Abstract
Corticotropin-releasing hormone (CRH) is a hypothalamic neuropeptide belonging to a family of neuropeptides that includes urocortins, urotensin I, and sauvagine in vertebrates. CRH and urocortin act as anorexigenic factors for satiety regulation in fish. In a goldfish model, intracerebroventricular (ICV) administration of CRH has been shown to affect not only food intake, but also locomotor and psychomotor activities. In particular, CRH elicits anxiety-like behavior as an anxiogenic neuropeptide in goldfish, as is the case in rodents. This paper reviews current knowledge of CRH and its related peptides derived from studies of teleost fish, as representative non-mammals, focusing particularly on the role of the CRH system, and examines its significance from a comparative viewpoint.
Keywords: goldfish, CRH, ICV injection, food intake, anorexigenic action, psychomotor activity, anxiogenic-like action
Introduction
Corticotropin-releasing hormone (CRH), a 41-amino-acid neuropeptide present in the brains of vertebrates, was first isolated and characterized from the ovine hypothalamus (Vale et al., 1981), and then subsequently identified in non-mammalian brains (Lovejoy and Balment, 1999). CRH is a member of a family of related peptides that includes urotensin-I (UI), sauvagine, and urocortin/stresscopin in vertebrates (Lovejoy and Balment, 1999; Boorse and Denver, 2006). In mammals, CRH is known to induce the release of adenohypophyseal hormones such as adrenocorticotropic hormone (ACTH), β-endorphin, and α-melanocyte-stimulating hormone (α-MSH) from the pituitary, and there is ample evidence that CRH and its related peptides play multiple roles in animal development and also in physiological and behavioral adaptation to environmental changes and energy balance (Tonon et al., 1986; Hauger et al., 1988, 2006; Lowry and Moore, 2006; Cooper and Huhman, 2007; Denver, 2009; Papadimitriou and Priftis, 2009; Chen et al., 2012; Kubota et al., 2012).
In non-mammalian vertebrates such as amphibians and teleosts, CRH acts as a potent stimulator of corticotropin, thyrotropin, and α-MSH release (Boorse and Denver, 2004, 2006; Calle et al., 2005; Ito et al., 2006; Okada et al., 2007). CRH and its related peptides also act as regulators of feeding behavior and stress responses in vertebrates including mammals, birds, amphibians, and fish (Kalra et al., 1999; Bernier and Peter, 2001; Ohgushi et al., 2001; Hillebrand et al., 2002; Tachibana et al., 2004; Saito et al., 2005; Lowry and Moore, 2006; Carr et al., 2010; Matsuda et al., 2010b; Morimoto et al., 2011; Khan et al., 2013). It has been reported that, in the goldfish, intracerebroventricular (ICV) administration of CRH or UI exerts an anorexigenic action (de Pedro et al., 1997; Bernier and Peter, 2001; Volkoff et al., 2005; Matsuda, 2009), which is blocked by treatment with a CRH 1/CRH 2 receptor antagonist, α-helical CRH(9−41) (de Pedro et al., 1997; Bernier and Peter, 2001; Bernier, 2006; Maruyama et al., 2006). In fish, ICV administration of CRH also affects locomotor activity (Clements and Schreck, 2004; Maruyama et al., 2006; Carpenter et al., 2007; Backström et al., 2011a; Ghisleni et al., 2012; Matsuda et al., 2013b), suggesting that CRH exerts psychophysiological effects in fish. Recent reports indicate that a fish's swimming pattern can be used to evaluate psychomotor activities, notably anxiety-like behavior (Faganello and Mattioli, 2007; Grossman et al., 2010; Maximino et al., 2010a,b; Matsuda et al., 2011a,b, 2013b; Blaser and Rosemberg, 2012; Maaswinkel et al., 2012). Therefore, the present mini-review summarizes recent advances in knowledge about the regulation of feeding behavior and locomotor or psychomotor activity by CRH and its related peptides in fish, especially with reference to the goldfish model.
Control of food intake by CRH and its related peptides in fish
The effects of ICV administration of neuropeptides on food intake in goldfish have been extensively studied. For example, ICV-injected ghrelin, neuropeptide Y, and orexin increase food consumption whereas CRH, UI, proopiomelanocortin (POMC)-derived peptides such as α-MSH, pituitary adenylate cyclase-activating polypeptide (PACAP), cholecystokinin (CCK), neuromedin U (NMU), and diazepam-binding inhibitor-derived peptides such as octadecaneuropeptide (ODN) decrease food intake (Matsuda, 2009). These neuropeptides are not independently involved in the control of feeding behavior, but mutually interact with each other. The anorexigenic actions of PACAP and NMU are abolished by treatment with α-helical CRH(9−41), and CCK- and ODN-evoked anorexigenic actions are also attenuated by treatment with the melanocortin 4 receptor (MC4R) antagonist HS024 (Maruyama et al., 2006, 2009; Kang et al., 2010; Matsuda et al., 2010a). These findings suggest that CRH and α-MSH mediate the actions of PACAP and NMU, and CCK and ODN, respectively. In goldfish, α-MSH-containing nerve fibers or endings lie in close apposition to CRH-containing neurons in a specific region of the hypothalamus, the nucleus posterioris periventricularis (NPPv). The anorexigenic action of the α-MSH agonist melanotan II (MT II) is abolished by treatment with α-helical CRH(9−41) whereas the anorexigenic action of CRH is not affected by treatment with HS024 (Matsuda et al., 2008a). These observations indicate that, in goldfish, α-MSH-induced anorexigenic action is mediated by the CRH-signaling pathway, and that CRH plays a crucial role in the regulation of feeding behavior as an integrated anorexigenic neuropeptide in this species.
The distribution of CRH in the brain of teleost fish including the goldfish, has been well-reported: CRH-containing neuronal cell bodies are localized in various hypothalamic regions, including the preopticus periventricularis (NPP), the nucleus preopticus (NPO), the lateral part of the nucleus lateralis tuberis (NLTl) and the NPPv, and CRH-containing fibers or endings are distributed throughout the brain, and in the neurohypophysis (Olivereau et al., 1984, 1988; Yulis et al., 1986; Yulis and Lederis, 1987). For example, in goldfish, neuronal cell bodies exhibiting CRH-like immunoreactivity are located mainly in the preoptic parvocellular areas comprising the NPP and NPO, the NLTl, and paraventricular organ areas such as the NPPv, and their fibers are distributed in the diencephalon, mesencephalon, and neurohypophysis. CRH-containing neurons that originate in the NPP and NPO parvocellular population seem to innervate the pituitary. As described above, studies of the effect of CRH on feeding behavior in goldfish have shown that it acts as a powerful hypothalamic anorexigenic peptide (de Pedro et al., 1993, 1997; Bernier et al., 1999, 2004; Bernier and Peter, 2001; Maruyama et al., 2006). Interestingly, we and others have found that ICV injection of gonadotropin-releasing hormone 2 (GnRH2, also known as chicken GnRH II) affects food consumption, and that GnRH2 decreases food intake (Hoskins et al., 2008; Matsuda et al., 2008b). Subsequently it has been indicated that the anorexigenic actions of CRH and α-MSH are blocked by treatment with the GnRH type I receptor antagonist Antide, suggesting that GnRH2 mediates the actions of other anorexigenic neuropeptides examined so far, and that GnRH2 acts as a key neuropeptide exerting satiety control (Kang et al., 2011).
Psychophysiological effect of CRH in fish
Recent studies have shown that several neuropeptides such as CRH, GnRH2, ODN, PACAP, NPY, ghrelin, and orexin affect not only food intake but also locomotor activity in fish (Table 1): ICV injection of CRH enhances swimming distance, and stimulates locomotor activity (Maruyama et al., 2006; Carpenter et al., 2007; Backström et al., 2011a,b; Matsuda et al., 2013b). Psychophysiological compounds including diazepam, serotonin, a selective serotonin reuptake inhibitor Fluoxetin, a central-type benzodiazepine receptor inverse agonist FG-7142, and an N-methyl-d-aspartate receptor antagonist MK-801 also modify locomotor activity (Kang et al., 2010; Matsuda et al., 2011b, 2013b; Winder et al., 2012). Recent reports have indicated that the swimming pattern of a fish in a tank can be used to evaluate psychomotor activity (Faganello and Mattioli, 2007; Cachat et al., 2010; Grossman et al., 2010; Maximino et al., 2010a,b; Khor et al., 2011, 2013; Matsuda et al., 2011a; Piato et al., 2011). The scototaxis test (light/dark preference test) has been developed, and used for measuring psychomotor activity (Faganello and Mattioli, 2007; Blaser and Rosemberg, 2012). Intact animals usually prefer the dark area to the light area, and psychophysiological substances affect this preference: treatment with diazepam increases the time spent in the light area, and treatment with FG-7142 increases the time spent in the dark area, suggesting that the former and latter treatments induce anxiolytic- and anxiogenic-like actions, respectively (Matsuda et al., 2011b). Since intact goldfish and zebrafish prefer the lower to the upper area of a tank, another preference test has also been developed to evaluate the effect of CRH or other substances on psychomotor activity (Khor et al., 2013; Matsuda et al., 2013b). ICV administration of CRH and FG-7142 both increase the time taken to move from the lower to the upper area, and the anxiogenic-like action of CRH is blocked by treatment with α-helical CRH(9−41) (Matsuda et al., 2013b). Recent studies of other fish have also indicated that CRH induces behavioral changes including anxiety and suppression of aggressive behavior (Lastein et al., 2008; Carpenter et al., 2009; Backström et al., 2011a,b; Ghisleni et al., 2012). These studies suggest that CRH exerts psychophysiological effects as an anxiogenic factor in addition to satiety control in fish. Figure 1 shows a schematic drawing of the anorexigenic signaling pathways mediated by CRH and other neuropeptides in the central nervous system of goldfish. As described above, CRH also evokes anxiogenic-like action in this species. Although it is unclear why regulation of food intake and the psychophysiological effects of CRH are closely linked, CRH appears to induce both anorexigenic- and anxiogenic-like actions in fish. Therefore, it is reasonable to suggest that the increased locomotor activity of fish in an experimental tank induced by CRH can be interpreted as escape behavior triggered by the anxiogenic-like action of CRH and subsequent stress response. Further study is warranted to clarify the function of CRH and its related peptides in the regulation of feeding and emotional activity in fish.
Table 1.
Effects of neuropeptides and psychophysiological compounds on food intake, locomotor activity, and emotional action in fish.
| Substances | Species | Food intake | Locomotor activity | Emotional action | References |
|---|---|---|---|---|---|
| CRH | Goldfish | Down | Up | Anxiogenic-like | Maruyama et al., 2006; Matsuda et al., 2013b |
| Rainbow trout | Up | Anxiogenic-like | Carpenter et al., 2007; Backström et al., 2011a,b | ||
| GnRH2 | Goldfish | Down | Up | Hoskins et al., 2008; Matsuda et al., 2008b | |
| Zebrafish | Down | Nishiguchi et al., 2012 | |||
| ODN | Goldfish | Down | Up | Anxiogenic-like | Matsuda et al., 2007, 2011b |
| PACAP | Goldfish | Down | Up | Anxiogenic-like | Matsuda et al., 2006a, 2013a |
| NPY | Goldfish | Up | Down | Anxiolytic-like | Matsuda et al., 2011a, 2012b |
| Zebrafish | Up | Yokobori et al., 2012 | |||
| Ghrelin | Goldfish | Up | Up or Down | Matsuda et al., 2006b; Yahashi et al., 2012 | |
| ORX | Goldfish | Up | Up | Nakamachi et al., 2006; Matsuda et al., 2012a | |
| Zebrafish | Up | Up | Yokogawa et al., 2007; Yokobori et al., 2011 | ||
| Diazepam | Goldfish | Down | Anxiolytic-like | Matsuda et al., 2011b | |
| Fluoxetine | Sheepshead minnow | Down | Winder et al., 2012 | ||
| Chinook salmon | Down | Clements and Schreck, 2007 | |||
| FG-7142 | Goldfish | Up | Anxiogenic-like | Matsuda et al., 2011b | |
| MK-801 | Goldfish | Up | Kang et al., 2011 |
Abbreviations
- CRH
corticotropin-releasing hormone
- GnRH2
gonadotropin-releasing hormone 2
- ODN
octadecaneuropeptide
- PACAP
pituitary adenylate cyclase-activating polypeptide
- NPY
neuropeptide Y
- ORX
orexin
- Fluoxetine
a selective serotonin reuptake inhibitor
- FG-7142
a central-type benzodiazepine receptor inverse agonist
- MK-801
an N-methyl-d-aspartate receptor antagonist.
Figure 1.
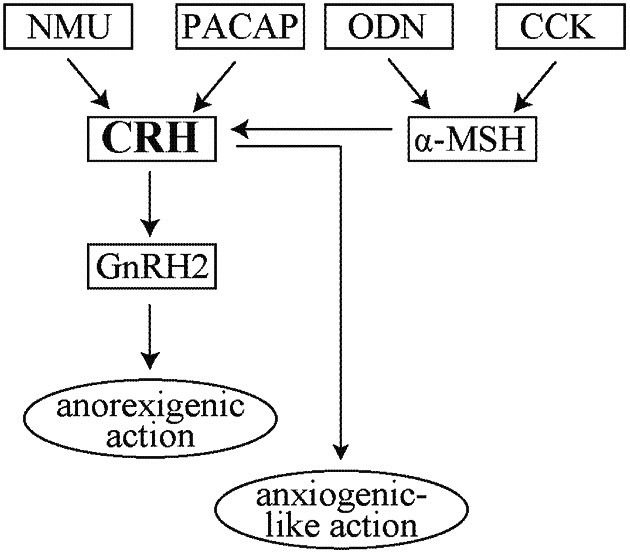
Schematic drawings of the neuronal signaling pathways of anorexigenic and anxiogenic-like action in goldfish. ODN and CCK-induced anorexigenic actions are mediated by α-MSH-signaling pathway, and the anorexigenic actions of NMU, PACAP, and α-MSH are mediated by CRH- and subsequent GnRH2-signaling pathways. CRH also evokes anxiogenic-like action. Abbreviations: NMU, neuromedin U; PACAP, pituitary adenylate cyclase-activating polypeptide; ODN, octadecaneuropeptide; CCK, cholecystokinin; CRH, corticotropin-releasing hormone; α-MSH, α-melanocyte-stimulating hormone; GnRH2, gonadotropin-releasing hormone 2.
Conclusion
In fish, CRH exerts potential effects on food intake, as well as locomotor and psychomotor activities, providing an example of a neuropeptide that regulates both feeding behavior and psychophysiological activity such as anxiogenic- or anxiolytic-like action.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (21370025 to Kouhei Matsuda), and by research grants from the University of Toyama (Kouhei Matsuda).
References
- Backström T., Pettersson A., Johansson V., Winberg S. (2011a). CRF and urotensin I effects on aggression and anxiety-like behavior in rainbow trout. J. Exp. Biol. 214, 907–914 10.1242/jeb.045070 [DOI] [PubMed] [Google Scholar]
- Backström T., Schjolden J., Øverli Ø., Thörnqvist P. O., Winberg S. (2011b). Stress effects on AVT and CRF systems in two strains of rainbow trout (Oncorhynchus mykiss) divergent in stress responsiveness. Horm. Behav. 59, 180–186 10.1016/j.yhbeh.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Bernier N. J. (2006). The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen. Comp. Endocrinol. 146, 45–55 10.1016/j.ygcen.2005.11.016 [DOI] [PubMed] [Google Scholar]
- Bernier N. J., Bedard N., Peter R. E. (2004). Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish. Gen. Comp. Endocrinol. 135, 230–240 10.1016/j.ygcen.2003.09.016 [DOI] [PubMed] [Google Scholar]
- Bernier N. J., Lin X., Peter R. E. (1999). Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. Gen. Comp. Endocrinol. 116, 461–477 10.1006/gcen.1999.7386 [DOI] [PubMed] [Google Scholar]
- Bernier N. J., Peter R. E. (2001). The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129, 639–644 10.1016/S1096-4959(01)00360-8 [DOI] [PubMed] [Google Scholar]
- Blaser R. E., Rosemberg D. B. (2012). Measures of anxiety in zebrafish (Danio rerio): dissociation of black/white preference and novel tank test. PLoS ONE 7:e36931 10.1371/journal.pone.0036931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorse G. C., Denver R. J. (2004). Expression and hypophysiotropic actions of corticotropin-releasing factor in Xenopus laevis. Gen. Comp. Endocrinol. 137, 272–282 10.1016/j.ygcen.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Boorse G. C., Denver R. J. (2006). Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen. Comp. Endocrinol. 146, 9–18 10.1016/j.ygcen.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Cachat J., Stewart A., Grossman L., Gaikwad S., Kadri F., Chung K. M., et al. (2010). Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 5, 1786–1799 10.1038/nprot.2010.140 [DOI] [PubMed] [Google Scholar]
- Calle M., Corstens G. J., Wang L., Kozicz T., Denver R. J., Barendregt H. P., et al. (2005). Evidence that urocortin I acts as a neurohormone to stimulate α MSH release in the toad Xenopus laevis. Brain Res. 1040, 14–28 10.1016/j.brainres.2004.12.056 [DOI] [PubMed] [Google Scholar]
- Carpenter R. E., Korzan W. J., Bockholt C., Watt M. J., Forster G. L., Renner K. J., et al. (2009). Corticotropin releasing factor influences aggression and monoamines: modulation of attacks and retreats. Neuroscience 158, 412–425 10.1016/j.neuroscience.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter R. E., Watt M. J., Forster G. L., Øverli Ø., Bockholt C., Renner K. J., et al. (2007). Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity. Horm. Behav. 52, 600–611 10.1016/j.yhbeh.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. A., Lustgarten J., Ahmed N., Bergfeld N., Bulin S. E., Shoukfeh O., et al. (2010). The organization of CRF neuronal pathways in toads: evidence that retinal afferents do not contribute significantly to tectal CRF content. Brain Behav. Evol. 76, 71–86 10.1159/000319555 [DOI] [PubMed] [Google Scholar]
- Chen Y. W., Rada P. V., Bützler B. P., Leibowitz S. F., Hoebel B. G. (2012). Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience 206, 155–166 10.1016/j.neuroscience.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Clements S., Schreck C. B. (2004). Central administration of corticotropin-releasing hormone alters downstream movement in an artificial stream in juvenile chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 137, 1–8 10.1016/j.ygcen.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Clements S., Schreck C. B. (2007). Chronic administration of fluoxetine alters locomotor behavior, but does not potentiate the locomotor stimulating effects of CRH in juvenile Chinook salmon (Oncorhynchus tshawytscha). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 43–49 10.1016/j.cbpa.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Cooper M. A., Huhman K. L. (2007). Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology 194, 297–307 10.1007/s00213-007-0849-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver R. J. (2009). Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann. N.Y. Acad. Sci. 1163, 1–16 10.1111/j.1749-6632.2009.04433.x [DOI] [PubMed] [Google Scholar]
- de Pedro N., Alonso-Gomez A. L., Gancedo B., Delgado M. J., Alonso-Bedate M. (1993). Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish. Physiol. Behav. 53, 517–520 10.1016/0031-9384(93)90146-7 [DOI] [PubMed] [Google Scholar]
- de Pedro N., Alonso-Gómez A. L., Gancedo B., Valenciano A. I., Delgado M. J., Alonso-Bedate M. (1997). Effect of alpha-helical-CRF[9-41] on feeding in goldfish: involvement of cortisol and catecholamines. Behav. Neurosci. 111, 398–403 10.1037/0735-7044.111.2.398 [DOI] [PubMed] [Google Scholar]
- Faganello F. R., Mattioli R. (2007). Anxiolytic-like effect of chlorpheniramine in inhibitory avoidance in goldfish submitted to telencephalic ablation. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 269–274 10.1016/j.pnpbp.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Ghisleni G., Capiotti K. M., Da Silva R. S., Oses J. P., Piato Â. L., Soares V., et al. (2012). The role of CRH in behavioral responses to acute restraint stress in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 176–182 10.1016/j.pnpbp.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Grossman L., Utterback E., Stewart A., Gaikwad S., Chung K. M., Suciu C., et al. (2010). Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 214, 277–284 10.1016/j.bbr.2010.05.039 [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Millan M. A., Lorang M., Harwood J. P., Aguilera G. (1988). Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology 123, 396–405 10.1210/endo-123-1-396 [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Risbrough V., Brauns O., Dautzenberg F. M. (2006). Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol. Disord. Drug Targets 5, 453–479 10.2174/187152706777950684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand J. J., de Wied D., Adan R. A. (2002). Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides 23, 2283–2306 10.1016/S0196-9781(02)00269-3 [DOI] [PubMed] [Google Scholar]
- Hoskins L. J., Xu M., Volkoff H. (2008). Interactions between gonadotropin-releasing hormone (GnRH) and orexin in the regulation of feeding and reproduction in goldfish (Carassius auratus). Horm. Behav. 54, 379–385 10.1016/j.yhbeh.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Ito Y., Okada R., Takahashi N., Kikuyama S. (2006). Cloning and distribution of the bullfrog type 1 and type 2 corticotropin-releasing factor receptors. Gen. Comp. Endocrinol. 146, 291–295 10.1016/j.ygcen.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Dube M. G., Pu S., Xu B., Horvath T. L., Kalra P. S. (1999). Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20, 68–100 10.1210/er.20.1.68 [DOI] [PubMed] [Google Scholar]
- Kang K. S., Shimizu K., Azuma M., Ui Y., Nakamura K., Uchiyama M., et al. (2011). Gonadotropin-releasing hormone II (GnRH II) mediates the anorexigenic actions of α-melanocyte-stimulating hormone (α-MSH) and corticotropin-releasing hormone (CRH) in goldfish. Peptides 32, 31–35 10.1016/j.peptides.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Kang K. S., Yahashi S., Azuma M., Matsuda K. (2010). The anorexigenic effect of cholecystokinin octapeptide in a goldfish model is mediated by the vagal afferent and subsequently through the melanocortin- and corticotropin-releasing hormone-signaling pathways. Peptides 31, 2130–2134 10.1016/j.peptides.2010.07.019 [DOI] [PubMed] [Google Scholar]
- Khan M. S., Cline M. A., Aramaki T., Ueda H., Tachibana T. (2013). Feeding response following central administration of chicken vasoactive intestinal peptide in chicks. Gen. Comp. Endocrinol. 184, 61–66 10.1016/j.ygcen.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Khor B.-S., Jamil M. F. A., Adenan M. I., Shu-Chien A. C. (2011). Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. Plos ONE 6:e28340 10.1371/journal.pone.0028340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor Y. M., Soga T., Parhar I. S. (2013). Caffeine neuroprotects against dexamethasone-induced anxiety-like behaviour in the Zebrafish (Danio rerio). Gen. Comp. Endocrinol. 181, 310–315 10.1016/j.ygcen.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Kubota N., Amemiya S., Motoki C., Otsuka T., Nishijima T., Kita I. (2012). Corticotropin-releasing factor antagonist reduces activation of noradrenalin and serotonin neurons in the locus coeruleus and dorsal raphe in the arousal response accompanied by yawning behavior in rats. Neurosci. Res. 72, 316–323 10.1016/j.neures.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Lastein S., Höglund E., Øverli Ø., Døving K. B. (2008). Effects of antalarmin, a CRF receptor 1 antagonist, on fright reaction and endocrine stress response in crucian carp (Carassius carassius). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194, 1007–1012 10.1007/s00359-008-0372-9 [DOI] [PubMed] [Google Scholar]
- Lovejoy D., Balment R. (1999). Evolution and physiology of the corticotrophin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen. Comp. Endocrinol. 115, 1–22 10.1006/gcen.1999.7298 [DOI] [PubMed] [Google Scholar]
- Lowry C. A., Moore F. L. (2006). Regulation of behavioral responses by corticotorpin-releasing factor. Gen. Comp. Endocrinol. 146, 19–27 10.1016/j.ygcen.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Maaswinkel H., Zhu L., Weng W. (2012). The immediate and the delayed effects of buspirone on zebrafish (Danio rerio) in an open field test: a 3-D approach. Behav. Brain Res. 234, 365–374 10.1016/j.bbr.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Maruyama K., Miura T., Uchiyama M., Shioda S., Matsuda K. (2006). Relationship between anorexigenic action of pituitary adenylate cyclase-activating polypeptide (PACAP) and that of corticotropin-releasing hormone (CRH) in the goldfish, Carassius auratus. Peptides 27, 1820–1826 10.1016/j.peptides.2006.01.013 [DOI] [PubMed] [Google Scholar]
- Maruyama K., Wada K., Ishiguro K., Shimakura S. I., Wakasugi T., Uchiyama M., et al. (2009). Neuromedin U-induced anorexigenic action is mediated by the corticotropin-releasing hormone receptor-signaling pathway in goldfish. Peptides 30, 2483–2486 10.1016/j.peptides.2009.08.013 [DOI] [PubMed] [Google Scholar]
- Matsuda K. (2009). Recent advances in the regulation of feeding behavior by neuropeptides in fish. Ann. N.Y. Acad. Sci. 1163, 241–250 10.1111/j.1749-6632.2008.03619.x [DOI] [PubMed] [Google Scholar]
- Matsuda K., Azuma M., Kang K. S. (2012a). Orexin system in teleost fish. Vitam. Horm. 89, 341–361 10.1016/B978-0-12-394623-2.00018-4 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Sakashita A., Yokobori E., Azuma M. (2012b). Neuroendocrine control of feeding behavior and psychomotor activity by neuropeptide Y in fish. Neuropeptides 46, 275–283 10.1016/j.npep.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Azuma M., Maruyama K., Shioda S. (2013a). Neuroendocrine control of feeding behavior and psychomotor activity by pituitary adenylate cyclase-activating polypeptide (PACAP) in vertebrates. Obes. Res. Clin. Pract. 7, e1–e7 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Hagiwara Y., Shibata H., Wada K. (2013b). Ovine corticotropin-releasing hormone (CRH) exerts an anxiogenic-like action in the goldfish, Carassius auratus. Gen. Comp. Endocrinol. [Epub ahead of print]. 10.1016/j.ygcen.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Kang K. S., Sakashita A., Yahashi S., Vaudry H. (2011a). Behavioral effect of neuropeptides related to feeding regulation in fish. Ann. N.Y. Acad. Sci. 1220, 117–126 10.1111/j.1749-6632.2010.05884.x [DOI] [PubMed] [Google Scholar]
- Matsuda K., Wada K., Azuma M., Leprince J., Tonon M. C., Sakashita A., et al. (2011b). The octadecaneuropeptide exerts an anxiogenic-like action in goldfish. Neuroscience 181, 100–108 10.1016/j.neuroscience.2011.02.058 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Kojima K., Shimakura S. I., Wada K., Maruyama K., Uchiyama M., et al. (2008a). Corticotropin-releasing hormone mediates α-melanocyte-stimulating hormone-induced anorexigenic action in goldfish. Peptides 29, 1930–1936 10.1016/j.peptides.2008.06.028 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Nakamura K., Shimakura S. I., Miura T., Kageyama H., Uchiyama M., et al. (2008b). Inhibitory effect of chicken gonadotropin-releasing hormone II on food intake in the goldfish, Carassius auratus. Horm. Behav. 54, 83–89 10.1016/j.yhbeh.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Kojima K., Wada K., Maruyama K., Leprince J., Tonon M. C., et al. (2010a). The anorexigenic action of the octadecaneuropeptide ODN in goldfish is mediated through the MC4R- and subsequently the CRH receptor-signaling pathways. J. Mol. Neurosci. 42, 74–79 10.1007/s12031-010-9346-9 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Morimoto N., Hashimoto K., Okada R., Mochida H., Uchiyama M., et al. (2010b). Changes in the distribution of corticotropin-releasing factor (CRF)-like immunoreactivity in the larval bullfrog brain and the involvement of CRF in the cessation of food intake during metamorphosis. Gen. Comp. Endocrinol. 168, 280–286 10.1016/j.ygcen.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Maruyama K., Nakamachi T., Miura T., Shioda S. (2006a). Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake and locomotor activity in the goldfish, Carassius auratus. Ann. N.Y. Acad. Sci. 1070, 417–421 10.1196/annals.1317.054 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Miura T., Kaiya H., Maruyama K., Uchiyama M., Kangawa K., et al. (2006b). Stimulatory effect of n-octanoylated ghrelin on locomotor activity in the goldfish, Carassius auratus. Peptides 27, 1335–1340 10.1016/j.peptides.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Wada K., Miura T., Maruyama K., Shimakura S. I., Uchiyama M., et al. (2007). Effect of the diazepam-binding inhibitor-derived peptide, octadecaneuropeptide, on food intake in goldfish. Neuroscience 150, 425–432 10.1016/j.neuroscience.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Maximino C., Marcues de Brito T., Colmanetti R., Pontes A. A., de Castro H. M., de Lacerda R. I., et al. (2010a). Parametric analyses of anxiety in zebrafish scototaxis. Behav. Brain Res. 210, 1–7 10.1016/j.bbr.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Maximino C., Marcues de Brito T., Dias C. A., Gouveia A., Jr., Morato S. (2010b). Scotaxis as anxiety-like behavior in fish. Nat. Protoc. 5, 209–216 10.1038/nprot.2009.225 [DOI] [PubMed] [Google Scholar]
- Morimoto N., Hashimoto K., Okada R., Mochida H., Uchiyama M., Kikuyama S., et al. (2011). Inhibitory effect of corticotropin-releasing factor on food intake in the bullfrog, Aquarana catesbeiana. Peptides 32, 1872–1875 10.1016/j.peptides.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Nakamachi T., Matsuda K., Maruyama K., Miura T., Uchiyama M., Funahashi H., et al. (2006). Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J. Neuroendocrinol. 18, 290–297 10.1111/j.1365-2826.2006.01415.x [DOI] [PubMed] [Google Scholar]
- Nishiguchi R., Azuma M., Yokobori E., Uchiyama M., Matsuda K. (2012). Gonadotropin-releasing hormone 2 suppresses food intake in the zebrafish, Danio rerio. Front. Endocrinol. (Lausanne) 3:122 10.3389/fendo.2012.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgushi A., Bungo T., Shimojo M., Masuda Y., Denbow D. M., Furuse M. (2001). Relationships between feeding and locomotion behaviors after central administration of CRF in chicks. Physiol. Behav. 72, 287–289 10.1016/S0031-9384(00)00377-2 [DOI] [PubMed] [Google Scholar]
- Okada R., Miller M. F., Yamamoto K., De Groef B., Denver R. J., Kikuyama S. (2007). Involvement of the corticotropin-releasing factor (CRF) type 2 receptor in CRF-induced thyrotropin release by the amphibian pituitary gland. Gen. Comp. Endocrinol. 150, 437–444 10.1016/j.ygcen.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Olivereau M., Moons L., Olivereau J., Vandesande F. (1988). Coexistence of corticotropin-releasing factor-like immunoreactivity and vasotocin in perikarya of the preoptic nucleus in the eel. Gen. Comp. Endocrinol. 70, 41–48 10.1016/0016-6480(88)90092-5 [DOI] [PubMed] [Google Scholar]
- Olivereau M., Ollevier F., Vandesande F., Verdonck W. (1984). Immunocytochemical identification of CRF-like and SRIF-like peptides in the brain and the pituitary of cyprinid fish. Cell Tissue Res. 237, 379–382 10.1007/BF00217162 [DOI] [PubMed] [Google Scholar]
- Papadimitriou A., Priftis K. N. (2009). Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16, 265–271 10.1159/000216184 [DOI] [PubMed] [Google Scholar]
- Piato A. L., Capiotti K. M., Tamborski A. R., Oses J. P., Barcellos L. J. G., Bogo M. R., et al. (2011). Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 561–567 10.1016/j.pnpbp.2010.12.018 [DOI] [PubMed] [Google Scholar]
- Saito E. S., Kaiya H., Tachibana T., Tomonaga S., Denbow D. M., Kangawa K., et al. (2005). Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul. Pept. 125, 201–208 10.1016/j.regpep.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Tachibana T., Saito E. S., Takahashi H., Saito S., Tomonaga S., Boswell T., et al. (2004). Anorexigenic effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide in the chick brain are mediated by corticotrophin-releasing factor. Regul. Pept. 120, 99–105 10.1016/j.regpep.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Tonon M. C., Cuet P., Lamacz M., Jégou S., Côté J., Gouteaux L., et al. (1986). Comparative effects of corticotropin-releasing factor, arginine vasopressin, and related neuropeptides on the secretion of ACTH and alpha-MSH by frog anterior pituitary cells and neurointermediate lobes in vitro. Gen. Comp. Endocrinol. 61, 438–445 [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. (1981). Characterization of a 41 residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213, 1394–1397 10.1126/science.6267699 [DOI] [PubMed] [Google Scholar]
- Volkoff H., Canosa L. F., Unniappan S., Cerdá-Reverter J. M., Bernier N. J., Kelly S. P., et al. (2005). Neuropeptides and the control of food intake in fish. Gen. Comp. Endocrinol. 142, 3–19 10.1016/j.ygcen.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Winder V. L., Pennington P. L., Hurd M. W., Wirth E. F. (2012). Fluoxetine effects on sheepshead minnow (Cyprinodon variegatus) locomotor activity. J. Environ. Sci. Health B 47, 51–58 10.1080/03601234.2012.607767 [DOI] [PubMed] [Google Scholar]
- Yahashi S., Kang K. S., Kaiya H., Matsuda K. (2012). GHRP-6 mimics ghrelin-induced stimulation of food intake and suppression of locomotor activity in goldfish. Peptides 34, 324–328 10.1016/j.peptides.2012.01.025 [DOI] [PubMed] [Google Scholar]
- Yokobori E., Azuma M., Nishiguchi R., Kang K. S., Uchiyama M., Matsuda K. (2012). Neuropeptide Y stimulates food intake in the zebrafish, Danio rerio. J. Neuroendocrinol. 24, 766–773 10.1111/j.1365-2826.2012.02281.x [DOI] [PubMed] [Google Scholar]
- Yokobori E., Kojima K., Azuma M., Kang K. S., Maejima S., Uchiyama M., et al. (2011). Stimulatory effect of intracerebroventricular administration of orexin A on food intake in the zebrafish, Danio rerio. Peptides 32, 1357–1362 10.1016/j.peptides.2011.05.010 [DOI] [PubMed] [Google Scholar]
- Yokogawa T., Marin W., Faraco J., Pézeron G., Appelbaum L., Zhang J., et al. (2007). Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 5:e277 10.1371/journal.pbio.0050277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulis C. R., Lederis K. (1987). Co-localization of the immunoreactivities of corticotropin-releasing factor and arginine vasotocin in the brain and pituitary system of the teleost Catostomus commersoni. Cell Tissue Res. 247, 267–273 10.1007/BF00218308 [DOI] [PubMed] [Google Scholar]
- Yulis C. R., Lederis K., Wong K. L., Fisher A. W. (1986). Localization of urotensin I- and corticotropin-releasing factor-like immunoreactivity in the central nervous system of Catostomus commersoni. Peptides 7, 79–86 [DOI] [PubMed] [Google Scholar]


