Abstract
We describe here the derivation, characterization, and use of clonal cadmium-resistant (Cdr) strains of the Chinese hamster cell line CHO which differ in their metallothionein (MT) induction capacity. By nondenaturing polyacrylamide gel electrophoresis, we showed that the stable Cdr phenotype is correlated with the augmented expression of both isometallothioneins (MTI and MTII). In cells resistant to concentrations of CdCl2 exceeding 20 microM, coordinate amplification of genes encoding both isometallothioneins was demonstrated by using cDNA MT-coding sequence probes and probes specific for 3'-noncoding regions of Chinese hamster MTI and MTII genes. Molecular and in situ hybridization analyses supported close linkage of Chinese hamster MTI and MTII genes, which we have mapped previously to Chinese hamster chromosome 3. This suggests the existence of a functionally related MT gene cluster in this species. Amplified Cdr variants expressing abundant MT and their corresponding Cds parental CHO cells should be useful for future studies directed toward elucidating the mechanisms that regulate expression of the isometallothioneins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Warren R., Sarthy A., Palmiter R. D. Regulation of metallothionein--thymidine kinase fusion plasmids injected into mouse eggs. Nature. 1982 Mar 4;296(5852):39–42. doi: 10.1038/296039a0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Chang E. H., Lander M. R., Ellis R. W., Scolnick E. M., Lowy D. R. Amplification and rearrangement of onc genes in mammalian species. Nature. 1982 Mar 25;296(5855):361–363. doi: 10.1038/296361a0. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Palmiter R. D. The metallothionein-I gene maps to mouse chromosome 8: implications for human Menkes' disease. Hum Genet. 1983;64(1):61–64. doi: 10.1007/BF00289481. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P., Ruddle F. H. Somatic cell genetics and gene families. Science. 1983 May 27;220(4600):919–924. doi: 10.1126/science.6573776. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
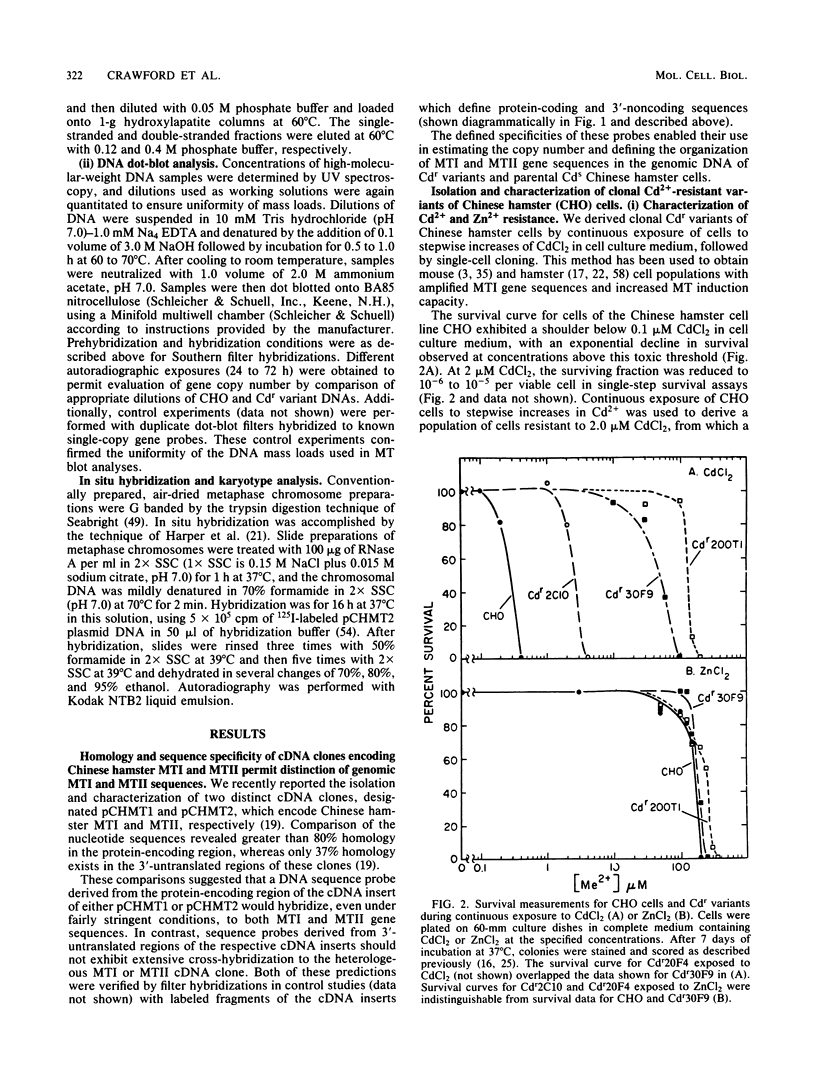
- Enger M. D., Ferzoco L. T., Tobey R. A., Hildebrand C. E. Cadmium resistance correlated with cadmium uptake and thionein binding in CHO cell variants Cdr20f4 and Cdr30f9. J Toxicol Environ Health. 1981 May;7(5):675–690. doi: 10.1080/15287398109530011. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Rall L. B., Hildebrand C. E. Thionein gene expression in Cd++-variants of the CHO cell: correlation of thionein synthesis rates with translatable mRNA levels during induction, deinduction, and superinduction. Nucleic Acids Res. 1979 Sep 11;7(1):271–288. doi: 10.1093/nar/7.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gick G. G., McCarty K. S., Sr Amplification of the metallothionein-I gene in cadmium- and zinc-resistant Chinese hamster ovary cells. J Biol Chem. 1982 Aug 10;257(15):9049–9053. [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
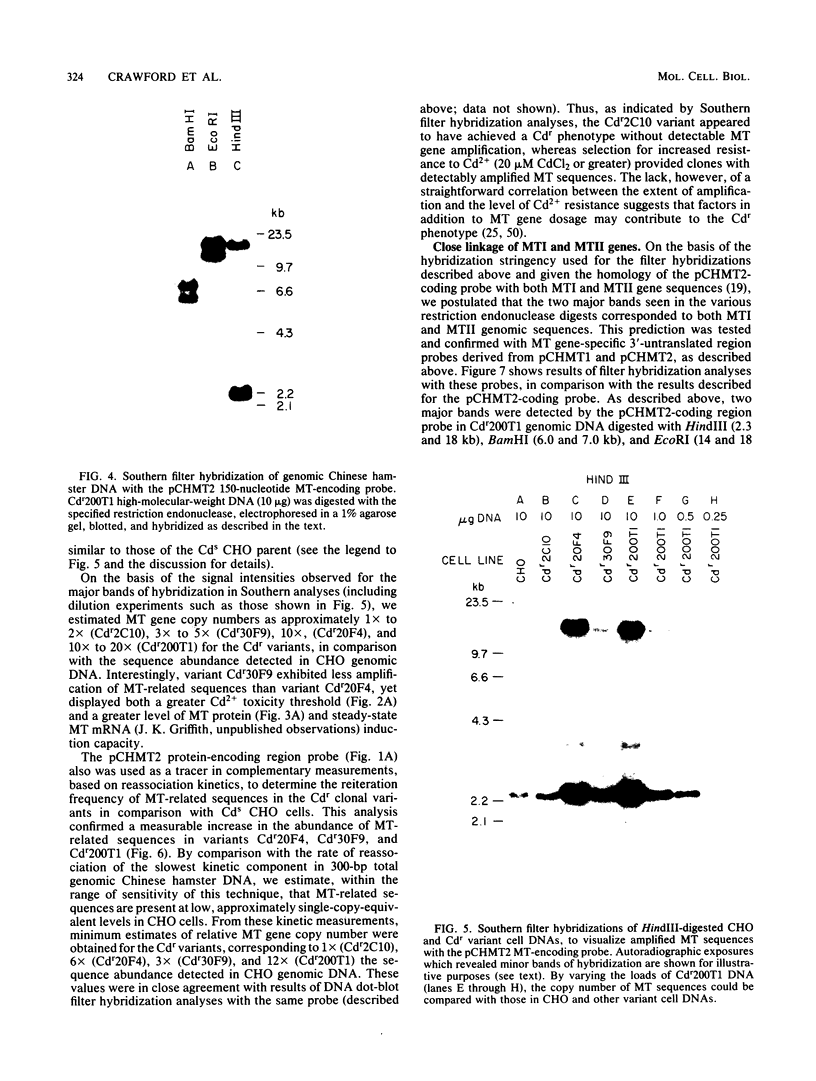
- Griffith B. B., Walters R. A., Enger M. D., Hildebrand C. E., Griffith J. K. cDNA cloning and nucleotide sequence comparison of Chinese hamster metallothionein I and II mRNAs. Nucleic Acids Res. 1983 Feb 11;11(3):901–910. doi: 10.1093/nar/11.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Walling M. Regulation in vivo of a cloned mammalian gene: cadmium induces the transcription of a mouse metallothionein gene in SV40 vectors. J Mol Appl Genet. 1982;1(4):273–288. [PubMed] [Google Scholar]
- Harper M. E., Ullrich A., Saunders G. F. Localization of the human insulin gene to the distal end of the short arm of chromosome 11. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4458–4460. doi: 10.1073/pnas.78.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Fujiki H., Sugimura T. Effects of tumor promoters on the frequency of metallothionein I gene amplification in cells exposed to cadmium. Cancer Res. 1983 Nov;43(11):5433–5436. [PubMed] [Google Scholar]
- Hayden T. L., Turner J. E., Williams M. W., Cook J. S., Hsie A. W. A model for cadmium transport and distribution in CHO cells. Comput Biomed Res. 1982 Apr;15(2):97–110. doi: 10.1016/0010-4809(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Enger M. D. Regulation of Cd2+/Zn2+-stimulated metallothionein synthesis during induction, deinduction, and superinduction. Biochemistry. 1980 Dec 9;19(25):5850–5857. doi: 10.1021/bi00566a029. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Tobey R. A., Campbell E. W., Enger M. D. A cadmium-resistant variant of the Chinese hamster (CHO) cell with increased metallothionein induction capacity. Exp Cell Res. 1979 Dec;124(2):237–246. doi: 10.1016/0014-4827(79)90199-x. [DOI] [PubMed] [Google Scholar]
- House W., Waddell A. Detection of mycoplasma in cell cultures. J Pathol Bacteriol. 1967 Jan;93(1):125–132. doi: 10.1002/path.1700930112. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Turner J. E. The interaction of cadmium and certain other metal ions with proteins and nucleic acids. Toxicology. 1980;16(1):1–37. doi: 10.1016/0300-483x(80)90107-9. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Cathala G., Slater E., Baxter J. D. Activation of a heterologous promoter in response to dexamethasone and cadmium by metallothionein gene 5'-flanking DNA. Cell. 1984 Feb;36(2):371–379. doi: 10.1016/0092-8674(84)90230-7. [DOI] [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Dexamethasone stimulation of metallothionein synthesis in HeLa cell cultures. Science. 1979 Apr 13;204(4389):176–177. doi: 10.1126/science.432639. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Lieberman M. W., Beach L. R., Palmiter R. D. Ultraviolet radiation-induced metallothionein-I gene activation is associated with extensive DNA demethylation. Cell. 1983 Nov;35(1):207–214. doi: 10.1016/0092-8674(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981 Mar 25;256(6):2621–2624. [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of the mouse metallothionein I gene is selectively lost following amplification of the gene. J Biol Chem. 1982 Mar 25;257(6):3061–3067. [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Lewis J. A., Biedler J. L., Hession C. Antifolate-resistant Chinese hamster cells. Evidence for dihydrofolate reductase gene amplification among independently derived sublines overproducing different dihydrofolate reductases. J Biol Chem. 1980 Jul 25;255(14):7024–7028. [PubMed] [Google Scholar]
- Montgomery K. T., Biedler J. L., Spengler B. A., Melera P. W. Specific DNA sequence amplification in human neuroblastoma cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5724–5728. doi: 10.1073/pnas.80.18.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Bonnet J., Li D. W., Ts'o P. O. An alternative view of mammalian DNA sequence organization. I. Repetitive sequence interspersion in Syrian hamster DNA: a model system. J Mol Biol. 1981 Dec 25;153(4):841–864. doi: 10.1016/0022-2836(81)90455-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Hamer D. H. Regulation of a metallothionein-growth hormone hybrid gene in bovine papilloma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(2):397–401. doi: 10.1073/pnas.80.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Jolicoeur-Paquet L. Metallothionein accumulation may account for intracellular copper retention in Menkes' disease. J Biol Chem. 1982 Apr 25;257(8):4639–4645. [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Seagrave J., Hildebrand C. E., Enger M. D. Effects of cadmium on glutathione metabolism in cadmium sensitive and cadmium resistant Chinese hamster cell lines. Toxicology. 1983 Dec;29(1-2):101–107. doi: 10.1016/0300-483x(83)90042-2. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981 Nov;27(1 Pt 2):193–201. doi: 10.1016/0092-8674(81)90373-1. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Munk A. C., Longmire J. L., Hildebrand C. E., Crawford B. D. Assignment of genes encoding metallothioneins I and II to Chinese hamster chromosome 3: evidence for the role of chromosome rearrangement in gene amplification. Mol Cell Biol. 1984 Dec;4(12):2932–2936. doi: 10.1128/mcb.4.12.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Rubnitz J. Co-amplification of rRNA genes with CAD genes in N-(phosphonacetyl)-L-aspartate-resistant Syrian hamster cells. Mol Cell Biol. 1983 Nov;3(11):2066–2075. doi: 10.1128/mcb.3.11.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]