Abstract
Objective
This study aimed to assess the demographic, behavioral, and clinical factors associated with HIV and syphilis infection among a sample of men attending a sexually transmitted infection clinic during 2009 to 2010 in San Juan, Puerto Rico (PR).
Methods
A sample of 350 clinical records from men visiting the clinic for the first time during 2009 to 2010 was reviewed. Descriptive statistics were used to describe the study sample, and bivariate analyses were performed separately for HIV and syphilis to identify factors associated with these infectious diseases. Variables that were significantly associated (p<0.05) with HIV and syphilis in the bivariate analysis were considered for inclusion in the logistic regression models.
Results
Overall, 11.2% and 14.1% of the men were infected with HIV and syphilis, respectively, and 5.1% were coinfected with HIV and syphilis. In multivariate logistic regression models, ever injecting drugs (POR = 8.1; 95%Cl 3.0, 21.8) and being a man who has sex with men (MSM) (POR = 5.3; 95%CI 2.3, 11.9) were positively associated with HIV infection. Being a man older than 45 years (POR = 4.0; 95%CI: 1.9, 8.9) and being an MSM (POR = 2.5; 95%CI: 1.3, 4.9) were both significantly associated with syphilis infection.
Conclusion
These findings reinforce the need for greater education and prevention efforts for HIV and other STIs among men in PR, particularly those who are MSM. However, there is a need to make an a priori assessment of the level of health literacy in the members of this group so that a culturally sensitive intervention can be provided to the men who attend this STI clinic.
Keywords: HIV, Syphilis, Epidemiology, MSM, Men, Puerto Rico
Sexually transmitted infections (STIs) are a significant public health problem worldwide (1). A disproportionate burden of HIV is carried by Puerto Ricans as compared to the general US population and the overall Hispanic US population (2). As of December 2010, the Puerto Rico HIV/AIDS Surveillance System reported 43,682 cases of HIV infection in PR, and ranked 4th among US states and territories reporting the highest number of diagnosis of HIV infection among adults and adolescents in 2010. (3). Disparities in HIV infection may be correlated with social determinants of health, such as poverty, high rates of illicit drug use, and complex patterns of risky sexual behavior (4). Social and epidemiological data suggest that the high prevalence of HIV in PR is concentrated among vulnerable and underserved populations. High-risk sexual practices in these populations compound the problem by increasing the rate of disease transmission. Furthermore, the lack of screening in these populations, in terms of long-term health problems associated with HIV, creates an even larger disparity between these populations and those with adequate access to medical care (5).
In addition, the incidence of syphilis is increasing worldwide (6). Syphilis is highly infectious but easily curable in its early stages. If untreated, it can lead to serious long-term complications, including neurological problems, cardiovascular disease, organ damage, and even death (7). In the US, the rate of primary and secondary syphilis decreased throughout the 1990s, and in 2000 it reached an all-time low (8). However, in the past 8 years the syphilis incidence rate in the US, as well as that in PR, has been increasing, primarily among men (9). Additionally, the disparity in syphilis infection rates between males and females has grown consistently throughout this period (10). For example, in PR, the rates of syphilis among males and females were almost equivalent in 2001, but by 2008 it was estimated that the rate was six times higher among males (10). It is suggested that this disparity may in part be due to the increasing incidence rate of syphilis in men who have sex with other men (MSM) (11).
Because of the reported increase in syphilis rates among men and given that the presence of syphilis increases susceptibility to HIV transmission (12), it is important to make every effort to curb the spread of syphilis and, thus, HIV infection, particularly in high-risk populations. In order to better understand the characteristics and behaviors associated with syphilis and HIV infection among high-risk men attending an STI clinic in PR, our aim was twofold: 1) to describe the proportion of individuals infected with HIV and syphilis and 2) to investigate the correlates of HIV and syphilis infection among the men who attended an urban STI clinic in PR from 2009 to 2010.
Methods
The Latin American Center for Sexually Transmitted Diseases (CLETS, by its initials in Spanish) is one of the largest publicly funded STI clinics in PR (13). A census conducted by our research group confirmed that the clinic received approximately 3,223 new patients between 2009 and 2010. As part of their initial examination, patients in this STI clinic are subject to the following routine procedures: First, the clinician determines which a series of STI tests to administer to each new patient based on that patient’s symptoms, age, medical history, and reason for visiting. If the patient reports little to no sexual history, the patient is tested only for gonorrhea and chlamydia. If the patient reports some sexual history, he is given the full series of tests, which includes tests for gonorrhea, chlamydia, HIV, and syphilis. A full series of tests will also be run on any patient who specifically requests an HIV test. Active primary and secondary syphilis are diagnosed at the local laboratory using rapid plasma reagin (RPR) card test. HIV testing is performed with a rapid enzyme immunoassay test, and positive results are confirmed with Western blot and ELISA. Chlamydia and gonorrhea are both assessed by a nucleic acid amplification test (NAAT).
Study population
This is a secondary data analysis of the parent cross-sectional study entitled Epidemiological Profile of Genital Warts in High-Risk Men and Women Attending an STI Clinic in Puerto Rico, aimed at assessing the prevalence of genital warts among men and women attending the CLETS clinic for the first time from 2009 to 2010. To accomplish this, 3,223 non-electronic medical records of the patients who had attended the CLETS clinic in this time period were assessed. A simple random sampling method was used to ensure an equal chance of selection so that there would be a representative sample of the patients who had visited the clinic. A total of 597 new patients were selected for a retrospective chart review. Each of these selected men and women had made at least one primary care visit to the clinic from 2009 to 2010. For purposes of this secondary data analysis, the medical records of the 350 men (58.6%) in the total sample were examined.
Data collection procedures and study variables
Trained personnel from the research study collected information from two sources: First, a standard form which includes sociodemographic and behavioral information. This form is included in all the medical records and is completed by a physician as part of the medical assessment. Sociodemographic information such as age, marital status (married or living with consensual partner/single), education level (less than high school/high school or higher), employment status (employed/unemployed) and having the government health plan (currently known as “Mi Salud”) (yes/no) was collected from this form. Behavioral risk factors such as lifetime use of cigarettes, intravenous drug use (IDU), or non-injected drugs (yes/no), and ever having sex with an intravenous drug user (yes/no) was also collected. Second, clinical information was gathered from the patient tests results for HIV and syphilis. All of the tests used for diagnosis in this study were serum based. Diagnostic modalities were all considered acceptable methods of detecting the respective STIs according to the most recent CDC and WHO guidelines (14, 15). The study was approved by the Medical Sciences Campus, University of Puerto Rico, Institutional Review Board (IRB).
Statistical analyses
Frequency distributions and descriptive statistics were used to characterize the study population and describe the proportion of men infected with HIV and syphilis, as well as that of those who were co-infected. Contingency tables and chi-square tests were used to describe the sociodemographic, behavioral, and clinical characteristics associated with HIV and syphilis infection. Risk factors significantly associated with HIV and syphilis (p<0.05) in the bivariate analysis were included in separate multivariate logistic regression models to estimate the adjusted prevalence odds ratio (POR). First, crude logistic regression analyses were performed to identify risk factors independently associated with HIV and syphilis (Model 1). Next, multivariate logistic regression models were constructed to determine the predictors of HIV and syphilis, in separate models, after controlling for all of the independent variables in the model (Model 2). Finally, all potential first-order interactions in the multivariate model were also evaluated with the likelihood ratio test. All statistical analyses were performed using a statistical package called SAS (Version 9.1, Cary, NC).
Results
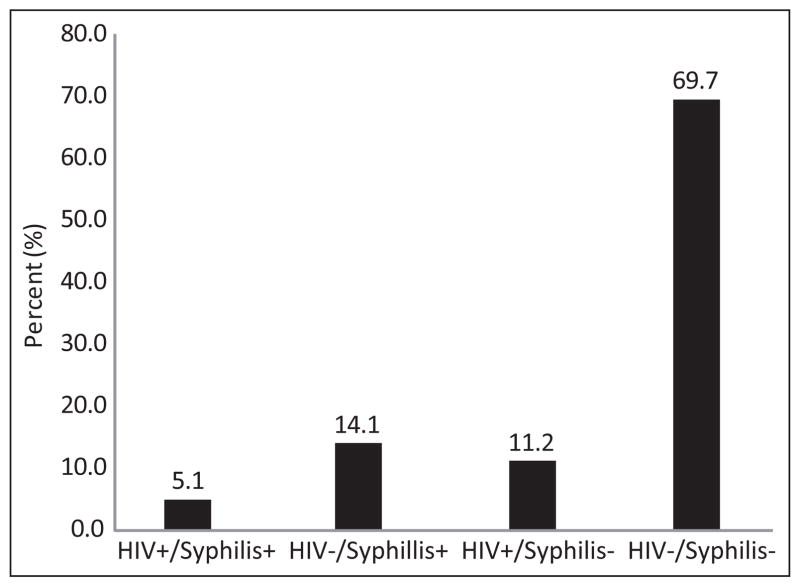
Demographic, behavioral, and clinical characteristics for the study sample are shown in Table 1. The mean age of the men sampled (n = 350) was 30.7±12.8 years. The majority of the men in the study sample were not married (72.0%) and had less than a high school education (67.5%). More than a third of them reported being unemployed at the time of the visit (37.0%) (Table 1). The three most frequently cited primary reasons for visiting the STI clinic were contact with a person infected with an STI (46.2%), voluntary examination (26.5%), and referral (24.1%) (data not shown). Overall, 11.2% of men were infected with HIV and 14.1% were infected with syphilis; 5.1% of these were coinfected with HIV and syphilis (Figure 1).
Table 1.
HIV infection in relationship to demographic and risk-related characteristics among male patients attending an STD clinic in Puerto Rico, 2009–2010.
| Characteristic | Total | HIV negative % (n) | HIV positive % (n) | p-value |
|---|---|---|---|---|
| Age group | ||||
| <45 years | 80.6 (274) | 83.9 (244) | 61.2 (30) | <0.01 |
| ≥45 years | 19.4 (66) | 16.2 (47) | 38.8 (19) | |
| Education | ||||
| <High school | 67.7 (220) | 69.3 (187) | 55.6 (25) | 0.07 |
| ≥High school | 32.3 (105) | 30.7 (83) | 44.4 (20) | |
| Health care plan (“Mi Salud”) | ||||
| Yes | 51.0 (288) | 63.0 (174) | 51.1 (24) | >0.10 |
| No | 49.0 (277) | 37.0 (102) | 48.9 (23) | |
| Lifetime alcohol use | ||||
| Yes | 58.4 (188) | 58.6 (160) | 54.4 (25) | >0.10 |
| No | 41.6 (134) | 41.4 (113) | 45.7 (21) | |
| Lifetime cigarette use | ||||
| Yes | 45.7 (147) | 44.0 (120) | 54.4 (25) | >0.10 |
| No | 54.4 (175) | 56.0 (153) | 45.7 (21) | |
| IDU | ||||
| Yes | 9.3 (30) | 5.9 (16) | 30.4 (14) | <0.01 |
| No | 90.7 (292) | 94.1 (257) | 70.0 (32) | |
| Ever used non-injecting drugs | ||||
| Yes | 33.9 (109) | 33.0 (90) | 37.0 (17) | >0.10 |
| No | 66.2 (213) | 67.0 (183) | 63.0 (29) | |
| MSM | ||||
| Yes | 26.6 (85) | 22.4 (61) | 53.3 (24) | <0.01 |
| No | 73.4 (235) | 77.6 (211) | 46.7 (21) | |
| Syphilis | ||||
| Yes | 19.6 (55) | 16.8 (39) | 31.1 (14) | 0.03 |
| No | 80.4 (225) | 83.2 (193) | 68.9 (31) | |
Figure 1.
HIV and Syphilis infection among men attending an STI clinic in Puerto Rico for the first time: 2009–2010.
A significant proportion of men with HIV infection were older than 45 years (p<0.05). An evaluation of the behavioral characteristics of the study participants showed that a high percentage of the HIV-infected men were both intravenous drug users and MSM. Also, a high proportion of syphilis co-infection was observed among the HIV+ men in this sample. Health-care coverage and ever using non-intravenous drugs were not associated with HIV infection in bivariate analyses (p>0.05) (Table 1). Multivariate logistic regression analysis showed that ever using injecting drugs (POR = 8.2; 95%Cl 3.0, 21.8) and being an MSM (POR = 5.3; 95%Cl 3.3, 11.9), infection remained significantly and positively associated with HIV infection. No first-order interactions were observed in the model (p>0.05) (Table 2).
Table 2.
Logistic regression analysis for factors associated with HIV infection among male patients attending an STI clinic in Puerto Rico, 2009–2010.
| Variable | POR*Crude (95% CI) | POR†Adjusted (95% CI) |
|---|---|---|
| Age group | ||
| <45 years | 1.0 | 1.0 |
| ≥45 years | 3.3 (1.7, 6.3) | 2.3 (0.9, 5.7) |
| IDU | ||
| Yes | 7.0 (3.1, 15.7) | 8.1 (3.0, 21.8) |
| No | 1.0 | 1.0 |
| MSM | ||
| Yes | 4.0 (2.1,7.6) | 5.3 (2.3, 11.9) |
| No | 1.0 | 1.0 |
| Syphilis | ||
| Yes | 2.2 (1.1, 4.6) | 1.5 (0.6, 3.4) |
| No | 1.0 | 1.0 |
Model 1: Crude model,
Model 2: Multivariate analysis
Regarding syphilis infection, a high proportion of men infected with syphilis were aged 45 or older and were MSM (p<0.05) (Table 3). In the multivariate logistic regression models, being a man older than 45 years (POR = 4.0; 95% CI 1.9, 8.9) and being a MSM (POR = 2.5; 95%CI: 1.3, 4.9) remained significantly associated with having syphilis (Table 4). No first-order interactions were observed in this model (p>0.05).
Table 3.
Syphilis infection in relationship to demographic and risk-related characteristics among male patients attending an STI clinic in Puerto Rico, 2009–2010.
| Characteristic | Syphilis negative % (n) | Syphilis positive % (n) | p-value |
|---|---|---|---|
| Age group | |||
| <45 years | 84.4 (190) | 61.8 (34) | <0.01 |
| ≥45 years | 15.6 (35) | 38.2 (21) | |
| Education | |||
| <High school | 67.9 (142) | 57.4 (31) | >0.10 |
| ≥High school | 32.1 (67) | 42.6 (23) | |
| Health care plan | |||
| Yes | 60.4 (131) | 62.3 (33) | |
| No | 39.7 (86) | 37.7 (20) | >0.10 |
| Lifetime alcohol use | |||
| Yes | 61.2 (131) | 54.7 (29) | >0.10 |
| No | 38.8 (83) | 45.3 (24) | |
| Lifetime cigarette use | |||
| Yes | 46.7 (100) | 45.3 (24) | >0.10 |
| No | 53.3 (114) | 54.7 (29) | |
| IDU | |||
| Yes | 9.3 (20) | 15.1 (8) | >0.10 |
| No | 90.7 (194) | 84.9 (45) | |
| Ever used non-injecting drugs | |||
| Yes | 32.71 (70) | 35.85 (19) | >0.10 |
| No | 67.29 (144) | 64.15 (34) | |
| MSM | |||
| Yes | 25.82 (55) | 43.40 (23) | 0.01 |
| No | 74.18 (158) | 56.60 (30) | |
Table 4.
Logistic regression analysis for factors associated with syphilis among male patients attending an STI clinic in Puerto Rico, 2009–2010.
| Variable | PORCrude* (95% CI) | PORAdjusted† (95% CI) |
|---|---|---|
| Age group | ||
| <45 years | 1.0 | 1.0 |
| ≥45 years | 3.5 (1.7, 6.4) | 4.0 (1.9, 8.3) |
| MSM | ||
| Yes | 2.2 (1.2, 4.1) | 2.5 (1.3, 4.9) |
| No | 1.0 | 1.0 |
| HIV | ||
| Yes | 2.2 (1.1, 4.6) | 1.4 (0.6, 3.2) |
| No | 1.0 | 1.0 |
Model 1: Crude model,
Model 2: Multivariate analysis
Discussion
Our study showed that 14.4% of the men surveyed at CLETS during the period ranging from 2009 to 2010 were infected with HIV and that 19.6% were infected with syphilis. This estimate is higher than those found in other studies performed in an STI clinic in Jamaica (16) but is lower than those seen in other STI clinics in countries such as Trinidad (17), India (18), and China (19). Although previous studies describe risk factors for the transmission of HIV in PR (20, 21, 22) and the US (23, 24), our results show that MSM attending this clinic were five times more likely to be infected with HIV and had twice the probability of being infected with syphilis than did the non-MSM who attended the clinic. Similar findings among patients at STI clinics in nine cities in the US (25) were reported previously. In PR, however, despite the growing impact of the HIV epidemic, limited epidemiological research on MSM has been conducted (26, 27). Descriptive studies have found a number of high-risk sexual behaviors within this group, including, for example, having a high number of lifetime sexual partners; a high prevalence of herpes virus-2 (32%), and a high prevalence of recent illicit drug use such as marijuana and cocaine (25). Findings from our study along with descriptive data published previously contribute to identifying high-risk subgroups and their equally high-risk sexual practices so that targeted intervention programs for STI prevention and control among men in PR might be developed. The goals of these interventions should include preventing HIV infection in those already infected with syphilis as well as preventing the transmission of HIV and syphilis to other sexual partners.
Injection drug use, another known risk factor for HIV infection, has been repeatedly studied in PR, and it is the primary risk factor for HIV infection among men on the island (2, 4). Consistent with the findings reported in the literature, our study outcomes linked injection drug use and HIV infection. This finding is important for our research context since STI clinics can be excellent venues at which specific harm-reduction strategies for injection drug users can be employed. These interventions should aim to reduce high-risk sexual practices as well as promote safe injection practices in the setting of an STI clinic.
Consistent with what has been previously reported, this study indicates that a notable proportion of people who attend STI clinics are older than 45 years old (28, 29, 30), supporting the observation that STIs and their related high-risk sexual behaviors are not confined to young people (31, 32). In this study, there is also significant evidence that the prevalence of syphilis is higher within this older age group than it is in the other age groups, which evidence remains significant even after multivariate adjustment. All of which highlights the need for comprehensive prevention messages on sexual health promotion and syphilis prevention for this age group of men in particular. In addition, since low educational attainment is reported in this study, both an a priori assessment of the level of health literacy within this group and the provision of culturally sensitive health literacy interventions to the men attending this clinic are necessary.
Consistent with a previous study (33), the main reasons that our population gave for attending the clinic for the first time were perceived or potential contact with an STI (reported by the patient due to his suspicion that his sexual partner may have an STI or because of that partner’s STI disclosure after sexual activity) followed by this being a voluntary visit to the clinic for an STI examination. These data have significant implications for health education efforts, since even though most of the men had attended the clinic as a result of their perceived contact with an STI, more than half were negative for both HIV and syphilis according to their medical records. In PR, then, public STI clinics provide excellent settings for targeted HIV-prevention efforts. As previously reported by other investigators (18), and reported in this study as well, STI clinic patients represent a major risk group for HIV acquisition and transmission, a group that can easily be approached within this setting. In these venues, behavioral and screening interventions aimed at the prevention and control of HIV and syphilis might be feasible since patients at STI clinics may be willing to undergo HIV testing in light of their high-risk sexual behaviors and may desire to participate in behavioral interventions in a convenient and confidential clinical setting (18).
There are limitations to our study that need to be considered. The data from this study were clinic-based; therefore, results are not necessarily generalizable to the population of PR as a whole. Additionally, the small number of co-infected patients precluded the assessment of covariates of HIV/syphilis co-infection in multivariate analysis. Since the evaluation of risk factors in this study came strictly from the retrospective evaluation of medical records, we did not have detailed information about the sexual behavior (i.e., number of sexual partners, condom use, type of sexual practice) or sexual partners (paid sex, spontaneous encounters, multiple partners) of the patients: such information is not part of the medical records. Consequently, we were not able to assess these characteristics in our study. Also, all of the information gathered in this study was collected from the medical charts that were completed by the physicians at the clinic. Potential ascertainment bias might lead to an underreporting of the high-risk behavioral practices assessed. However, in which group this underreporting would be higher (younger or older age group, MSM, IDUs) is difficult to ascertain with the available data. Multidisciplinary efforts should be made to increase the clinic’s capacity to gather valuable research data in a sustainable manner. Despite the above limitations, this study provides useful information on the burden of STI in one of the largest and most frequented STI clinics in PR.
In summary, this study highlights the importance of utilizing STI clinics for monitoring the burden and correlates of HIV and syphilis within male subgroups. Since both of these STIs have complex, long-term consequences, multidisciplinary health resources should be developed in STI clinics to account for these risk factors. More specific behavioral risk factors, regarding sexual and drug use practices, particularly in a more recent timeframe, are needed as part of a multidisciplinary initiative. Furthermore, training that targets the health care professionals who provide services to this population needs to be developed in order to decrease STI comorbidities, related long-term sequelae, and the transmission of STIs within this group.
Acknowledgments
The project described above was fully supported by NIH/NIDA A-START 1R03DA031590-01and partially supported by the CDC’s Cooperative Agreement: Enhancing Professional, Education, and Capacity Building U50/CC325128-05. Funding also came from the National Center for Research Resources (2G12-RR003051) and the National Institute on Minority Health and Health Disparities (8G12-MD007600) of the National Institutes of Health. We wish to express our gratitude to the medical and clinical staff of the Centro Latino Americano de Enfermedades de Transmisión Sexual, especially Sol Merced, who provided guidance and help in evaluating the medical records for this study. Also, we thank Jorge Ballester, Sullymar Vélez and Dr. Kai Bullard for their assistance and suggestions on earlier versions of this manuscript.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Uusküla A, Puur A, Toompere K, DeHovitz J. Trends in the epidemiology of bacterial sexually transmitted infections in eastern Europe, 1995–2005. Sex Transm Infect. 2010;86:6–14. doi: 10.1136/sti.2009.037044. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Incidence and diagnoses of HIV infection, Puerto Rico, 2006. MMWR Morb Mortal Wkly Rep. 2009;58:589–591. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) [Accessed September 19, 2012];HIV Surveillance Report. 2010 22 Available at: Url: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published March 2012. [Google Scholar]
- 4.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol Rev. 2004;26:22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 5.Reyes JC, Robles RR, Colón HM, et al. Homelessness and HIV risk behaviors among drug injectors in Puerto Rico. J Urban Health. 2005;82:446–455. doi: 10.1093/jurban/jti073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjekić M, Marković M, Sipetić S. Clinical manifestations of primary syphilis in homosexual men. Braz J Infect Dis. 2012;16:387–389. doi: 10.1016/j.bjid.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Zeltser R, Kurban AK. Syphilis. Clin Dermatol. 2004;22:461–468. doi: 10.1016/j.clindermatol.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Sexually Transmitted Disease Surveillance, 2007. Atlanta, GA, US: Department of Health and Human Services; Dec, 2008. [Google Scholar]
- 9.Puerto Rico Health Department. OCASET Program of STD/HIV/AIDS Prevention. Sexually transmitted diseases reported by municipality and regions, Office of STD Surveillance System. 2008. [Google Scholar]
- 10.Centers for Disease and Control (CDC) [Accessed October 12, 2011];Trends in Reportable Sexually Transmitted Diseases in the United States, 2007 National Surveillance Data for Chlamydia, Gonorrhea, and Syphilis. 2007 Available at: Url: http://www.cdc.gov/std/stats07/trends.htm.
- 11.Zetola NM, Klausner JD. HIV/AIDS: Syphilis and HIV Infection: An Update. Clin Infect Dis. 2007;44:1222–1228. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 12.Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008;42:226–236. doi: 10.1345/aph.1K086. [DOI] [PubMed] [Google Scholar]
- 13.Clatts MC, Rodríguez-Díaz CE, García H, et al. Sexually transmitted infections clinics as strategic venues for targeting high-risk populations for HIV research and sexual health interventions. P R Health Sci J. 2011;30:101–108. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) [Accessed: November 11, 2011];Sexually Transmitted Diseases. Available at: Url: http://www.cdc.gov/std/general/default.htm.
- 15.World Health Organization (WHO) Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. [Accessed: November 18, 2011];Breaking the chain of transmission. Available at: Url: http://www.who.int/reproductivehealth/publications/rtis/9789241563475/en/index.html.
- 16.Figueroa JP, Brathwaite A, Morris J, et al. Rising HIV-1 prevalence among sexually transmitted disease clinic attenders in Jamaica: traumatic sex and genital ulcers as risk factors. J Acquir Immune Defic Syndr. 1994;7:310–316. [PubMed] [Google Scholar]
- 17.Cleghorn FR, Jack N, Murphy JR, et al. HIV-1 prevalence and risk factors among sexually transmitted disease clinic attenders in Trinidad. AIDS. 1995;9:389–394. [PubMed] [Google Scholar]
- 18.Rodrigues JJ, Mehendale SM, Shepherd ME, et al. Risk factors for HIV infection in people attending clinics for sexually transmitted diseases in India. BMJ. 1999;311:283–286. doi: 10.1136/bmj.311.7000.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang QQ, Chen XS, Yin YP, et al. HIV/STD pattern and its associated risk factors among male STD clinic attendees in China: a foci for HIV intervention. BMC Public Health. 2011;11:955. doi: 10.1186/1471-2458-11-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Báez Feliciano DV, Gómez MA, Fernández-Santos DM, et al. Profile of Puerto Rican HIV/AIDS patients with early and non-early initiation of injection drug use. Ethn Dis. 2008;18(2 Suppl 2):S2-99–104. [PubMed] [Google Scholar]
- 21.Báez-Feliciano DV, Quintana R, Gómez MA, et al. Trends in the HIV and AIDS epidemic in a Puerto Rican cohort of patients: 1992–2005. Bol Asoc Med PR. 2006;98:174–183. [PubMed] [Google Scholar]
- 22.Gomez MA, Fernandez DM, Otero JF, et al. The shape of the HIV/AIDS epidemic in Puerto Rico. Rev Panam Salud Publica. 2000;7:377–383. doi: 10.1590/s1020-49892000000600004. [DOI] [PubMed] [Google Scholar]
- 23.Hall HI, Song R, Rhodes P, et al. HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Prevalence and awareness of HIV infection among men who have sex with men - 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1201–1207. [PubMed] [Google Scholar]
- 25.Weinstock H, Dale M, Gwinn M, Satten GA, Kothe D, Mei J, et al. HIV seroincidence among patients at clinics for sexually transmitted diseases in nine cities in the United States. J Acquir Immune Defic Syndr. 2002;29:478–483. doi: 10.1097/00126334-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Colón-López V, Rodríguez-Díaz CE, Ortiz AP, et al. HIV-related risk behaviors among a sample of men who have sex with men in Puerto Rico: an overview of substance use and sexual practices. PR Health Sci J. 2011;30:65–68. [PMC free article] [PubMed] [Google Scholar]
- 27.Clatts MC, Rodriguez-Diaz CE, Garcia H, et al. A Preliminary Profile of HIV Risk in a Clinic-Based Sample of MSM in Puerto Rico: Implications for Health Promotion Interventions. P R Health Sci J. 2012;3:154–160. [PubMed] [Google Scholar]
- 28.Cooperman NA, Arnsten JH, Klein RS. Current sexual activity and risky sexual behavior in older men with or at risk for HIV infection. AIDS Educ Prev. 2007;19:321–333. doi: 10.1521/aeap.2007.19.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dougan S, Payne LJ, Brown AE, et al. Past it? HIV and older people in England, Wales and Northern Ireland. Epidemiol Infect. 2004;132:1151–1160. doi: 10.1017/s0950268804002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodley-Tickell AT, Olowokure B, Bhaduri S, et al. Trends in sexually transmitted infections (other than HIV) in older people: analysis of data from an enhanced surveillance system. Sex Transm Infect. 2008;84:312–317. doi: 10.1136/sti.2007.027847. [DOI] [PubMed] [Google Scholar]
- 31.Tan HH, Chan RK, Goh CL. Sexually transmitted diseases in the older population in Singapore. Ann Acad Med Singapore. 2002;31:493–496. [PubMed] [Google Scholar]
- 32.Bourne C, Minichiello V. Sexual behaviour and diagnosis of people over the age of 50 attending a sexual health clinic. Australas J Ageing. 2009;28:32–36. doi: 10.1111/j.1741-6612.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 33.Gindi RM, Erbelding EJ, Page KR. Sexually transmitted infection prevalence and behavioral risk factors among Latino and non-Latino patients attending the Baltimore City STD clinics. Sex Transm Dis. 2010;37:191–196. doi: 10.1097/OLQ.0b013e3181bf55a0. [DOI] [PMC free article] [PubMed] [Google Scholar]