Abstract
Although it is hypothesized that surface (like surface charge) and physical characteristics (like particle size) play important roles in cellular interactions of nanoparticles (NPs), a systematic study probing this issue is missing. Hence, a comparative cytotoxicity study quantifying nine different cellular endpoints, was performed with a broad series of monodisperse, well characterized silicon (Si) and germanium (Ge) NPs with various surface functionalizations. Human colonic adenocarcinoma Caco-2 and rat alveolar macrophage NR8383 cells were used, to clarify the toxicity of this series of NPs. The surface coatings on the NPs appeared to dominate the cytotoxicity: the cationic NPs exhibited cytotoxicity, whereas the carboxylic acid-terminated and hydrophilic PEG- or dextran-terminated NPs did not. Within the cationic Si NPs, smaller Si NPs were more toxic than bigger ones. Manganese-doped (1 % Mn) Si NPs did not show any added toxicity, which favors their further development for bioimaging. Iron-doped (1 % Fe) Si NPs showed some added toxicity, which may be due to the leaching of Fe3+ ions from the core. A silica coating seemed to impart toxicity, in line with the reported toxicity of silica. Intracellular mitochondria seem to be a target organ for the toxic NPs since a dose-, surface charge- and size-dependent imbalance of the mitochondrial membrane potential was observed. Such imbalance led to a series of other cellular events for cationic NPs, like decreased mitochondrial membrane potential (ΔΨm) and ATP production, induction of ROS generation, increased cytoplasmic Ca2+ content, production of TNF-α and enhanced caspase-3 activity. Taken together, the results explain the toxicity of Si NPs/Ge NPs largely by their surface characteristics, provide insight in the mode of action underlying the observed cytotoxicity, and give directions on synthesizing biocompatible Si and Ge NPs, as this is crucial for bioimaging and other applications in for example the field of medicine.
Introduction
Nanoparticles (NPs), with their unconventional properties1 and ability to interact with a wide variety of biomolecules, have the potential to revolutionize medicine, including diagnostics and therapeutics.2, 3 Unfortunately, often the applicabilities of different NPs are hampered by their toxicity.4, 5 Hence, there is a rapidly growing interest in understanding the toxicity of such NPs in more detail, with the aim to control and minimize this toxicity.
Semiconductor quantum dots (SCQDs), like silicon NPs (Si NPs) or germanium NPs (Ge NPs), have recently received significant attention because they can be made into multipotent and biocompatible NPs.6, 7 Si or Ge NPs can be used as vehicles for drug delivery,8, 9 but perhaps the most exciting application of SCQDs can be foreseen in the field of bioimaging.10, 11 The Si NPs or Ge NPs, due to their very small sizes (< 5 nm) and intrinsic fluorescence, enjoy an edge over other NP (like polymer NPs/PNPs)12 and carbon NPs13) that need to be functionalized to be fluorescent.
The potential to provide intrinsically non-toxic NPs also makes Si and Ge NPs highly interesting systems. In biological systems the Si NPs may possibly degrade to silicates, although the alkyl surface groups have been reported to retard such breakdown especially in case of porous Si NPs.14 For example, in human beings, Si is converted to orthosilicic acid/Si(OH)4 and is excreted in the urine.15, 16 Moreover, since they can be synthesized with diameters < 5 nm, the sizes of Si NPs and Ge NPs are often below the size threshold for renal clearance for NP (< 5.5 nm) enabling elimination.17 In addition, unlike the popular but intrinsically toxic Cd-based bioimaging agents, such as CdSe/ZnS quantum dots (QDs),15 Si or Ge are mostly non-toxic. In line with this, it has been reported for a set of well-characterized, monodisperse (size 1.6 ± 0.2 nm) and fluorescent Si NPs18, 19 that Si NPs may display toxicity or non-toxicity, against human colonic adenocarcinoma Caco-2 and rat alveolar macrophage NR8383 cells, depending on their surface functionalization (Si-C3H6-NH3+, Si-C4H8-N3 and Si-C11H22-COO−)10, 18, 20, 21 although many other variations of synthesis of Si NPs exist.22-33 It was found that cationic Si NP-NH3+ were toxic,34 while the anionic Si NP-COO− displayed no discernible toxicity in two different toxicity tests (MTT and BrdU).21
It has been proposed that the surface properties of NP determine their interactions with biological systems,35-37 although little data based on systematic investigations are available. Fortunately, a wide array of synthetic methods has become available for the preparation of surface-functionalized Si and Ge NPs.38, 39 Therefore, a comparative study is now possible, in which the toxicity of the different Si NPs and Ge NPs can be determined with respect to their surface characteristics. This can help to gain insight in how surface factors of these NPs can influence the toxicity, although n-alkyl terminated uncharged and water-insoluble Si NPs were also reported to show adequate cellular uptake without causing toxicity.40 To obtain a generally applicable hypothesis, we therefore performed a systematic investigation in which a large series of water-soluble Si NPs and Ge NPs with different properties (sizes, synthetic origin and surface functionalities) were tested for their possible adverse cellular effects.
Rat alveolar macrophage NR8383 and human colonic adenocarcinoma Caco-2 cells provide two adequate in vitro testing models for the Si NPs and Ge NPs. The NR8383 lung cells, being macrophages, act as the first line of defense against air-borne foreign pathogens and a toxic effect imparted on them by the NP can give an idea on how the NP can influence the innate immune system. Similarly, eyeing the increasing number of food-based applications of different NP (like silica NP), Caco-2 cells being a human colonic cell line, can be an excellent model to test cytotoxicity and extrapolate the data to in vivo situations. Due to the ample reported data on the toxicity of NP on these two cell lines,12, 20, 41 a systematic and comparative toxicity testing with a mechanistic perspective can be performed. It is yet not fully clear what might be the mechanism of cytotoxicity for NP. Although oxidative stress had been recognized as a mechanism,42, 43 some recent reports counter this view and identified intracellular mitochondria as the target and perhaps the starting point of cytotoxicity. Bhattacharjee et al.20 have shown that an isolated mitochondrial fraction from rat liver produced reactive oxygen species (ROS), after being exposed to cationic Si NP-NH3+. A recent hypothesis to explain the cytotoxicity of especially the cationic NP is that the cationic NP can interfere with the mitochondrial membrane and decouple the electron transport chain (ETC).44, 45 This in turn may lead to an induction of intracellular ROS (like superoxides, peroxides, hydroxyls) production as well as to leaching out of the sequestered calcium from the mitochondria. Interestingly, this can lead to a cytoplasmic calcium overload and initiation of apoptotic cascades. Additionally, a de-coupling of the ETC can cause a decreased cellular ATP production, which may compromise the cellular viability.
The objective of the current paper is thus to investigate the cytotoxicity of a broad series of Si and Ge NPs. To this aim the following features were measured in both the NR8383 and Caco-2 cells after 24 h exposure to the different Si NPs or Ge NPs (see the quoted references for more detailed expositions of these nine endpoints): 1) cell viability by MTT assay,46 2) cell proliferation by BrdU assay,21 3) induction of ROS from isolated rat liver mitochondrial fraction by DCFH-DA assay,20 4) change in mitochondrial membrane potential (ΔΨm),44 5) cellular ATP content, 44 6) cellular ROS production by DCFH-DA assay,20 7) cytoplasmic free Ca2+ concentration,47 8) caspase-3 activity,44 as a biomarker for apoptotic pathways, and 9) production of TNF-α,48 as a pro-inflammatory marker. Finally, the obtained data were assembled to propose a series of events that ultimately lead to the toxicity (or lack thereof) of surface-functionalized Si and Ge NPs, and to provide directions to obtain non-toxic Si and Ge NPs.
Materials and Methods
Si NPs and Ge NPs
The fully characterized different Si NPs and Ge NPs were obtained as aqueous dispersions. Exposure ranges of the different Si NPs and Ge NPs (concentration 0-100 μg/ml) were prepared by mixing the aqueous dispersion of NP with cell culture media (F12-K or DMEM).
NR8383 cells
Rat alveolar macrophage (NR8383) cells were obtained from ATCC (Manassas, VA). The NR8383 cells were cultured in 150 cm2 cell culture flasks with 25 ml F12-K culture medium (Gibco 21127) supplemented with 10 % (v/v) heat inactivated fetal calf serum (FCS) in a humidified atmosphere containing 5 % CO2 at 37 °C. The cells were sub-cultured every two weeks. Cells with passage numbers 30-40 were used.
Caco-2 cells
The Caco-2 cells were obtained from ATCC (Rockville, MD) and were cultured in DMEM medium (Gibco), fortified with 10 % (v/v) heat-inactivated fetal calf serum (FCS) and 50 mg/ml gentamicin, in a humidified atmosphere at 5 % CO2 and 37 °C in 75 cm2 flasks.20 After reaching a ~70 % confluence, the Caco-2 cells were sub-cultured, after rinsing with phosphate buffered saline (PBS), using trypsin (Gibco, Paisley, UK). Only cells within passage numbers 30-40 were used.
MTT assay
NR8383 cells
In this assay, the mitochondrial activity is determined photometrically by measuring the amount of MTT salt converted to insoluble formazan crystals by mitochondrial reductase enzymes. An NR8383 cell suspension was collected and centrifuged at 140 g for 5 min before resuspending the cell pellet in medium followed by counting and adjusting the cellular concentration to 2 × 105 cells/ml.20 The cells were then seeded in a 96-well plate (50 μl/well) and the plate was kept in a 5 % CO2 incubator at 37 °C for 24 h. Next day, 50 μl of serial dilutions of Si NPs or Ge NPs were added to the cells to obtain the required final concentration range (0-100 μg/ml) of Si NPs or Ge NPs, and then the plates were incubated for 24 h. After 24 h 5 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution in PBS (5 mg/ml) was added to each well and the plate was incubated for another 4 h. Then 100 μl of pure DMSO was added to each well to dissolve the formazan crystals. Now the absorption of each well was measured at 562 nm in a 96-well plate reader and the background absorption at 612 nm was subtracted. Mitochondrial metabolic activity for each concentration of Si NPs was expressed as % of the value of the corresponding negative control. F12-K medium without NPs and medium with Triton-X (0.01%) were used as negative and positive controls respectively. Control experiments were done to exclude a possible reaction between MTT salt and Si NPs or Ge NPs by mixing test concentration of the Si NPs or Ge NPs with MTT reagent.
Caco-2 cells
The Caco-2 cells were plated at a concentration of 105 cells/ml in a 96-well plate (100 μl/well) and were incubated for 24 h.20 Then the Si NPs or Ge NPs were added to the cells in a total volume of 100 μl at final exposure concentrations of 0-100 μg/ml. After this, the cells were incubated for another 24 h. After 24 h exposure to the Si NPs or Ge NPs, 5 μl MTT solution in PBS (5 mg/ml) was added to each well and incubated for 4 h. Then, the medium was removed and 100 μl of DMSO was added to dissolve the formed formazan crystals. The plates were put in the plate shaker for 5 min. The absorbance at both 562 nm and 612 nm was measured. The mitochondrial metabolic activity was expressed as the mean percentage of the negative control values (0 μg/ml). 0.01% Triton-X was used as positive control and DMEM medium without Si NPs or Ge NPs was used as negative control. Control tests were also done to exclude interfering reactions between the NPs and the MTT solution.
BrdU assay
NR8383 cells
The NR8383 cells were plated and exposed to Si NPs or Ge NPs (final exposure concentration 0-100 μg/ml) as described before. Cell proliferation was quantified using the colorimetric BrdU (5-bromo-2-deoxyuridine) assay (catalogue no. 647229001, Roche Diagnostics, Penzberg, Germany). BrdU acts as a structural analogue of thymidine and will be incorporated in newly synthesized DNA during cell replication and hence indicates cell proliferation.21 After 24 h incubation with NP and BrdU, 100 μl of the BrdU labeling solution was added to each well followed by an incubation for 4 h. The immunoassay was then performed as instructed by the manufacturer. A 0.01% Triton-X solution in F12-K medium was used as positive control and F12-K medium without Si NPs or Ge NPs was used as negative control.
Caco-2 cells
The Caco-2 cells were plated and exposed to Si NP or Ge NP as described before.21 After incubation of the Caco-2 cells with the Si NPs or Ge NPs and BrdU for 24 h, the medium containing the Si NPs or Ge NPs and BrdU was removed, and the BrdU labeling solution was added to the wells and incubated for 4 h. Subsequently, the immunoassay was performed according to the protocol of the manufacturer. Results were expressed as the mean % of the negative control (0 μg/ml) values. 0.01% Triton-X in DMEM medium and DMEM medium without Si NPs or Ge NPs were used as positive and negative controls, respectively.
Induction of ROS from isolated mitochondrial fraction from rat liver by DCFH-DA assay
An isolated mitochondrial fraction from rat liver tissue was prepared as described before.20 The isolated mitochondrial fraction (3 mg pellet/ml in PBS) was plated in a 96-well plate (50 μl/well) and serial dilutions of Si NPs or Ge NPs and 5 μl of 20 mM DCFH-DA (2′,7′-dichlorofluorescein diacetate) probe (catalogue no. D6883/ Sigma Aldrich Chemie BV) were added. The plate was incubated for 90 min at 37 °C in a humidified 5 % CO2 atmosphere. The plate was then measured at λex = 485 nm and λem = 538 nm. Medium without Si NPs or Ge NPs and with 75 μM DNP in DMSO were used as negative and positive controls, respectively. Results were expressed as % of negative control (0 μg/ml).
Measurement of the mitochondria membrane potential (ΔΨm)
The NR8383 and Caco-2 cells were plated as described above and exposed to serial test concentrations of NPs (0 - 100 μg/ml). The mitochondrial membrane potential (ΔΨm) was then measured by a commercially available kit from Invitrogen (MitoProbe™ Transition Pore Assay Kit; catalogue no. M34153) and the results were expressed as % of the negative control (0 μg/ml). A 100 μM solution of F12-K or DMEM medium containing ionomycin and medium without NPs were used as positive and negative controls, respectively.
Measurement of intracellular ATP content
The NR8383 and Caco-2 cells were seeded in a 96-well plate and exposed to different Si NPs or Ge NPs as mentioned before. After 24 h, the intracellular ATP content of each well was measured by a commercial ATP measuring kit (Sigma Aldrich, Product No. FLASC) and results were expressed as % of the negative control (0 μg/ml). Cells exposed to medium without NPs and to medium with 75 mM 2,4-DNP (2,4-dinitrophenol) were used as negative and positive controls, respectively.
Measurement of cytoplasmic free Ca2+ content
The NR8383 and Caco-2 cells were plated and exposed to the serial test concentration range before measuring the cytoplasmic free calcium content by a commercially available kit from the Invitrogen (Fluo-4 Direct™ Calcium Assay Kit; catalogue no. F10472). Only F12-K or DMEM medium without NPs (0 μg/ml) was used as negative control and the results were expressed as % of the negative control.
Measurement of intracellular ROS by DCFH-DA assay
NR8383 cells
The cell suspension was adjusted to 2 × 105 cells/ml and seeded in a 96-well plate (50 μl/well) in F12-K medium. 50 μl/well of serial dilutions of Si NPs or Ge NPs in F12-K medium were added to obtain the required final concentrations of Si NPs or Ge NPs. A final concentration of 10 mM H2O2 in F12-K medium was used as positive control and F12-K medium without NP as negative control. After 6 h of exposure to the Si NPs or Ge NPs, 5 μl of a 20 mM solution of DCFH-DA were added to each well and the plates were incubated for another 18 h in a 5 % CO2 atmosphere at 37 °C. The fluorescence was then measured at λex = 485 nm and λem = 538 nm. The fluorescence induction factor for each concentration of Si NPs or Ge NPs was then calculated by dividing the reading of each well with the average reading of the negative control (0 μg/ml) and expressed as %. Control experiments were performed by incubating the Si NPs or Ge NPs at their test concentrations with DCFH-DA in the absence of cells to check the possibility of a positive fluorescence reading caused by reaction with NP alone.
Caco-2 cells
The cells were suspended in DMEM medium to a concentration of 1 × 105 cells/ml after trypsinization and were plated in a 96-well plate (100 μl/well). After 24 h the cells were exposed to 100 μl/well of final concentrations of Si NPs or Ge NPs. Following another 6 h, 5 μl of a 20 mM solution of DCFH-DA in DMSO was added to each well. The plate was further incubated for 18 h before measurement of the fluorescence was carried out as described above. Control experiments were performed by incubating the Si NPs or Ge NPs at their test concentrations with DCFH-DA in the absence of cells to check the possibility of a positive fluorescence reading caused by reaction with Si NPs or Ge NPs alone.
Measurement of TNF-α
The NR8383 and Caco-2 cells were seeded in a 96-well plate and exposed to different Si NPs or Ge NPs as mentioned before. After 24 h, the TNF-α content of each well was measured by a commercial TNF-α measuring kit (Invitrogen, catalogue no. KRC3011) and results were expressed as % of negative control (0 μg/ml). Medium without NPs and medium with 0.1 μg/ml lipopolysaccharide were used as negative and positive controls, respectively.
Measurement of caspase-3 activity
With prior plating and exposure of the NR8383 and Caco-2 cells to serial test concentrations of Si NPs or Ge NPs, the caspase-3 levels were measured by a commercially available kit from Sigma Aldrich Chemie BV (CASP3C). Results were expressed as % of negative control (0 μg/ml).
Statistical analysis
The data were analyzed and plotted with the Origin Pro (Version 8.0) software. The results were presented as arithmetic mean (n = 3) ± standard error of mean (SEM).
Results and Discussion
Si NPs and Ge NPs
The Si NPs and Ge NPs under investigation were obtained via four different synthetic approaches10, 18, 21, 49-52 in order to have a diverse mix of NPs with a wide range of surface properties. These NPs were all prepared from a Si or Ge core, which were subsequently surface-functionalized with different groups. The detailed characterization data including the abbreviations used for each of them are given in Table 1. This collection of Si NPs and Ge NPs could be classified into four groups based on their source: (1) Si NPs (1.6 ± 0.2 nm), synthesized from SiCl4, with surface functionalizations of amine (Si(1.6) NP-NH2), carboxylic acid (Si(1.6) NP-COOH) and azide (Si(1.6) NP-N3).10, 18, 21 These Si(1.6) NP showed emission in the blue region upon excitation with UV light and their toxicity, or lack thereof, has been reported before;20, 21 these data are added for reference. The Si(1.6) NP-NH2 were the only cytotoxic NPs within these three Si(1.6) NP, whereas the Si(1.6) NP-COOH did not show any cytotoxicity up to 3 μg/ml.20, 21 The Si(1.6) NP-N3 were toxic only at higher concentrations of > 2 μg/ml. (2) Amine-terminated Si NP (3.9 ± 1.3 nm) synthesized from Zintl salts (NaSi1-xMx, x = 0.0, 0.05, 0.1, 0.15; M = Mn, Fe), further on referred to as: Si(3.9) NP-NH2. The synthetic route used allows the doping of these Si NP with 1 % manganese (SiMn(3.9) NP-NH2), or 1 % iron (SiFe(3.9) NP-NH2). These three Si NPs were then also coated with dextran, referred to as Si(3.9) NP-NH2-Dex, SiMn(3.9) NP-NH2-Dex and SiFe(3.9) NP-NH2-Dex. (3) Ge NPs (average size 5.5 ± 2.5 nm) with surfaces functionalized with polyethylene glycol (Ge NP-PEG) or N,N,N-trimethyl-3(1-propyne) ammonium iodide (Ge NP-TMPA) and Si NPs, surface-functionalized with PEG (Si NP-PEG).52 (4) Si NPs (average size 2.5 ± 0.5 nm) functionalized with undecenylic acid (Si NP-UDA),53 or linked via a dodecyl chain to a coating of poly(maleic anhydride)-based amphiphilic polymer (Si NP-Pol),51 or silica (Si NP-Sil)54 with average sizes of 17.8 ± 0.4 nm and 35 ± 5 nm, respectively. All the Si NPs and Ge NPs were well characterized with the dynamic light scattering (DLS) data on the dextran coated Si NP provided as Electronic Supplementary Information (ESI). More extensive characterization of the NP involved, can be found elsewhere.21, 49, 50 For the cluster of Si(1.6) NP-NH2, Si(1.6) NP-COOH and Si(1.6) NP-N3, the published reports20, 21 already provide us with knowledge on the toxicity which will be discussed in later sections. In the next group comprising Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2 and dextran coated Si(3.9) NP-NH2-Dex, SiMn(3.9) NP-NH2-Dex, SiFe(3.9) NP-NH2-Dex, the comparison can be useful in several ways. As this group contained Si(3.9) NP-NH2 bigger in size than Si(1.6) NP-NH2, an indication of the influence of NP size on their cytotoxicity can be obtained. The toxicity of the Mn- or Fe-doped Si(3.9) NP can be compared to both Si(1.6) NP-NH2 and Si(3.9) NP-NH2 to find if the Mn or Fe dopants had any added toxic effects. This is important to learn, as the SiMn(3.9) NP-NH2 and SiFe(3.9) NP-NH2 have tremendous potential to be developed as bioimaging agents in the future and hence, an exacerbated toxicity is undesirable.
Table 1.
Data on different Si NPs and Ge NPs used in this study
| NP | Surface Functionalization |
Dopant | Diameter (nm) |
Abbreviations used |
Graphical presentation |
|---|---|---|---|---|---|
| Si NP | Si-C3-NH2 | No | 1.6 ± 0.2 | Si(1.6) NP-NH3+10 |
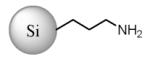
|
| Si-C11-N3 | Si(1.6) NP-N3 |
|
|||
| Si-C4-COOH | Si(1.6) NP-COO−21 |
|
|||
| Si NP | SiM-C3-NH2 | No | 3.9 ± 1.3 | Si(3.9) NP-NH3+50 |
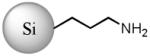
|
| Mn (1 %) |
SiMn(3.9) NP-NH3+49 |
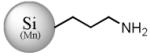
|
|||
| Fe (1 %) |
SiFe(3.9) NP-NH3+ |
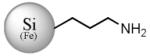
|
|||
| Si NP | SiM-C3-NH2-Dex | No | 3.9 ± 1.3 | Si(3.9) NP-NH2- Dex |
|
| Mn (1 %) |
SiMn(3.9) NP-NH2- Dex |
|
|||
| Fe (1 %) |
SiFe(3.9) NP-NH2- Dex |
|
|||
| Ge NP | Ge-PEG | No | 5.5 ± 2.5 | Ge NP-PEG52 |
|
| Ge-TMPA | Ge NP-TMPA52 |
|
|||
| Si NP | Si-PEG | Si NP-PEG52 |
|
||
| Si NP | Si-undecenylic acid | 2.9 ± 0.5 (core) |
Si NP-UDA53 |
|
|
| Si-C12-Pol | 17.8 ± 0.4 (hydro- dynamic) |
Si NP-Pol51 |

|
||
| Si-C12-Sil | 35 ± 5 | Si NP-Sil |

|
The dextran-coated Si NPs gave an interesting scope to compare the toxicity and find out whether the dextran coating can alleviate the toxicity. For the following group of Ge NP-PEG, Ge NP-TMPA and Si NP-PEG, a comparative investigation may reveal several important things. It has been claimed before that the balance between the hydrophilicity and hydrophobicity of NP surface coatings is important for the NP to exert cytotoxicity.55, 56 The Ge NP-PEG, Ge NP-TMPA, Si NP-Pol and Si NP-PEG differ in hydrophilicity and hence, an idea can be developed on the role of hydrophilicity on the toxic effects of Si NPs or Ge NPs. Furthermore, to investigate what effect the addition of PEG to the surface has, the toxicity of Si NP-PEG, can be compared with Si(1.6) NP-NH2 or Si(3.9) NP-NH2. In case of the group of Si NP-UDA and Si NP-Sil, it is relevant to investigate if toxicity is detected in spite of the fact that the Si NP-Sil had a non-toxic Si core, as silica is often reported to be toxic.57, 58
MTT assay
The MTT assay measures the mitochondrial metabolic activity of the cells and this can be expressed as % cell viability of the negative control. In this study, the MTT assay was performed on both NR8383 and Caco-2 cells exposed to serial dilutions of Si NP or Ge NP (Fig. 1) for 24 h. From the results, it could be seen that only Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2, Ge NP-TMPA and Si NP-Sil caused a dose-dependent reduction in cell viability, whereas the remaining Si NP/Ge NP did not show any toxicity within the tested concentration range (0 - 100 μg/ml). In the study by Bhattacharjee et al.,20, 21 among the Si(1.6) NP-NH2, Si(1.6) NP-N3 and Si(1.6) NP-COOH, only Si(1.6) NP-NH2 was found to be toxic, followed by Si(1.6) NP-N3, but only at comparatively high concentrations (> 2 μg/ml).
Fig. 1. MTT assay on NR8383 and Caco-2 cells after 24 h exposure to.
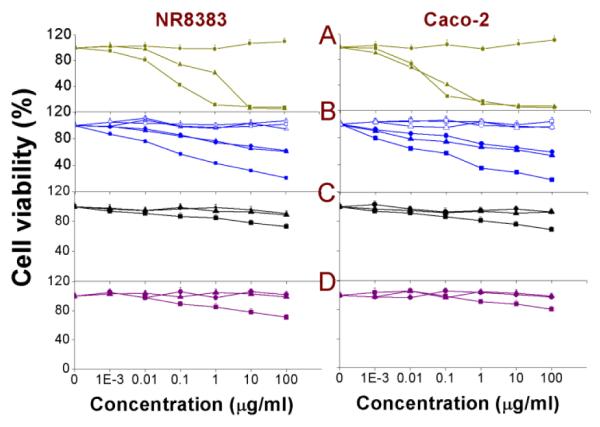
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●),SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●),SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± standard error of mean (SEM) (n = 3).
). Results are shown as mean ± standard error of mean (SEM) (n = 3).
While the toxicity of amine-terminated NPs is thus in line with previous observations, it is noteworthy that the addition of Mn doping did not yield any increased toxicity of SiMn(3.9) NP-NH2 in comparison to Si(3.9) NP-NH2. Such an increased toxicity upon doping was, however, observed for the iron-doped SiFe(3.9) NP, which may be attributed to the leaching out of Fe ions from the Si NPs core to the cellular environment or to the NPs surfaces, being responsible for the concomitant increase in overall toxicity. Perhaps the most relevant finding of this test was that coating the NP with PEG or dextran reduced the toxicity to nearly nil, even if the NP was doped with Fe or Mn. Apparently, the coating effectively blocked any leaching out in the case of Fe-doped Si NP and/or removes any toxicity of the remaining amine-groups or surfaced Fe ions ‘hidden’ under the polymeric coating. The EC50 values of all the NPs measured by the MTT assay are given in Table 2 for comparison.
Table 2.
The EC50 values (μg/ml) for different Si NPs/Ge NPs obtained for the various endpoints reported in this article
| Experiment | Fig. | Si(1.6) NP-NH2 | Si(1.6) NP-N3 | Si(3.9) NP-NH2 | SiMn(3.9) NP-NH2 | SiFe(3.9) NP-NH2 | Ge NP-TMPA | Si NP-Sil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR8383 | Caco-2 | NR8383 | Caco-2 | NR8383 | Caco-2 | NR8383 | Caco-2 | NR8383 | Caco-2 | NR8383 | Caco-2 | NR8383 | Caco-2 | ||
| MTT | 1 | 0.01220 | 0.01421 | 0.2720 | 0.3121 | 0.38 | 0.16 | 0.4 | 0.17 | 0.07 | 0.03 | 0.17 | 0.16 | 0.12 | 0.58 |
| BrdU | 2 | 0.02 | 0.01121 | 0.26 | 0.2821 | 0.15 | 0.12 | 0.16 | 0.14 | 0.08 | 0.05 | 0.22 | 0.21 | 0.18 | 0.37 |
| Ψ m | 4 | 0.02 | 0.017 | 0.18 | 0.31 | 0.19 | 0.21 | 0.18 | 0.22 | 0.09 | 0.08 | 0.17 | 0.22 | 0.23 | 0.41 |
| ATP | 5 | 0.01 | 0.02 | 0.11 | 0.14 | 0.16 | 0.15 | 0.17 | 0.16 | 0.04 | 0.06 | 0.19 | 0.2 | 0.33 | 0.45 |
| Cytoplasmic free Ca2+ |
6 | 0.05 | 0.04 | 0.19 | 0.17 | 0.18 | 0.21 | 0.13 | 0.18 | 0.08 | 0.07 | 0.15 | 0.27 | 0.28 | 0.39 |
| ROS (DCFH-DA) |
7 | 0.02220 | 0.01820 | 0.1720 | 0.3120 | 0.21 | 0.22 | 0.2 | 0.23 | 0.09 | 0.08 | 0.18 | 0.19 | 0.29 | 0.34 |
| TNF-α | 8 | 0.1 | 0.2 | 0.18 | 0.28 | 0.18 | 0.19 | 0.22 | 0.21 | 0.08 | 0.11 | 0.19 | 0.21 | 0.33 | 0.39 |
| Caspase-3 | 9 | 0.11 | 0.24 | 0.21 | 0.26 | 0.16 | 0.24 | 0.18 | 0.23 | 0.09 | 0.15 | 0.22 | 0.18 | 0.27 | 0.48 |
| DCFH-DA (isolated mitochondrial fraction) |
3 | 0.0820 | 1.0520 | 0.28 | 0.29 | 0.12 | 0.11 | 0.34 | |||||||
BrdU assay
5-Bromo-2-deoxyuridine (BrdU) is a structural analogue of the thymidine base of DNA and gets incorporated within the strands of the DNA of proliferating cells and the amount of incorporated BrdU can then be measured spectrophotometrically.59 As an adjunct to the MTT assay, which gives an idea on the effect on mitochondrial metabolic activity, the BrdU assay displays a DNA-based degree of continuing cell divisions, with the results presented as % of the negative control. The results obtained after 24 h exposure of NR8383 and Caco-2 cells towards different Si NP and Ge NP are shown in Fig. 2 and the EC50 values are given in Table 2. From Figure 2, it can be seen that apart from the amine-terminated Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2 and Ge NP-TMPA, only Si NP-Sil were slightly cytotoxic, which matched well with the MTT assay data. The SiMn(3.9) NP-NH2 did not show any added toxicity over Si(3.9) NP-NH2, which encourages their further development as imaging agents.
Fig. 2. BrdU assay on NR8383 and Caco-2 cells after 24 h exposure to.
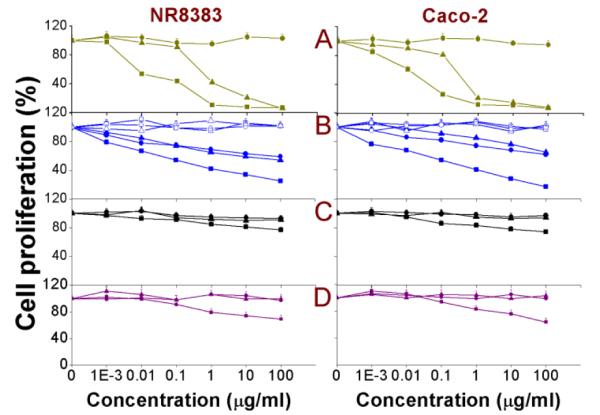
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
The SiFe(3.9) NP-NH2 again showed enhanced toxicity compared to the Si(3.9) NP-NH2, which – as discussed with the MTT assay – can be an effect of the leaching Fe3+ ions from the core to the cellular environment or NP surfaces. In line with what was observed in the MTT assay, the dextran coating curbed the toxicity of not only SiFe(3.9) NP-NH2 but also of the Si(3.9) NP-NH2 and SiMn(3.9) NP-NH2. The slight toxicity of Si NP-Sil can be attributed to the silica shell of these NP. All the other Si NP/ Ge NP were found to be non-toxic. In contrast to the positively charged NP, the -OH or -COOH terminated Si NP/ Ge NP did not show any effect on cell proliferation.60 A possible reason behind lesser cytotoxicity is the comparatively reduced cellular uptake of anionic NPs compared to the cationic NPs. Various groups have observed such surface charge-dependent cellular uptake59, 60 and this phenomenon could also affect the toxicity of NP and the role of surface properties in it.
Induction of Reactive Oxygen Species (ROS) from isolated rat liver mitochondrial fraction by DCFH-DA assay
The mitochondrial fraction of liver tissue from a Wistar rat was prepared as reported before,20 and these isolated mitochondria were incubated with serial dilutions of Si NPs and Ge NPs. DCFH-DA was used for the detection of reactive oxygen species (ROS). DCFH-DA is cleaved by nonspecific intramitochondrial esterases61 to form DCFH. DCFH is further oxidized by ROS to form the fluorescent compound DCF (2′,7′-dichlorofluorescein), which was then measured (λex = 485 nm; λem = 538 nm); the results are shown in Fig. 3 and the EC50 values are given in Table 2.
Fig. 3. DCFH-DA assay on isolated rat liver mitochondrial fraction after 1.5 h exposure to.
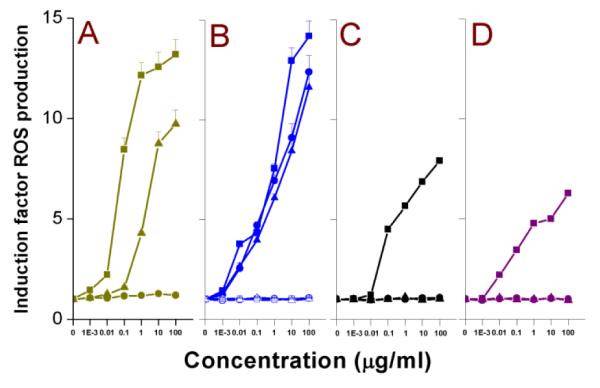
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
From our data, it is clear that only the cationic Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2, Ge NP-TMPA and the hydroxyl-capped Si NP-Sil induced ROS production upon incubation with the isolated mitochondrial fraction. PEG-terminated Ge and Si NPs displayed no discernible ROS production. The ROS production induced by all the Si(3.9) NP-NH2, SiMn(3.9) NP-NH2 and SiFe(3.9) NP-NH2 was decreased to almost none by the covalently bound dextran coating. How cationic NP show enhanced ROS production when incubated with isolated mitochondria is not fully understood, although as hypothesized for the outer cell membrane, electrostatic interaction between the negative lipid bilayer membranes and positive NP may be a cause. Such interaction between mitochondria and especially cationic polystyrene NPs has been noted before,44 although to the best of our knowledge this is one of the first cases where such an interaction between intracellular mitochondria and semiconductor quantum dots were shown.
Measurement of mitochondrial membrane potential (ΔΨm)
The change of the mitochondrial membrane potential (ΔΨm) can be an important parameter in understanding the mechanism of toxicity of NPs. A change in ΔΨm indirectly shows the alteration in the mitochondrial membrane permeability and might cause disruption of the electron transport chain (ETC). This may subsequently yield a decrease in ATP production and induction of ROS production. The ΔΨm in both the NR8383 and Caco-2 cell lines was measured after 24 h exposure to different Si NPs and Ge NPs, and the results along with the corresponding EC50 values are shown in Fig. 4 and Table 2, respectively. Only exposure of the cells to the cationic amine-terminated NP as well as to the Si NP-Sil resulted in a decrease in the ΔΨm in contrast to exposure of the cells to anionic or PEG-terminated Si NP and Ge NP. Interestingly, a dextran coating over the Si(3.9) NP-NH2, SiMn(3.9) NP-NH2 and SiFe(3.9) NP-NH2 minimizes also the effects on ΔΨm.
Fig. 4. Mitochondrial membrane potential (Ψm) in NR8383 and Caco-2 cells after 24 h exposure to.
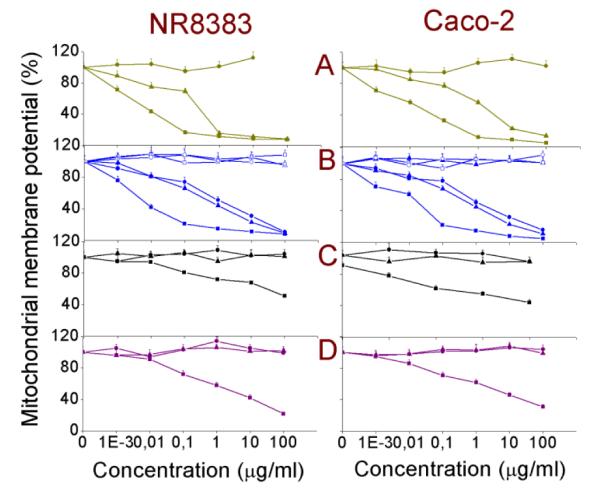
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
Measurement of intracellular ATP production
To investigate more deeply the effects of the interaction of the different Si NPs and Ge NPs with mitochondrial membranes and the probable disruption of the ETC, the intracellular ATP content was measured. The results are shown in Fig. 5 and the EC50 values are given in Table 2. In line with the observations made before, only exposure to the cationic Si(1.6) NP-NH2, Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2, Ge NP-TMPA, apart from the Si(1.6) NP-N3 and Si NP-Sil, resulted in a decrease in intracellular ATP production. This further strengthens our hypothesis that the interaction of cationic NPs with the outer layer of mitochondrial membranes disrupts the ETC, which then results in a decreased ATP production. Interestingly, depletion of ATP can be a contributing factor to the toxicity of the Si NPs or Ge NPs. In line with the MTT and the BrdU assay data, the dextran coating basically removed the toxicity of Si(3.9) NP-NH2, SiMn(3.9) NP-NH2 and SiFe(3.9) NP-NH2. While similar findings have been reported for 60-300 nm polystyrene NP44 and polydisperse (6—20 nm) starch-coated Ag NP,62 this is the first report of the effect of NP with different surface charges on the cellular ATP production for NP that are smaller than the critical diameter of 5.5 nm, which is required for efficient renal clearance.18
Fig. 5. Cellular ATP content in NR8383 and Caco-2 cells after 24 h exposure to.
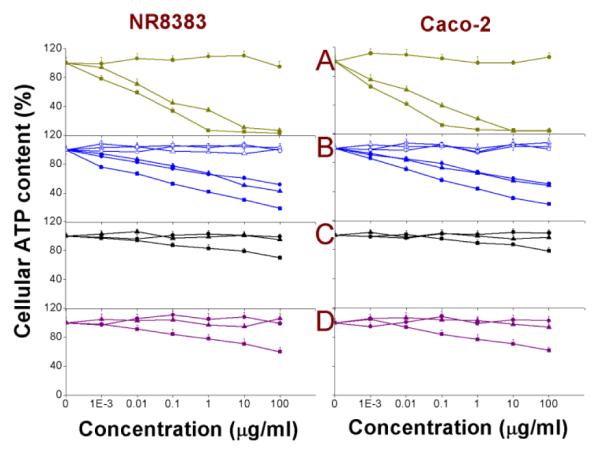
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
Measurement of cytoplasmic free Ca2+
The cytoplasmic free calcium concentration is important in many aspects regarding the physiology of the cells. An increased free calcium content can not only disturb the ionic contents (K+, Na+, etc.) of the cellular cytoplasm, but can also trigger the apoptotic cascade that leads to programmed cell death. Here, the NR8383 and Caco-2 cells were exposed for 24 h to different test concentrations (0-100 μg/ml) of Si NPs and Ge NPs and cytoplasmic free Ca2+ levels were quantified. The results are shown in Fig. 6. The corresponding EC50 values are given in Table 2. The cationic NP and Si NP-Sil showed mild to moderate increase in cytoplasmic free calcium (order: (SiFe(3.9) NP-NH2 > Si(1.6) NP-NH2 = SiMn(3.9) NP-NH2 > Si(1.6) NP-N3 > Ge NP-TMPA > Si NP-Sil) whereas no such increase could be seen for the anionic NP or PEG-terminated and dextran-coated NPs.
Fig. 6. Cellular free calcium in NR8383 and Caco-2 cells after 24 h exposure to.
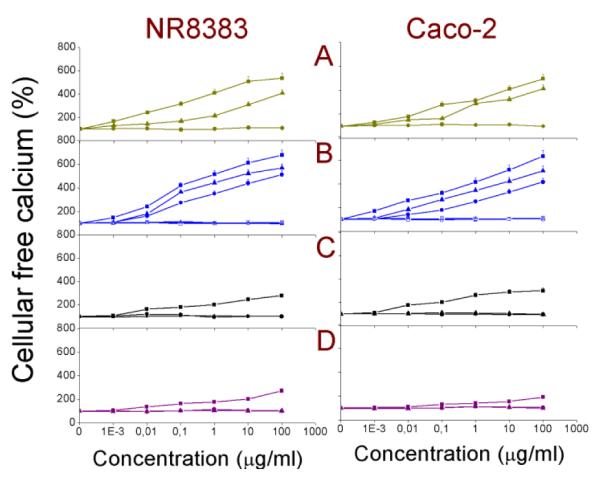
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
In order to better understand our findings of especially cationic NPs causing induction of ROS production in mitochondria along with causing a reduction in the cellular ATP concentration, the intracellular ROS concentration was measured. With data pointing towards possible damage caused by cationic Si NPs and Ge NPs on the mitochondrial membrane and decoupling of the electron transport chain, it is possible that the exaggerated production of ROS may cause oxidative stress. Several groups have hypothesized oxidative stress as the mechanism of NP cytotoxicity,64-66 although the source of ROS is still not clear.
It is possible that damaged mitochondria with compromised outer membrane integrity can be a source for the production of intracellular ROS. This would also suggest that intracellular oxidative stress is rather a secondary mechanism, which appears as a follow-up event to that of the mitochondrial interaction with cationic Si NPs or Ge NPs. The results of the DCFH-DA assay performed to measure the intracellular ROS production are shown in Fig. 7, with the EC50 values given in Table 2. In line with previous results, exposure of the cells to Si NP-Sil and the cationic Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2 and Ge NP-TMPA resulted in an increase in intracellular ROS production. The negatively charged Si (1.6) NP-COOH, Si NP-UDA, and the dextran- or PEG-coated Si or Ge NPs did not induced ROS production.
Fig. 7. DCFH-DA assay on NR8383 and Caco-2 cells after 24 h exposure to.
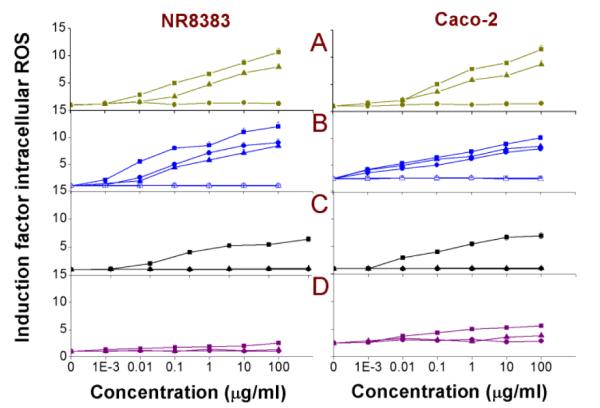
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n = 3).
). Results are shown as mean ± SEM (n = 3).
Measurement of TNF-α
As a result of the oxidative stress and the injury inflicted by the different ROS radicals, an inflammatory response can be anticipated. The cytokine TNF-α (tumor necrosis factor-alpha or cachectin) is a pro-inflammatory biomarker that can be measured to identify an inflammatory response (see Fig. 8). Only the amine-terminated NP (Si(1.6) NP-NH2, Si(3.9) NP-NH2, SiMn(3.9) NP-NH2, SiFe(3.9) NP-NH2, Ge NP-TMPA) and Si NP-Sil caused an induction in the TNF-α production. The corresponding EC50 values are given in Table 2.
Fig. 8. Cellular TNF-α in NR8383 and Caco-2 cells after 24 h exposure to.
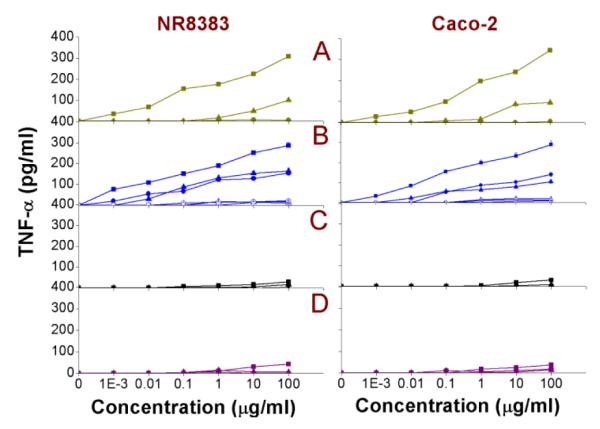
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n=3).
). Results are shown as mean ± SEM (n=3).
Measurement of caspase-3 enzyme
The caspase-3 enzyme is an important biomarker for the apoptotic (self-programmed cell death) cascade. The caspase-3 activity was measured in NR8383 and Caco-2 cells after 24 h exposure to the different Si NP and Ge NP and the results are shown in Fig. 9 with the EC50 values enlisted in Table 2. In line with the data obtained from the previously mentioned results in this paper, apart from the Si NP-Sil, only exposure to the cationic Si NPs/Ge NPs resulted in an increase of the caspase-3 activity. The observed order was SiFe(3.9) NP-NH2 > Si(1.6) NP-NH2 = SiMn(3.9) NP-NH2 > Si(1.6) NP-N3 > Si(3.9) NP-NH2 > Ge NP-TMPA > Si NP-Sil, but the differences between these NPs were all relatively small. Characteristically, the Fe-containing NPs yielded the highest caspase-3 induction. On the other hand, none of the anionic NP, dextran-coated or PEG-ylated NPs showed any increase of the caspase-3 activity.
Fig. 9. Cellular caspase-3 activity in NR8383 and Caco-2 cells after 24 h exposure to.
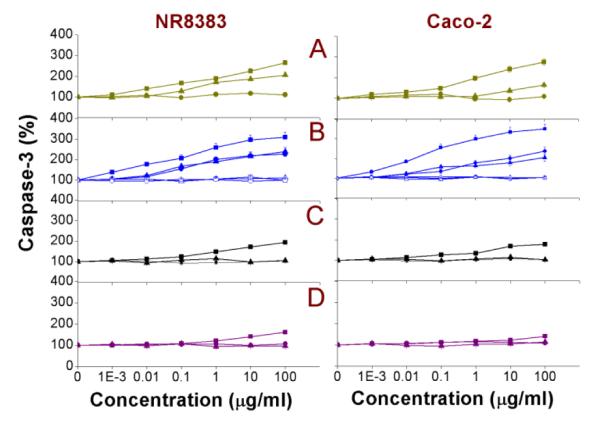
(A) Si(1.6) NP-NH2 ( ), Si(1.6) NP-N3 (
), Si(1.6) NP-N3 ( ) and Si(1.6) NP-COOH (
) and Si(1.6) NP-COOH ( ); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex (
); (B) SiFe(3.9) NP-NH2 (∎), Si(3.9) NP-NH2 (▴), SiMn(3.9) NP-NH2 (●), SiFe(3.9) NP-NH2-Dex ( ), Si(3.9) NP-NH2-Dex (
), Si(3.9) NP-NH2-Dex ( ) and SiMn(3.9) NP-NH2-Dex (
) and SiMn(3.9) NP-NH2-Dex ( ); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil (
); (C) Ge NP-TMPA (∎), Ge NP-PEG (▴) and Si NP-PEG (●); (D) Si NP-Sil ( ), Si NP-UDA (
), Si NP-UDA ( ) and Si NP-Pol (
) and Si NP-Pol ( ). Results are shown as mean ± SEM (n=3).
). Results are shown as mean ± SEM (n=3).
Analysis of toxicity tests
Upon comparison, it can be seen that, apart from Si NP-Sil, only cationic Si NPs/Ge NPs induced signs of cytotoxicity and effects on the nine endpoints evaluated in this study. The surface charge of NP has been hypothesized to be an important factor in cytotoxicity of NP.37, 63-65 Typically, positive NPs were found to be toxic whereas the anionic ones were not, which is in agreement with the current data. A detailed discussion on why positive surface charge-containing NPs are toxic in contrast with the negative NPs is beyond the scope of this article. However, it can be stated that positive NPs get electrostatically attracted towards the negative cell membrane,66 thereby initiating cell membrane-bound receptor-mediated interactions and possibly also cell signaling cascades.44 Within the group of amine-terminated Si NPs, the smaller Si(1.6) NP-NH2, in line with previously reported relationships between the size and toxicity of NP,67-70 were found to be more toxic than the bigger Si(3.9) NP-NH2. It should be mentioned here that it has been reported that it is the cationic charge carried by the amine groups in aqueous dispersions (as confirmed by their pKa values12) rather than the amine group itself that contributes to the toxicity. For example, it has been reported that replacing the terminal amine groups with glucose moieties significantly reduced the toxicity of silicon nanowires in mouse stroma cells.71 One possible explanation for this size-dependence of toxicity of NPs is that smaller NPs can have better access to different parts of the cells.
Additionally, smaller NP have more reactive surface area.72 It was interesting to find that Mn-doped SiMn(3.9) NP-NH2 did not show a higher cytotoxicity than either of the Si(3.9) NP-NH2. This result is exciting in view of the potential of Mn-doped Si NP for bimodal bioimaging.49 However, the Fe-doped SiFe(3.9) NP-NH2 did show a higher cytotoxicity than Si(3.9) NP-NH2. It was already reported that Fe has a tendency to leach out from the core of NP as ions and cause toxicity.73 The toxicity caused by the Fe3+ ions can be due to first their reduction to Fe2+ (by acidic lysosomes) and then reacting with mitochondrial and nuclear hydrogen peroxide to produce ROS via Fenton reaction.74, 75 Remarkably, the toxicity could be diminished significantly when the SiFe(3.9) NP-NH2 was coated by a covalently linked dextran coating. The dextran coating also abolished the toxicity of the other two toxic Si NPs: Si(3.9) NP-NH2 and SiMn(3.9) NP-NH2. This showed that a surface coating with a biocompatible material, like dextran, can strongly reduce the overall toxicity, and perhaps even turn the NP (nearly) non-toxic. The Si NP-Sil showed signs of toxicity although the toxicity was comparatively much lower. It has been reported that silica NP are toxic,57, 58, 76 and this can also be linked to the toxicity of Si NP-Sil. It was highly relevant to note that the carboxylic acid-terminated and hydroxyl-terminated Si NP-PEG, Ge NP-PEG, Si NP-UDA and Si NP-Pol did not show any toxicity within the tested concentration range. It has been reported that negative Si NPs were much less toxic compared to the positive ones.20, 21, 34 This may be due to the fact that negative NPs get repelled by the negatively charged cell membranes, which hinders their cellular interactions. It had also been observed that the positive and negative NPs follow different endocytic uptake patterns in cells via activation of distinct groups of cell membrane receptors.60, 70, 77, 78 Interestingly, the interactions between lipid bilayer membranes and cationic NPs are of the same nature as the interactions between mitochondria and charge-bearing NPs. It is reported that cationic NPs cause an imbalance of the normal electrochemical gradient (80-130 mV)79 across the outer mitochondrial membrane,80 and thus yield an ionic imbalance and increased permeability. This can cause decoupling of ETC, and cause an increase of intracellular ROS as well as a depletion of intracellular ATP production. Therefore we studied the effect of these NPs on the mitochondrial membrane potential and on the intracellular ATP production.
A silica coating on an intrinsically less or non-toxic Si-core would be of both chemical and toxicological interest investigating the effect of surface coating on toxicity of NP as silica NP have been reported to be toxic.81 For such hybrid SiO2/Si NPs, the presence of an organic coating is relevant, as this allows tuning of the hydrophilicity/hydrophobicity of the surfaces, which is reported to play an important role in the cellular interaction and uptake. Silica NP have been reported to cause induction of intracellular ROS,82, 83 increase in the cytosolic free calcium concentration,84 and increased damage to the intra-nuclear DNA.85 It is reported that cationic NP (Si21 and ZnO/CeO234) induced intracellular ROS production, and this matches with the current data. However, how these ROS are formed is yet unclear. It is possible that with reactive nature, NPs can react with a wide variety of biomolecules inducing production of oxygen and nitrogen radicals or that they are the result of uncoupling of oxidative phosphorylation as such. A mitochondrial involvement in such induction of ROS production seems to be a feasible explanation. It should also be mentioned here that the high induction of ROS production following the exposure to the SiFe(3.9) NP-NH2 may be due to the leaching of Fe3+ ions from the core to the NPs surface or cellular environment. The results obtained from the TNF-α measurements point towards damage caused by the ROS and show that the inflammatory behavior of the cells is a response to the toxic effects caused by the NPs. It is documented in literature that especially cationic NPs (of different compositions, like lipids 86 or gelatin 87) can cause an induction of TNF-α. Our data on the currently studied inorganic NPs are in line with the available literature.
Interestingly, an inverse relationship between the inflicted toxicity and size of the NPs can also be observed here, as the induction of TNF-α was found to be larger for the smaller Si(1.6) NP-NH2 compared to the bigger Si(3.9) NP-NH2, which is in line with literature available for polystyrene88 or metallic NPs.63 The induction of TNF-α by the cationic Si NPs, found in the present study, also strengthened the idea that the inflammatory responses of the cells are caused by various radicals and ions. This can be pivotal in understanding the mechanism of cytotoxicity of different NPs.
Compilation of the available data and strategy to design more biocompatible Si NPs/Ge NPs
An analysis of the reported data in this article can not only lead to a better understanding of the mode of action underlying the cytotoxicity of these NPs, but may also help to develop smarter Si NPs or Ge NPs in future. In this article, we tried to show that the cell-NPs interaction can be evaluated on the basis of their surface properties, and hence it is of utmost importance that the surface chemistry of both the exposed cells and the NPs are known in detail. It has been reported that NPs interact preferentially with cell membrane-bound receptors.12 In fact, by computational chemistry, the characteristics of these types of interactions between the cell membrane and NP have recently been probed.89 Besides that, cationic surface charge has been recognized as an important factor in causing toxicity of NPs, a surface charge-dependent cellular uptake pattern, with cationic NPs showing higher cellular uptake compared to the anionic ones,12 has also been observed. Interestingly, these two phenomena may be counteracting each other, as for targeted drug delivery it is important that the NPs combine a high cellular uptake with minimal toxicity. Hence, the finding that cationic NPs are usually more toxic can be a limiting factor for their possible applications in biological systems.
Recently, it was shown that an alleviation of the toxicity of cationic NP could be achieved by increasing the steric bulk around the positive charge of the NP.12 In the current study, the positive charge on the Ge NP-TMPA was also sterically hindered, and by simple comparison of EC50 values it can be stated that in equivalent amounts, the toxicity of these Ge NP-TMPA was smaller than that of the other amine-terminated NPs. Although this is just only the second example of such reduced toxicity, increasing the steric bulk around positive surface charges may thus be an interesting way of decreasing the toxicity of cationic NPs. More research is surely desired here to further delineate this phenomenon.
It is also important to have an idea of the surface functionalization of the NPs, as from our data it can be observed that a silica coating over a Si-core imparted toxic effects. Similarly, a coating with biocompatible dextran almost annulled the toxic effects of amine-terminated Si(3.9) NP-NH2, SiMn(3.9) NP-NH2 and SiFe(3.9) NP-NH2. It is possible that the cells recognize the dextran moieties on the NPs surface and hence the cell-NPs interactions are immediately channeled in a different route, like activation of a different set of receptors. This is important to note, as it may provide some initial guidelines for functionalizing the surfaces of NPs which are targeted for biological applications. A review of literature on the toxicity of the coating materials as well as some control experiments with only the coating material can give initial predictions on the toxicity of NP coated with the respective material.
Taken together, an indication towards surface reactivity-oriented interactions between the NPs and the cells can be obtained. It suggests to analyze the interactions between the cells and the NPs on the basis of chemical interactions possible between them. It is reported in literature that different cell lines show dissimilar responses after being exposed to similar doses of the same NPs for the same time points,44 which can be due to the fact that diverse cell lines express different cell membrane-bound receptors in varying quantities.90 The interaction of NPs with cell membrane-bound receptors are quite specific and a variation in the amount of receptor protein expressed can also result in a variation of the exhibited toxicity. The battery of tests performed in the present study also enables us in getting a clearer picture of the mechanism of toxicity of NPs.
A schematic diagram showing the proposed mechanism of cytotoxicity of cationic Si NPs/Ge NPs is given in Fig. 10. It seems that the mitochondria play a pivotal role in the entire mechanistic cascade of toxicity, where especially the cationic NPs, by creating damage to the normal physiology of the outer membrane of mitochondria, propel a series of events (like dissipation of ATP production, induction of ROS generation, cytoplasmic free calcium upload, oxidative stress, inflammatory response and finally triggering of apoptotic reactions) that ultimately sum up as the observed toxicity.
Fig. 10.
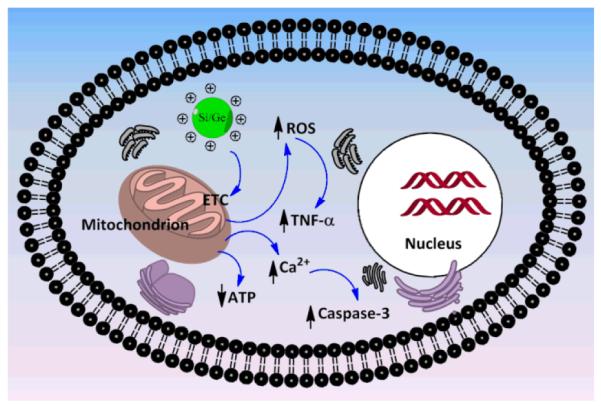
Schematic diagram showing the proposed mechanism of cytotoxicity for cationic Si and Ge NP.
In summary, in this article we have demonstrated by comparing the host of data obtained from a series of systematically varied Si NPs and Ge NPs that have been subjected to a systematic set of toxicological in vitro experiments, that the toxicity of Si NPs and Ge NPs is dominated by their surface chemistry. Whereas positively charged NPs displayed some toxicity, carboxylic acid-coated, dextran-coated and PEG-coated Si and Ge NPs display no toxicity in a rat lung and human colon cell lines. Such surface-functionalized Si NPs or Ge NPs are of interest because of their intrinsic fluorescence, modifiable surfaces, minimally toxic cores and tunable doping with MRI active elements such as Mn and Fe. Given the right coating, these are thus highly attractive materials for biological and medical applications. Of course, it is not only looks (outside), but also size that matters: only smaller NPs (< 5.5 nm) are typically effectively cleared via the kidneys.17 Therefore, further research into Si or Ge NPs with a relatively small core, some bio-inert, neutral coating, and possibly dopants for bimodal bioimaging seems highly attractive. In addition, if an effective renal clearance is undesired, Si NPs and Ge NPs do provide access to materials that combine a substantial larger size with minimal intrinsic toxicity. Finally, with this systematic set of toxicological investigations, a clearer idea on the mechanism of cytotoxicity could be achieved, which puts intracellular mitochondria as one of the important target organs for the toxicity of NPs.
Supplementary Material
Acknowledgements
The authors would like to thank Graduate school VLAG, the Wageningen UR strategic research program Bionanotechnology, QNano, NIH (project: EB008576-01), DOE (DESC0002289), Natural Science and Engineering Council of Canada and Alberta Innovates and NSF Grant CMMI-0726943 for funding, and thank Prof. Angelique Louie (UC Davis) for useful discussions.
Footnotes
Electronic Supplementary Information (ESI) available: Synthesis and Characterization of SiM-C3-NH2 and SiM-C3-NH2-Dex (M =0,Mn, Fe). See DOI: 10.1039/b000000x/
References
- 1.Luo XL, Morrin A, Killard AJ, Smyth MR. Electroanalysis. 2006;18:319–326. [Google Scholar]
- 2.Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Nat. Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin. Pharmacol. Ther. 2007;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 4.Buzea C, Pacheco I, Robbie K. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 5.Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P. Part. Fibre Toxicol. 2009:6. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iga AM, Robertson JHP, Winslet MC, Seifalian AM. J. Biomed. Biotechnol. 2007:1–10. doi: 10.1155/2007/76087. Article ID 76087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing Y, Rao JH. Cancer Biomark. 2008;4:307–319. doi: 10.3233/cbm-2008-4603. [DOI] [PubMed] [Google Scholar]
- 8.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Advanced Drug Delivery Reviews. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Chu PK. Small. 2010;6:2080–2098. doi: 10.1002/smll.201000543. [DOI] [PubMed] [Google Scholar]
- 10.Rosso-Vasic M, Spruijt E, Popovic Z, Overgaag K, van Lagen B, Grandidier B, Vanmaekelbergh D, Dominguez-Gutierrez D, De Cola L, Zuilhof H. J. Mater. Chem. 2009;19:5926–5933. [Google Scholar]
- 11.Oku T, Nakayama T, Kuno M, Nozue Y, Wallenberg LR, Niihara K, Suganuma K. Mater. Sci. Eng. B. 2000;74:242–247. [Google Scholar]
- 12.Bhattacharjee S, Ershov D, Gucht J. v. d., Alink GM, Rietjens IMCM, Zuilhof H, Marcelis ATM. Nanotoxicology. 2013;7:71–84. doi: 10.3109/17435390.2011.633714. [DOI] [PubMed] [Google Scholar]
- 13.Chandra S, Das P, Bag S, Laha D, Pramanik P. Nanoscale. 2011;3:1533–1540. doi: 10.1039/c0nr00735h. [DOI] [PubMed] [Google Scholar]
- 14.Hon NK, Shaposhnik Z, Diebold ED, Jalali F. Tamanoi and B. Journal of Biomedical Materials Research Part A. 2012;100A:3416–3421. doi: 10.1002/jbm.a.34294. [DOI] [PubMed] [Google Scholar]
- 15.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popplewell JF, King SJ, Day JP, Ackrill P, Fifield LK, Cresswell RG, DiTada ML, Liu K. J. Inorg. Biochem. 1998;69:177–180. doi: 10.1016/s0162-0134(97)10016-2. [DOI] [PubMed] [Google Scholar]
- 17.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosso-Vasic M, Spruijt E, van Lagen B, De Cola L, Zuilhof H. Small. 2008;4:1835–1841. doi: 10.1002/smll.200800066. [DOI] [PubMed] [Google Scholar]
- 19.Warner JH, Hoshino A, Yamamoto K, Tilley RD. Angew. Chem. Int. Edi. 2005;44:4550–4554. doi: 10.1002/anie.200501256. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee S, Haan L. H. J. de, Evers NM, Jiang X, Marcelis ATM, Zuilhof H, Rietjens IMCM, Alink GM. Part. Fibre Toxicol. 2010;7:25. doi: 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruizendaal L, Bhattacharjee S, Pournazari K, Rosso-Vasic M, Haan L. H. J. de, Alink GM, Marcelis ATM, Zuilhof H. Nanotoxicology. 2009;3:339–347. [Google Scholar]
- 22.Peng K-Q, Wang X, Li L, Wu X-L, Lee S-T. J Am Chem Soc. 2010;132:6872–6873. doi: 10.1021/ja910082y. [DOI] [PubMed] [Google Scholar]
- 23.Shiohara A, Prabakar S, Faramus A, Hsu C-Y, Lai P-S, Northcote PT, Tilley RD. Nanoscale. 2011;3:3364–3370. doi: 10.1039/c1nr10458f. [DOI] [PubMed] [Google Scholar]
- 24.Shiohara A, Hanada S, Prabakar S, Fujioka K, Lim TH, Yamamoto K, Northcote PT, Tilley RD. J Am Chem Soc. 2009;132:248–253. doi: 10.1021/ja906501v. [DOI] [PubMed] [Google Scholar]
- 25.Prabakar S, Shiohara A, Hanada S, Fujioka K, Yamamoto K, Tilley RD. Chem Mat. 2009;22:482–486. [Google Scholar]
- 26.Maier-Flaig F, Henderson EJ, Valouch S, Klinkhammer S, Kübel C, Ozin GA, Lemmer U. Chemical Physics. 2012;405:175–180. [Google Scholar]
- 27.Anthony R, Kortshagen U. Phys. Rev. B. 2009;80:115407. [Google Scholar]
- 28.Liu C-Y, Holman ZC, Kortshagen UR. Nano Letters. 2008;9:449–452. doi: 10.1021/nl8034338. [DOI] [PubMed] [Google Scholar]
- 29.Stegner AR, Pereira RN, Lechner R, Klein K, Wiggers H, Stutzmann M, Brandt MS. Phys. Rev. B. 2009;80:165326. doi: 10.1103/PhysRevLett.100.026803. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Swihart MT, Wiggers H. Adv Func Mat. 2009;19:696–703. [Google Scholar]
- 31.Erogbogbo F, Yong K-T, Roy I, Hu R, Law W-C, Zhao W, Ding H, Wu F, Kumar R, Swihart MT, Prasad PN. ACS Nano. 2010;5:413–423. doi: 10.1021/nn1018945. [DOI] [PubMed] [Google Scholar]
- 32.Dohnalová K, Fučíková A, Umesh CP, Humpolíčková J, Paulusse JMJ, Valenta J, Zuilhof H, Hof M, Gregorkiewicz T. Small. 2012;8:3185–3191. doi: 10.1002/smll.201200477. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Liu Y, Peng F, Chen C, He Y, Ma H, Cao L, Sun S. Small. 2012;8:2430–2435. doi: 10.1002/smll.201102627. [DOI] [PubMed] [Google Scholar]
- 34.Shiohara A, Hanada S, Prabakar S, Fujioka K, Lim TH, Yamamoto K, Northcote PT, Tilley RD. J. Am. Chem. Soc. 2009;132:248–253. doi: 10.1021/ja906501v. [DOI] [PubMed] [Google Scholar]
- 35.Asati A, Santra S, Kaittanis C, Perez JM. ACS Nano. 2010;4:5321–5331. doi: 10.1021/nn100816s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaeublin NM, Braydich-Stolle LK, Schrand AM, Miller JM, Hutchison J, Schlager JJ, Hussain SM. Nanoscale. 2011;3:410–420. doi: 10.1039/c0nr00478b. [DOI] [PubMed] [Google Scholar]
- 37.Badawy A. M. El, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Environ. Sci. Technol. 2010;45:283–287. doi: 10.1021/es1034188. [DOI] [PubMed] [Google Scholar]
- 38.Shirahata N. Phys. Chem. Chem. Phys. 2011;13:7284–7294. doi: 10.1039/c0cp02647f. [DOI] [PubMed] [Google Scholar]
- 39.Siekierzycka JR, Rosso-Vasic M, Zuilhof H, Brouwer AM. J. Phys. Chem. C. 2011;115:20888–20895. [Google Scholar]
- 40.Alsharif NH, Berger CEM, Varanasi SS, Chao Y, Horrocks BR, Datta HK. Small. 2009;5:221–228. doi: 10.1002/smll.200800903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherbart AM, Langer J, Bushmelev A, van Berlo D, Haberzettl P, van Schooten FJ, Schmidt AM, Rose CR, Schins RPF, Albrecht C. Part. Fibre Toxicol. 2011;8 doi: 10.1186/1743-8977-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nel A, Xia T, Mädler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Zhou J, Cai Z. Mar. Pollut. Bull. 2011;63:334–338. doi: 10.1016/j.marpolbul.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. ACS Nano. 2007;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 45.Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uboldi C, Bonacchi D, Lorenzi G, Hermanns MI, Pohl C, Baldi G, Unger RE, Kirkpatrick CJ. Part. Fibre Toxicol. 2009;6 doi: 10.1186/1743-8977-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon M, Barberet P, Delville M-H, Moretto P, Seznec H. Nanotoxicology. 2011;5:125–139. doi: 10.3109/17435390.2010.502979. [DOI] [PubMed] [Google Scholar]
- 48.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Brynda M, Britt RD, Carroll EC, Larsen DS, Louie AY, Kauzlarich SM. J. Am. Chem. Soc. 2007;129:10668–10669. doi: 10.1021/ja074144q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XM, Neiner D, Wang SZ, Louie AY, Kauzlarich SM. Colloidal Quantum Dots for Biomedical Applications II. 2007;6448:44804–44804. [Google Scholar]
- 51.Hessel CM, Rasch MR, Hueso JL, Goodfellow BW, Akhavan VA, Puvanakrishnan P, Tunnel JW, Korgel BA. Small. 2010;6:2026–2034. doi: 10.1002/smll.201000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heintz AS, Fink MJ. B. S. Mitchell. 2007;19:3984–3988. [Google Scholar]
- 53.Clark RJ, Dang MKM. J. G. C. Veinot. 2010;26:15657–15664. doi: 10.1021/la102983c. [DOI] [PubMed] [Google Scholar]
- 54.Regli S, Kelly JA, Veinot JGC. Mater. Res. Soc. Symp. Proc. 2011;1359:149–154. [Google Scholar]
- 55.Hoshino Y, Koide H, Furuya K, Haberaecker WW, Lee S-H, Kodama T, Kanazawa H, Oku N, Shea KJ. Proc. Natl. Aca. Sci. 2012;109:33–38. doi: 10.1073/pnas.1112828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gil P. Rivera, Oberdörster G, Elder A, Puntes V, Parak WJ. ACS Nano. 2010;4:5527–5531. doi: 10.1021/nn1025687. [DOI] [PubMed] [Google Scholar]
- 57.Lin W, Huang Y.-w., Zhou X-D, Ma Y. Toxicol. Appl. Pharmacol. 2006;217:252–259. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Yu T, Malugin A, Ghandehari H. ACS Nano. 2011;5:5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prasad BR, Nikolskaya N, Connolly D, Smith TJ, Byrne SJ, Gerard VA, Gun’ko YK, Rochev Y. J. Nanobiotechnol. 2010;8:7. doi: 10.1186/1477-3155-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V. M. Thompson. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 61.Tazawa H, Fujita C, Machida K, Osada H, Ohta Y. Arch. Biochem. Biophys. 2009;481:59–64. doi: 10.1016/j.abb.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 62.AshaRani PV, Mun G. Low Kah, Hande MP, Valiyaveettil S. ACS Nano. 2008;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 63.Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Wires Nanomed Nanobi. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 64.Mura S, Hillaireau H, Nicolas J, Le Droumaguet B, Gueutin C, Zanna S, Tsapis N, Fattal E. Int. J. Nanomed. 2011;6:2591–2605. doi: 10.2147/IJN.S24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhattacharjee S, Ershov D, Islam MA, Kämpfer AM, Gucht J. v. d., Alink GM, Marcelis ATM, Zuilhof H, Rietjens IMCM. Submitted. 2012 [Google Scholar]
- 66.Verma A, Stellacci F. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 67.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 68.Prabhu BM, Ali SF, Murdock RC, Hussain SM, Srivatsan M. Nanotoxicology. 2010;4:150–160. doi: 10.3109/17435390903337693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin Y-S, Haynes CL. J. Am. Chem. Soc. 2010;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 70.Bhattacharjee S, Ershov D, Fytianos K, Gucht J. v. d., Alink GM, Rietjens IMCM, Marcelis ATM, Zuilhof H. Part. Fibre Toxicol. 2012;9 doi: 10.1186/1743-8977-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang K, Fan D, Belabassi Y, Akkaraju G, Montchamp J-L, Coffer JL. ACS Applied Materials & Interfaces. 2008;1:266–269. doi: 10.1021/am800219r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Auffan M, Rose J, Bottero J-Y, Lowry GV, Jolivet J-P, Wiesner MR. Nat. Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 73.Bouwmeester H, Poortman J, Peters RJ, Wijma E, Kramer E, Makama S, Puspitaninganindita K, Marvin HJP, Peijnenburg AACM, Hendriksen PJM. ACS Nano. 2011;5:4091–4103. doi: 10.1021/nn2007145. [DOI] [PubMed] [Google Scholar]
- 74.Voinov MA, Pagán JOS, Morrison E, Smirnova TI, Smirnov AI. J. Am. Chem. Soc. 2010;133:35–41. doi: 10.1021/ja104683w. [DOI] [PubMed] [Google Scholar]
- 75.Singh N, Jenkins GJ, Asadi R, Doak SH. Nano Rev. 2010;1 doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin Y, Kannan S, Wu M, Zhao JX. Chem. Res. Toxicol. 2007;20:1126–1133. doi: 10.1021/tx7001959. [DOI] [PubMed] [Google Scholar]
- 77.Lynch I, Salvati A, Dawson KA. Nat. Nanotechnol. 2009;4:546–547. doi: 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- 78.Vácha R, Martinez-Veracoechea FJ, Frenkel D. Nano Lett. 2011;11:5391–5395. doi: 10.1021/nl2030213. [DOI] [PubMed] [Google Scholar]
- 79.Kleiner D, Fitzke E. BBA-Biomembranes. 1981;641:138–147. doi: 10.1016/0005-2736(81)90577-0. [DOI] [PubMed] [Google Scholar]
- 80.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Bioconjugate Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 81.Ariano P, Zamburlin P, Gilardino A, Mortera R, Onida B, Tomatis M, Ghiazza M, Fubini B, Lovisolo D. Small. 2011;7:766–774. doi: 10.1002/smll.201002287. [DOI] [PubMed] [Google Scholar]
- 82.Napierska D, Thomassen LCJ, Lison D, Martens JA, Hoet PH. Part. Fibre Toxicol. 2010;7 doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merchant RK, Peterson MW, Hunninghake GW. J. Appl. Physiol. 1990;68:1354–1359. doi: 10.1152/jappl.1990.68.4.1354. [DOI] [PubMed] [Google Scholar]
- 84.Chen J, Armstrong LC, Liu SJ, Gerriets JE, Last JA. Toxicol. Appl. Pharmacol. 1991;111:211–220. doi: 10.1016/0041-008x(91)90025-a. [DOI] [PubMed] [Google Scholar]
- 85.Schins RPF, Duffin R, Hohr D, Knaapen AM, Shi TM, Weishaupt C, Stone V, Donaldson K, Borm PJA. Chem. Res. Toxicol. 2002;15:1166–1173. doi: 10.1021/tx025558u. [DOI] [PubMed] [Google Scholar]
- 86.Kedmi R, Ben-Arie N, Peer D. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 87.Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Pharm. Res. 2008;25:551–562. doi: 10.1007/s11095-007-9410-5. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Li W, Lao F, Liu Y, Wang L, Bai R, Zhao Y, Chen C. Biomaterials. 2011;32:8291–8303. doi: 10.1016/j.biomaterials.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 89.Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Adv. Mater. 2009;21:419–424. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auffan M, Rose J, Wiesner MR, Bottero J-Y. Environ. Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


