analysis, confounding by unmeasured factors is a possibility. While many potential confounders of interest were not available in all studies, six cohorts measured a large number of potential confounders including socioeconomic characteristics. When these factors were included in the maximally adjusted models, results remained unchanged. Another potential confounder is presence and severity of disease. Having an illness may alter a child’s nutritional status and may also increase mortality, leading to potential positive confounding. However, we could not adjust for disease status because this information was not available in most cohorts. Furthermore, specific causes of death were determined by verbal autopsy in most studies, and the possibility of misclassification cannot be excluded. We expect any such misclassification to be independent of exposure values and thus not introduce any bias in the reported hazard ratios. Finally, we may have observed larger effects for wasting compared with underweight or stunting because the true exposure window for underweight and stunting may be longer than the average duration between exposure and death in our study.
Our findings have important policy implications. While the anthropometric status of children in many developing countries has improved over the past three decades [4] and under-5 mortality has declined [61], some countries in sub-Saharan Africa and South Asia have not yet experienced adequate progress [4], [61], [62]. Recent estimates suggest that the current pace is inadequate to achieve the first Millennium Development Goal (MDG 1) of eradicating extreme poverty and hunger: developing countries as a group have less than a 5% chance of meeting the target, and the probability is less than 50% for more than half of the countries [4]. New initiatives such as the UN “Global Strategy for Women’s and Children’s Health” [19], [20] and the UNICEF’s “Committing to Child Survival: A Promise Renewed” [21] are attempting to refocus the attention of governments and partners on addressing health challenges of children worldwide. Findings from this study should motivate continued support for the implementation of interventions which have been shown to be effective, and encourage research that explores new interventions and delivery strategies. For instance, promoting exclusive breastfeeding, complementary feeding and the WHO’s ‘case management of severe acute malnutrition’ guidelines have been shown to improve survival and reduce stunting in children [63]. To further reduce child mortality globally, sustained commitment is required beyond the MDG target year of 2015.
Supporting Information
Additional baseline covariates adjusted for in maximally adjusted analyses.
(DOCX)
Study-specific and pooled hazard ratios (HR) for all-cause mortality using WHO 2006 standards for (A) weight-for-age; (B) height/length-for-age; and (C) weight-for-height/length.
(DOCX)
Mortality hazard ratios (HR) in several sensitivity analyses, WHO 2006 standards.
(DOCX)
Acknowledgments
Nutrition Impact Model Study (anthropometry cohort pooling)
Writing group and pooled analysis: Ibironke Olofin, Christine M. McDonald, Majid Ezzati, Seth Flaxman, Wafaie W. Fawzi, Laura E. Caulfield, Robert E. Black, Goodarz Danaei
Cohort investigators: Linda Adair PhD, University of North Carolina, Chapel Hill, NC, USA; Shams Arifeen MBBS, MPH, DrPH, : Centre for Child and Adolescent Health, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh; Nita Bhandari MD PhD, Centre for Health Research and Development, Society for Applied Studies, New Delhi, India; Michel Garenne PhD, Institut Pasteur, Paris, France, Institut de Recherche pour le Developpement, Unité Résiliences, Paris, France and University of the Witwatersrand, School of Public Health, Johannesburg, South Africa; Betty Kirkwood Ma MSc DIC FFPH FMedSci, London School of Hygiene and Tropical Medicine, London, United Kingdom; Kåre Mølbak MD, DMSc, Department of Infectious Disease Epidemiology, Statens Serum Institut, Copenhagen, Denmark; Joanne Katz ScD, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Alfred Sommer, MD MHS, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Keith P. West, Jr., DrPH, MPH, Center and Program in Human Nutrition, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Mary E Penny, MA, MBChB, Instituto de Investigación Nutricional, Lima, Peru.
Funding Statement
Ths study was supported by Bill & Melinda Gates Foundation; United Kingdom Medical Research Council (MRC); National Institute for Health Research Comprehensive Biomedical Research Centre at Imperial College London and Imperial College Healthcare NHS Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, et al. (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371(9608): 243–60. [DOI] [PubMed] [Google Scholar]
- 2. Scrimshaw NS, SanGiovanni JP (1997) Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr 66(2): 464S–77S. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF (1990) Strategy for improved nutrition of children and women in developing countries. New York: UNICEF. [DOI] [PubMed]
- 4. Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, et al. (2012) Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet 380(9844): 824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alam N, Wojtyniak B, Rahaman MM (1989) Anthropometric indicators and risk of death. Am J Clin Nutr 49(5): 884–8. [DOI] [PubMed] [Google Scholar]
- 6. Chen LC, Chowdhury A, Huffman SL (1980) Anthropometric assessment of energy-protein malnutrition and subsequent risk of mortality among preschool aged children. Am J Clin Nutr 33(8): 1836–45. [DOI] [PubMed] [Google Scholar]
- 7. El Samani EF, Willett WC, Ware JH (1988) Association of malnutrition and diarrhea in children aged under five years. A prospective follow-up study in a rural Sudanese community. Am J Epidemiol 128(1): 93–105. [DOI] [PubMed] [Google Scholar]
- 8. Kossmann J, Nestel P, Herrera MG, El Amin A, Fawzi WW (2000) Undernutrition in relation to childhood infections: a prospective study in the Sudan. Eur J Clin Nutr 54(6): 463–72. [DOI] [PubMed] [Google Scholar]
- 9. Lindtjorn B, Alemu T, Bjorvatn B (1993) Nutritional status and risk of infection among Ethiopian children. J Trop Pediatr 39(2): 76–82. [DOI] [PubMed] [Google Scholar]
- 10. Sepulveda J, Willett W, Munoz A (1988) Malnutrition and diarrhea. A longitudinal study among urban Mexican children. Am J Epidemiol 127(2): 365–76. [DOI] [PubMed] [Google Scholar]
- 11. Smith TA, Lehmann D, Coakley C, Spooner V, Alpers MP (1991) Relationships between growth and acute lower-respiratory infections in children aged less than 5 y in a highland population of Papua New Guinea. Am J Clin Nutr 53(4): 963–70. [DOI] [PubMed] [Google Scholar]
- 12. Tupasi TE, de Leon LE, Lupisan S, Torres CU, Leonor ZA, et al. (1990) Patterns of acute respiratory tract infection in children: a longitudinal study in a depressed community in Metro Manila. Rev Infect Dis 12 Suppl 8S940–9. [DOI] [PubMed] [Google Scholar]
- 13. Tupasi TE, Velmonte MA, Sanvictores ME, Abraham L, De Leon LE, et al. (1988) Determinants of morbidity and mortality due to acute respiratory infections: implications for intervention. J Infect Dis 157(4): 615–23. [DOI] [PubMed] [Google Scholar]
- 14. Garenne M, Maire B, Fontaine O, Briend A (2006) Distributions of mortality risk attributable to low nutritional status in Niakhar, Senegal. J Nutr 136(11): 2893–900. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier DL, Frongillo EA Jr, Schroeder DG, Habicht JP (1994) A methodology for estimating the contribution of malnutrition to child mortality in developing countries. J Nutr 124(10 Suppl): 2106S–22S. [DOI] [PubMed]
- 16.Fishman SM, Caulfield LE, De Onis M, et al.. (2004) Childhood and maternal underweight. In: Ezzati M, Lopez AD, Rodgers A, Murray CJ, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization. 39–161.
- 17.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF (1977) NCHS growth curves for children birth-18 years.United States. Vital Health Stat. 11(165): i-iv,1–74. [PubMed]
- 18.WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization (312 pages).
- 19.United Nations. Every Woman Every Child. Available: http: //everywomaneverychild.org/about. Accessed 2012 Aug 29.
- 20.United Nations Secretary-General Ban Ki-moon. Global Strategy for Women's and Children's Health. Available: http: //everywomaneverychild.org/images/content/files/global_strategy/full/20100914_gswch_en.pdf. Accessed 2012 Aug 29.
- 21.UNICEF. Committing to child survival: A promise renewed. Available: http: //www.apromiserenewed.org/A_Promise_Renewed.html. Accessed 2012 Aug 29.
- 22.United Nations Standing Committee on Nutrition. Scaling Up Nutrition (SUN). Available: http: //www.unscn.org/en/scaling_up_nutrition_sun/. Accessed 2012 Aug 29.
- 23. WHO/CHD Immunisation-Linked Vitamin A Supplementation Study Group (1998) Randomised trial to assess benefits and safety of vitamin A supplementation linked to immunisation in early infancy. WHO/CHD Immunisation-Linked Vitamin A Supplementation Study Group. Lancet 352(9136): 1257–63. [PubMed] [Google Scholar]
- 24. Adair L, Popkin BM, VanDerslice J, Akin J, Guilkey D, et al. (1993) Growth dynamics during the first two years of life: a prospective study in the Philippines. Eur J Clin Nutr 47(1): 42–51. [PubMed] [Google Scholar]
- 25. Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, et al. (2001) Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics 108(4): E67. [DOI] [PubMed] [Google Scholar]
- 26. Fawzi WW, Herrera MG, Spiegelman DL, el Amin A, Nestel P, et al. (1997) A prospective study of malnutrition in relation to child mortality in the Sudan. Am J Clin Nutr 65(4): 1062–9. [DOI] [PubMed] [Google Scholar]
- 27.Garenne M, Maire B, Fontaine O, Dieng K, Briend A (2000) Risques de décès associés à différents états nutritionnels chez l'enfant d'âge préscolaire. Etudes du CEPED n° 17, 192 pages.
- 28. Katz J, West KP Jr, Tarwotjo I, Sommer A (1989) The importance of age in evaluating anthropometric indices for predicting mortality. Am J Epidemiol 130(6): 1219–26. [DOI] [PubMed] [Google Scholar]
- 29. Mølbak K, Aaby P, Ingholt L, Hojlyng N, Gottschau A, et al. (1992) Persistent and acute diarrhoea as the leading causes of child mortality in urban Guinea Bissau. Trans R Soc Trop Med Hyg 86(2): 216–20. [DOI] [PubMed] [Google Scholar]
- 30. West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, et al. (1991) Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 338(8759): 67–71. [DOI] [PubMed] [Google Scholar]
- 31. Andersen PK, Gill RD (1982) Cox's regression model for counting processes: a large sample study. Ann Stat 10(4): 1100–20. [Google Scholar]
- 32. Cox D (1972) Regression models and life-tables. Journal of the Royal Statistical Society Series B. 34(2): 187–220. [Google Scholar]
- 33. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3): 177–88. [DOI] [PubMed] [Google Scholar]
- 34. Renaudin P (1997) Evaluation of the nutritional status of children less than 5 years of age in Moundou, Chad: correlations with morbidity and hospital mortality. Med Trop (Mars). 57(1): 49–54. [PubMed] [Google Scholar]
- 35. Randriamiharisoa FA, Razanamparany NJ, Ramialimanana V, Razanamparany MS (1993) Epidemiological data on children hospitalized with malaria from 1983 to 1992. Arch Inst Pasteur Madagascar. 60(1–2): 38–42. [PubMed] [Google Scholar]
- 36. Olumese PE, Sodeinde O, Ademowo OG, Walker O (1997) Protein energy malnutrition and cerebral malaria in Nigerian children. J Trop Pediatr 43(4): 217–9. [DOI] [PubMed] [Google Scholar]
- 37. Man WD, Weber M, Palmer A, Schneider G, Wadda R, et al. (1998) Nutritional status of children admitted to hospital with different diseases and its relationship to outcome in The Gambia, West Africa. Trop Med Int Health 3(8): 678–86. [DOI] [PubMed] [Google Scholar]
- 38. Faye O, Correa J, Camara B, Dieng T, Dieng Y, et al. (1998) Malaria lethality in Dakar pediatric environment: study of risk factors. Med Trop (Mars) 58(4): 361–4. [PubMed] [Google Scholar]
- 39. Wenlock RW (1979) The epidemiology of tropical parasitic diseases in rural Zambia and the consequences for public health. J Trop Med Hyg 82(5): 90–8. [PubMed] [Google Scholar]
- 40. Burgess HJ, Burgess AP, Driessen F (1975) The nutritional status of children ages 0–5 years in Nkhotakota, Malawi. Trop Geogr Med 27(4): 375–82. [PubMed] [Google Scholar]
- 41. Williams TN, Maitland K, Phelps L, Bennett S, Peto TE, et al. (1997) Plasmodium vivax: a cause of malnutrition in young children. QJM 90(12): 751–7. [DOI] [PubMed] [Google Scholar]
- 42. Goyal SC (1991) Protein energy malnutrition and cerebral malaria. J Trop Pediatr 37(3): 143–4. [DOI] [PubMed] [Google Scholar]
- 43. Edington GM (1954) Cerebral malaria in the Gold Coast African: four autopsy reports. Ann Trop Med Parasitol 48(3): 300–6. [DOI] [PubMed] [Google Scholar]
- 44. Murray MJ, Murray NJ, Murray AB, Murray MB (1975) Refeeding-malaria and hyperferraemia. Lancet 305(7908): 653–4. [DOI] [PubMed] [Google Scholar]
- 45. Murray MJ, Murray AB, Murray NJ, Murray MB (1978) Diet and cerebral malaria: the effect of famine and refeeding. Am J Clin Nutr 31(1): 57–61. [DOI] [PubMed] [Google Scholar]
- 46. Murray MJ, Murray AB, Murray MB, Murray CJ (1976) Somali food shelters in the Ogaden famine and their impact on health. Lancet 307(7972): 1283–5. [DOI] [PubMed] [Google Scholar]
- 47. Doolan DL, Dobano C, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22(1): 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tonglet R, Mahangaiko Lembo E, Zihindula PM, Wodon A, Dramaix M, et al. (1999) How useful are anthropometric, clinical and dietary measurements of nutritional status as predictors of morbidity of young children in central Africa? Trop Med Int Health 4(2): 120–30. [DOI] [PubMed] [Google Scholar]
- 49. Snow RW, Byass P, Shenton FC, Greenwood BM (1991) The relationship between anthropometric measurements and measurements of iron status and susceptibility to malaria in Gambian children. Trans R Soc Trop Med Hyg 85(5): 584–9. [DOI] [PubMed] [Google Scholar]
- 50. Genton B, Al-Yaman F, Ginny M, Taraika J, Alpers MP (1998) Relation of anthropometry to malaria morbidity and immunity in Papua New Guinean children. Am J Clin Nutr 68(3): 734–41. [DOI] [PubMed] [Google Scholar]
- 51. Cunningham-Rundles S, McNeeley DF, Moon A (2005) Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 115(6): 1119–28. [DOI] [PubMed] [Google Scholar]
- 52. Rodriguez L, Cervantes E, Ortiz R (2011) Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health 8(4): 1174–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Keusch GT (2003) The history of nutrition: malnutrition, infection and immunity. J Nutr 133(1): 336S–40S. [DOI] [PubMed] [Google Scholar]
- 54. Reddy V, Raghuramulu N, Bhaskaram C (1976) Secretory IgA in protein-calorie malnutrition. Arch Dis Child 51(11): 871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ha CL, Woodward B (1997) Reduction in the quantity of the polymeric immunoglobulin receptor is sufficient to account for the low concentration of intestinal secretory immunoglobulin A in a weanling mouse model of wasting protein-energy malnutrition. J Nutr 127(3): 427–35. [DOI] [PubMed] [Google Scholar]
- 56. Neyestani TR, Woodward B (2005) Blood concentrations of Th2-type immunoglobulins are selectively increased in weanling mice subjected to acute malnutrition. Exp Biol Med 230(2): 128–34. [DOI] [PubMed] [Google Scholar]
- 57. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, et al. (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394(6696): 897–901. [DOI] [PubMed] [Google Scholar]
- 58. Rodriguez L, Gonzalez C, Flores L, Jimenez-Zamudio L, Graniel J, et al. (2005) Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol 12(4): 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grover Z, Ee LC (2009) Protein energy malnutrition. Pediatr Clin North Am 56(5): 1055–68. [DOI] [PubMed] [Google Scholar]
- 60. Brown KH, Nyirandutiye DH, Jungjohann S (2009) Management of children with acute malnutrition in resource-poor settings. Nat Rev Endocrinol 5(11): 597–603. [DOI] [PubMed] [Google Scholar]
- 61. Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, et al. (2010) Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet 375(9730): 1988–2008. [DOI] [PubMed] [Google Scholar]
- 62.The Millennium Development Goals Report 2012. New York: United Nations. Available: http: //www.un.org/millenniumgoals/pdf/MDG%20Report%202012.pdf. Accessed 2012 Aug 28.
- 63. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, et al. (2008) What works? Interventions for maternal and child undernutrition and survival. Lancet 371(9610): 417–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional baseline covariates adjusted for in maximally adjusted analyses.
(DOCX)
Study-specific and pooled hazard ratios (HR) for all-cause mortality using WHO 2006 standards for (A) weight-for-age; (B) height/length-for-age; and (C) weight-for-height/length.
(DOCX)
Mortality hazard ratios (HR) in several sensitivity analyses, WHO 2006 standards.
(DOCX)