Abstract
Background:
Administration of daily recombinant human GH (rhGH) poses a considerable challenge to patient compliance. Reduced dosing frequency may improve treatment adherence and potentially overall treatment outcomes.
Objectives:
This study assessed the safety and tolerability and the potential for achieving IGF-I levels within the target range in adults with GH deficiency after a single dose of the long-acting rhGH analog, VRS-317.
Design:
This was a randomized, double-blind, placebo-controlled, single ascending dose study.
Patients:
Fifty adults with growth hormone deficiency (mean age, 45 years) were studied in 5 treatment groups of 10 subjects each (8 active drug and 2 placebo).
Setting:
The study was conducted in 17 adult endocrinology centers in North America and Europe.
Main Outcome Measures:
Adverse events, laboratory safety assessments, and VRS-317 pharmacokinetics and pharmacodynamics (IGF-I and IGF binding protein-3) were analyzed.
Results:
At 0.80 mg/kg, VRS-317 had a mean terminal elimination half-life of 131 hours. Single VRS-317 doses of 0.05, 0.10, 0.20, 0.40, and 0.80 mg/kg (approximately equivalent to daily rhGH doses of 0.3–5.0 μg/kg over 30 d) safely increased the amplitude and duration of IGF-I responses in a dose-dependent manner. After a single 0.80 mg/kg dose, serum IGF-I was maintained in the normal range between −1.5 and 1.5 SD values for a mean of 3 weeks. No unexpected or serious adverse events were observed.
Conclusions:
The elimination half-life for VRS-317 is 30- to 60-fold longer and stimulates more durable IGF-I responses than previously studied rhGH products. Prolonged IGF-I responses do not come at the expense of overexposure to high IGF-I levels. The pharmacokinetics and pharmacodynamics combined with the observed safety profile indicate the potential for safe and effective monthly dosing.
Achievement of treatment goals with recombinant human GH (rhGH) typically requires years of treatment. Maintaining full compliance with daily injections has been difficult for many patients and dose omissions occur frequently (1–4). In children, a lack of compliance with daily rhGH has been associated with significantly diminished growth velocities (5). A long-acting rhGH may reduce dosing frequency, improve compliance, and potentially improve overall treatment outcomes.
The potential benefits of a long-acting human GH (hGH) were recognized and explored more than 30 years ago (6) and a sustained-release rhGH (7–10) was approved for children but later withdrawn from distribution because of difficulties with manufacturing of the product. Since then, studies have been performed with a variety of modified rhGH molecules that may be suitable for weekly administration (11–18), but none has shown duration of action extending beyond 1 week and none has yet received approval from US or European Union regulatory authorities.
VRS-317 is a long-acting rhGH in development for use as long-term replacement therapy in adults and children with growth hormone deficiency (GHD). It is a fusion protein (molecular mass of 119 kDa) produced in Escherichia coli. The pharmacologically active portion is the rhGH domain (22 kDa), and the pharmacologically inactive domains are long chains of natural hydrophilic amino acids, referred to as XTEN (19). The XTEN domain enables extension of the half-life of rhGH by increasing the hydrodynamic size of rhGH and delaying receptor-mediated clearance. Although the in vitro potency of VRS-317 was reduced by approximately 12-fold compared with that of rhGH, the in vivo potency was increased because of the greatly prolonged exposure at the target tissues (20).
In light of previous pharmacokinetic (PK) and pharmacodynamic (PD) studies (10–15, 18) using sustained-release GH preparations, we performed a randomized, double-blind, placebo-controlled, multicenter, single ascending dose study, and we report the results of the safety, tolerability, and PK/PD of VRS-317 in adults with GHD.
Patients and Methods
Subject characteristics
Subjects had GHD as confirmed by a negative response to insulin (peak GH, <5.0 ng/mL), arginine-GHRH (peak GH based on body mass index [BMI]) (21, 22), glucagon (peak GH, <3.0 ng/mL) (23), or at least 3 other pituitary hormone deficiencies and a low IGF-I for age and sex (21). When GHD was due to a lesion in the sellar region, surveillance magnetic resonance imaging scans showed at least 6 months of stability. Treatments for other pituitary hormone deficiencies were stable for 2 months before study drug administration. Free T4 was in the normal range for all subjects when VRS-317 was administered. Each subject not receiving daily glucocorticoid treatment had normal responses to a standard-dose (250 μg) ACTH test to rule out secondary adrenal insufficiency. For female patients receiving estrogen, transdermal treatment was used and maintained throughout the study. Serum IGF-I responses to daily rhGH were characterized in all subjects before study drug administration. Key exclusion criteria included the presence of significant concurrent disease (eg, diabetes), active malignancy, anti-hGH antibodies at screening, pregnancy, or lactation or the use of oral estrogens.
Dose selection
Because in vivo potency was enhanced in monkeys (20), the VRS-317 dose range for the first dose in humans was selected to approximate the daily rhGH doses in the lower half of the typical dosing range for each 30-day interval (i.e., 0.03–0.5 mg rhGH/d or approximately 0.3–5.0 μg/kg/d). The VRS-317 doses selected were 0.05, 0.10, 0.20, 0.40, and 0.80 mg/kg administered as a single sc injection.
Study procedures and method of study
All patients provided signed informed consent before any study activity. The study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki with site-specific regulatory and ethics approval. The trial was performed in 17 endocrine centers under an investigational new drug application to the US Food and Drug Administration, clinical trial applications in Canada and the United Kingdom, and analogous clinical trial submissions in Sweden and Serbia. Initially, all subjects were given daily rhGH for a minimum of 28 days and until 2 successive IGF-I standard deviation scores (SDSs), measured at least 1 week apart, were within the range of −1.5 to 1.5 (+2.0 for men). Subjects were then withdrawn from daily rhGH until their IGF-I SDS decreased by at least 0.75 and had dropped to ≤−1.0. Subjects were then randomized to the treatment cohort enrolling at that time. On day 1, all subjects were admitted to the hospital and received a single sc dose of VRS-317 or placebo with an insulin syringe with a 29-gauge needle. Samples for PK/PD analysis were collected predose and at 0.5, 1.0, 2, 4, 8, 12, 24, 36 and 48 hours. Outpatient visits and additional PK/PD sampling were conducted on days 4, 8, 11, 15, 18, 22, 25, and 30. Glucose and lipid metabolism was assessed predose and on days 8, 15, 22, 30, 44, and 60. Testing for anti–VRS-317 antibodies was conducted predose and on days 30 and 60. Before proceeding to the next dosing level, safety data from at least 8 patients with a minimum of 7 days postdosing data were reviewed to ensure that the prespecified stopping criteria were not met before dose escalation. Adverse events were collected at each visit and graded using Common Terminology Criteria for Adverse Events (CTCAE) (24). Erythema and edema at injection sites were graded using the Draize technique (25).
Laboratory safety assessments were performed before and at selected times after dosing. Tests included standard blood counts, biochemistry analyses, fasting and postprandial glucose levels after a standard meal, hemoglobin A1c (HbA1c), and fasting levels of cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides.
Definition of patient populations
The safety population consisted of all 50 randomized subjects. The PK/PD population consisted of 48 subjects receiving either VRS-317 or placebo and excluded 2 subjects who received inappropriate doses for their weight (1 subject in the 0.80 mg/kg dose group and 1 subject in placebo group).
Assays
All assays were performed in central laboratories. VRS-317 concentrations were measured in plasma using an ELISA. The assay uses capture and detection antibodies to the XTEN and rhGH domains, respectively, to ensure detection of the intact molecule. Anti–VRS-317 antibodies were measured in samples taken predose and at days 30 and 60. Because of the potential for interference from high VRS-317 concentrations, samples were taken at the end of the dosing interval, and assays were performed using solid-phase extraction with acid dissociation followed by a direct electrochemiluminescence assay. Anti-rhGH antibodies were measured in a direct ELISA. VRS-317 concentration and antibody assays were developed and conducted at Intertek (San Diego, California). Serum IGF-I levels were measured to bioanalytical standards using an acid extraction, IGF-II blocking RIA at Esoterix (Calabasas Hills, California). The lower limit of quantitation for the IGF-I assay is 15 ng/ml. Serum IGF binding protein-3 (IGFBP-3) levels were also measured by RIA at Esoterix. The lower limit of quantitation for the IGFBP-3 assay is 0.3 mg/L. Assay-specific SDSs for IGF-I and IGFBP-3 were developed using power transformed normative data provided by Esoterix for the assays in use.
PK/PD analysis
VRS-317 PK parameters were estimated with noncompartmental techniques using WinNonlin professional version 5.3 (Pharsight Corporation, Mountain View, California). The IGF-I area under the curve (AUC) after a single sc dose of VRS-317 was calculated using the linear trapezoid rule, and average IGF-I was calculated by dividing IGF-I AUC by the time of the dosing interval.
Statistical analysis
Descriptive statistics and multivariate analyses were conducted according to a statistical analysis plan finalized before database lock. Laboratory parameters were analyzed for change from predose baseline by analysis of covariance (ANCOVA) with change as the dependent variable, treatment as a cofactor, and baseline value as a covariate. Values of P < 0.05 defined statistical significance. Data management, data quality assurance, and statistical analyses were conducted by Medpace, Inc (Cincinnati, Ohio).
Results
Patient disposition and characteristics
Sixty-nine subjects were screened; there were 19 screen failures, and 50 subjects were randomized to 5 groups, each consisting of 8 active drug– and 2 placebo-treated subjects. There were no withdrawals after randomization, and all 50 randomized subjects completed the 60-day dose evaluation period. There were 21 women and 29 men with mean (SD) age and BMI of 44.7 (12.9) years and 30.9 (6.2) kg/m2, respectively (Table 1). Age distributions were similar in each of the five dosing cohorts; however, some sex imbalance occurred between dosing arms (the placebo and 0.10 mg/kg cohorts included 6 men and 2 women, and the 0.80 mg/kg cohort had 3 men and 5 women). Daily rhGH doses in the stability phase were in the range of 0.4 to 0.6 mg/d (4.1–5.8 μg/kg/d) on average across all dose groups. For subjects randomized to VRS-317, the mean change in IGF-I SDS after rhGH withdrawal ranged from −1.7 to −2.4, and withdrawal criteria were satisfied within 7 days of rhGH withdrawal in 68% of subjects.
Table 1.
Characteristics of Randomized Subjects
| Characteristic | Treatment Group |
|||||
|---|---|---|---|---|---|---|
| 0.05 mg/kg (n = 8) | 0.10 mg/kg (n = 8) | 0.20 mg/kg (n = 8) | 0.40 mg/kg (n = 8) | 0.80 mg/kg (n = 8) | Placebo (n = 10) | |
| Age, y | 41.1 (29, 57) | 55.5 (48, 64) | 37.1 (27, 59) | 44.6 (29, 58) | 43.1 (26, 59) | 46.3 (26, 66) |
| Male sex, n (%) | 4 (50) | 6 (75) | 5 (62.5) | 5 (62.5) | 3 (37.5) | 6 (60) |
| BMI, kg/m2 | 34.2 (23, 45) | 29.5 (20, 43) | 33.7 (27, 44) | 30.8 (23, 38) | 27.5 (19, 34) | 30.0 (23, 39) |
| Height, cm | 173.1 (160,180) | 175.8 (160,185) | 173.1 (151,183) | 169.1 (153,178) | 170.0 (159, 188) | 172.9 (159,191) |
| Weight, kg | 103.2 (58,138) | 90.9 (61, 124) | 101.3 (70, 130) | 88.1 (66,115) | 80.7 (49,119) | 90.9 (61,144) |
| rhGH dose, μg/kg/d | 5.1 (1.5, 9.6) | 4.1 (2.5, 7.3) | 5.0 (1.9, 7.9) | 5.2 (2.5, 10.5) | 5.8 (2.5, 10.2) | 5.2 (2.3, 10.3) |
| IGF-I SDS in daily rhGH phase | 0.11 (−0.54,1.4) | 0.17 (−1.1,1.5) | −0.21 (−1.5,0.9) | −0.63 (−1.4, −0.1) | 0.02 (−1.3, 1.6) | −0.12 (−1.2, 0.9) |
| IGF-I SDS at baseline | −1.74 (−2.2, −0.8) | −2.27 (−2.9, −1.9) | −2.09 (−3.0, −1.4) | −2.30 (−2.90, −0.7) | −1.75 (−2.9, −1.1) | −1.52 (−3.1, −0.7) |
| Change in IGF-I SDS (daily to baseline) | −1.85 (−3.4, −0.6) | −2.44 (−3.4, −1.9) | −1.88 (−2.9, −0.5) | −1.67 (−2.4, −0.6) | −1.77 (−4.4, −0.7) | −1.40 (−2.6, −0.8) |
Values are means (minimum, maximum). Baseline is defined as the last measurement before study drug administration.
Pharmacokinetics
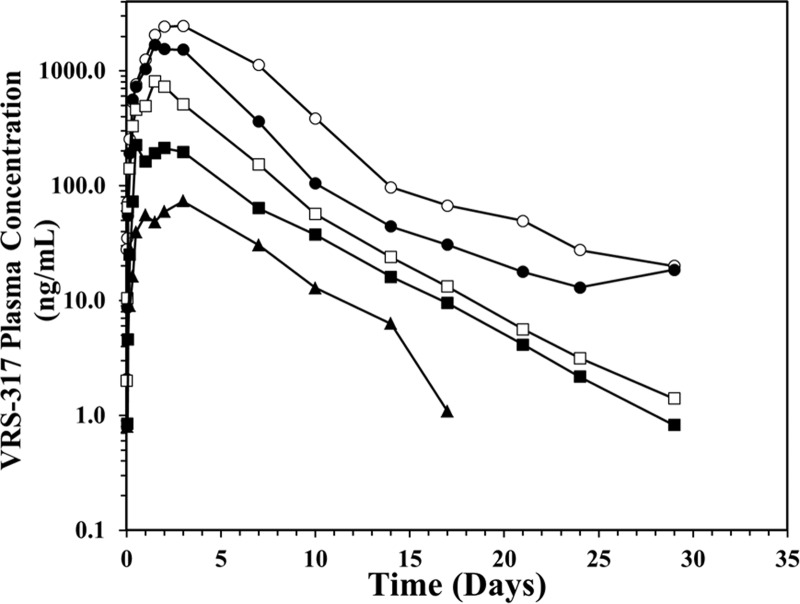
A single sc dose resulted in rapid absorption and prolonged serum exposure to VRS-317 (Figure 1). Mean maximal VRS-317 plasma concentrations (Cmax) were reached at 44 to 82 hours. VRS-317 exposure was directly proportional to dose. There was a general trend for VRS-317 elimination half-life (t1/2) to increase with increasing dose. The mean t1/2 was 131 hours at the highest dose tested (0.80 mg/kg) (Table 2). In multivariate analyses, the AUC0–t for VRS-317 was highly correlated to dose (P < .0001), but no significant age or sex effect was observed in this population.
Figure 1.
Time course of mean VRS-317 concentrations in adult subjects with GHD receiving a single sc dose on day 1. Subjects received a single sc dose of either 0.05 (▴), 0.10 (■), 0.20 (□), 0.40 (●) or 0.80 (○) mg/kg VRS-317.
Table 2.
VRS-317 PK Parameters in GH-Deficient Adults After a Single Subcutaneous Injection
| Dose | Cmax, ng/mL | Tmax, h | AUC0–t, ng·h/mL | AUC0–∞, ng·h/mL | t1/2, h |
|---|---|---|---|---|---|
| 0.05 mg/kg | 92 ± 29 | 46 ± 27 | 11,161 ± 3,395 | 11,706 ± 3,499 | 68 ± 18 |
| 0.10 mg/kg | 354 ± 368 | 44 ± 21 | 33,365 ± 16,410 | 33,822 ± 16,343 | 85 ± 34 |
| 0.20 mg/kg | 889 ± 606 | 50 ± 19 | 86,429 ± 67,201 | 87,291 ± 67,068 | 90 ± 50 |
| 0.40 mg/kg | 1968 ± 676 | 48 ± 17 | 241,280 ± 121,549 | 244,601 ± 125,167 | 109 ± 57 |
| 0.80 mg/kg | 2887 ± 1345 | 82 ± 39 | 402,541 ± 124,653 | 407,421 ± 124,915 | 131 ± 62 |
Values are means ± SD. The dose proportionality correlation coefficients (log:log) were 0.87 for Cmax and 0.93 for AUC0–t.
Abbreviations: AUC0–t, area under the curve from time 0 to the last measurable time point; AUC0-–∞, area under the curve from time 0 to infinity; Cmax, maximum concentration; Tmax, time to maximum concentration.
Pharmacodynamics
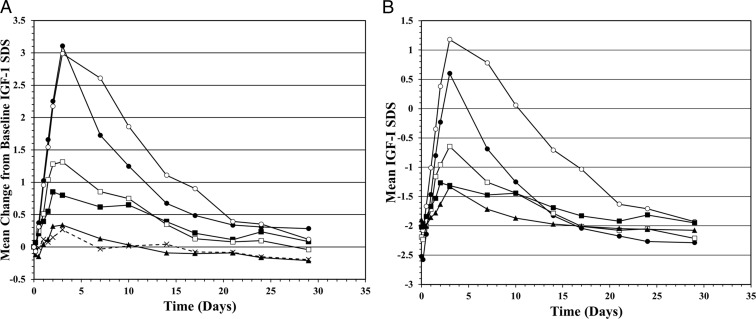
Serum IGF-I concentration was the primary PD marker for this study. The amplitude and duration of IGF-I exposure was directly proportional to the VRS-317 dose (Figure 2A and Table 3). The maxima for mean changes in IGF-I concentrations and IGF-I SDS were similar for the 0.40 and 0.80 mg/kg groups. The similarity may have been caused by the uneven distribution of subject characteristics affecting IGF-I responses to VRS-317. Therefore, an ANCOVA was used to examine the set of all postdose values of IGF-I concentration for dependencies on age, sex, treatment day, VRS-317 dose, treatment by day interaction (as factors), and baseline (predose) IGF-I concentration (as covariate). Dose, day, and dose and treatment × day interaction were all significant (P < .0001) as were age (P = .0034) and sex (P = .0224). Higher doses, male sex, and younger age were all associated with greater IGF-1 responses.
Figure 2.
A, Mean change in IGF-1 SDS for placebo and 5 VRS-317 dosing groups. Data represent the mean of subjects in a dose group for the differences for each subject between his or her baseline and each subsequent time point and are not the absolute mean IGF-1 SDS at each time point. Subjects received a single sc dose of either placebo (10 subjects, ×) or 0.05 (8 subjects, ▴), 0.10 (8 subjects, ■), 0.20 (8 subjects, □), 0.40 (8 subjects, ●), or 0.80 (7 subjects, ○) mg/kg VRS-317. B, Extent of normalization of IGF-I SDS after a single sc dose administration of VRS-317. Mean IGF-I SDS for subjects with a baseline IGF-I SDS of ≤ −1.5. Subjects received a single sc dose of either 0.05 (7 subjects, ▴), 0.10 (8 subjects, ■), 0.20 (7 subjects, □), 0.40 (7 subjects, ●), or 0.80 (5 subjects, ○) mg/kg VRS-317.
Table 3.
PD Response Summary of IGF-I to a Single Dose Administration of VRS-317 in the PK/PD Population (n = 48)
| Dose | No. | Stability, ng/mL | Baseline, ng/mL | Cmax, ng/mL | Cmax (SDS) | Tmax, d | AUC0–t, ng·h/mL | Average, ng/mL |
|---|---|---|---|---|---|---|---|---|
| Placebo | 9 | 188 ± 49 | 106 ± 47 | ND | ND | ND | ND | 102 ± 49 |
| 0.05 mg/kg | 8 | 212 ± 41 | 97 ± 47 | 137 ± 58 | −1.1 ± 0.7 | 6.4 ± 6.5 | 2837 ± 1330 | 95 ± 44 |
| 0.10 mg/kg | 8 | 170 ± 30 | 57 ± 18 | 105 ± 43 | −1.2 ± 0.9 | 5.0 ± 2.9 | 2214 ± 855 | 74 ± 29 |
| 0.20 mg/kg | 8 | 214 ± 68 | 86 ± 30 | 196 ± 58 | −0.5 ± 0.9 | 4.1 ± 1.8 | 3541 ± 1260 | 118 ± 42 |
| 0.40 mg/kg | 8 | 165 ± 44 | 70 ± 40 | 248 ± 87 | 0.9 ± 1.4 | 4.5 ± 1.4 | 3771 ± 1524 | 126 ± 51 |
| 0.80 mg/kg | 7 | 197 ± 76 | 89 ± 31 | 280 ± 103 | 1.4 ± 1.3 | 5.1 ± 2.0 | 4884 ± 915 | 163 ± 31 |
Stability refers to the time during which daily rhGH treatment was given. Baseline refers to day 1, before the dose of VRS-317 or placebo. The IGF-I AUC was calculated using the linear trapezoid rule. Average IGF-I was calculated by dividing AUC by the observation interval of 29 days. The dose proportionality correlation coefficients (log:log) were 0.76 for baseline corrected Cmax and 0.76 for baseline corrected AUC0–t.
Abbreviations: AUC0–t, area under the curve from time zero to the last measurable time point; Cmax, maximum concentration; ND, not determined; Tmax, time to maximum concentration.
The extent and duration to which IGF-I SDS were normalized were also VRS-317 dose dependent. An analysis of subjects having an IGF-I SDS less than −1.5 at the time of dosing indicated that VRS-317 increases IGF-I SDS into the target range of −1.5 to 1.5 in a dose-dependent manner (Figure 2B). IGF-I SDS was normalized for a mean of approximately 3 weeks for the 0.80 mg/kg group. This prolonged duration of normalization did not come at the expense of overexposure to IGF-I. The 40 VRS-317–treated patients had a total of 513 postdose IGF-I SDS determinations and only 8 values (1.6%) in 6 patients were above the normal range (SDS >+2). The individual IGF-I SDSs greater than +2 (ranging from 2.01 to 3.59) were observed only in the 0.40 and 0.80 mg/kg groups and usually occurred within 72 hours after dosing and had normalized by the subsequent sampling time.
IGFBP-3 SD scores were low at baseline (mean, −1.28; SD, 1.82) but increased with VRS-317 dosing. The time course of change in IGFBP-3 was similar to that of IGF-I. Maximal IGFBP-3 responses were generally observed at day 4 or day 8. The changes in IGFBP-3 levels were dose dependent. At day 8, the least square mean changes in IGFBP-3 were 0.05, 0.17, 0.55, 0.80, and 1.41 mg/L (IGFBP-3 SDS Cmax of −0.6 to 2.6) for the 0.05, 0.10, 0.20, 0.40, and 0.80 mg/kg dosing groups, respectively. In ANCOVA, IGFBP-3 responses were dependent on the VRS-317 dose, day, and baseline value (all P < .0001), but no effects of age or sex were observed. At baseline, the IGF-I/IGFBP-3 molar ratio was 0.22 ± 0.05 and did not differ among the different dosing groups (P = .49). Mean maximal molar ratio values were observed on day 4 and increased with increasing VRS-317 dose (P < .0001). The maximal mean molar ratio for the 0.80 mg/kg group was 0.47 ± 0.11. The maximal molar ratio value for any subject was 0.65.
Safety results
Per protocol, safety data from a minimum of 8 patients exposed for a minimum of 7 days were reviewed before patients were enrolled in the next higher dose level. None of the protocol-specified stopping criteria were met; consequently, all 5 planned dosing levels were studied. There were no unexpected adverse events (AEs) related to the study drug.
Nonlaboratory AEs considered to be related to the study drug by investigators were transient and mild (CTCAE grade 1 except for 2 cases of grade 2) and occurred in a minority of subjects (Table 4). Many related events (headache [4], arthralgia [3], myalgia [1], and edema [1]) were of the type typically observed when rhGH therapy is started in adult patients with GHD. The 0.40 and 0.80 mg/kg dosing groups had the greatest number of any related AEs (7 in each group), but no specific event had a clear dose relationship.
Table 4.
Treatment-Emergent Adverse Events Possibly, Probably, or Definitely Related to Study Drug Administration in the Safety Population (n = 50)
| Treatment Group |
||||||
|---|---|---|---|---|---|---|
| Placebo | 0.05 mg/kg | 0.10 mg/kg | 0.20 mg/kg | 0.40 mg/kg | 0.80 mg/kg | |
| No. subjects | 10 | 8 | 8 | 8 | 8 | 8 |
| No. subjects with any event | 2 | 1 | 4 | 4 | 7 | 7 |
| No. events | 2 | 1 | 2 | 3 | 4 | 6 |
| Headache | 0 | 0 | 1 | 1 | 2 | 0 |
| Cognitive disorder | 1 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 | 0 | 0 | 0 | 1 | 0 |
| Arthralgia | 0 | 0 | 1 | 0 | 0 | 2 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 1 |
| Muscle fatigue | 0 | 0 | 0 | 0 | 0 | 1 |
| Edema | 0 | 0 | 0 | 0 | 0 | 1 |
| Rash | 0 | 0 | 0 | 1 | 1 | 0 |
| Generalized pruritus | 0 | 1 | 0 | 0 | 0 | 0 |
| Paresthesia | 0 | 0 | 0 | 1 | 0 | 0 |
| Warm skin | 0 | 0 | 0 | 0 | 0 | 1 |
Injection site reactions and laboratory events are discussed in the text.
Injection site reactions were the most commonly reported drug-related AEs. Injection site erythema was noted in 30% of VRS-317–treated and 10% of placebo-treated subjects. Injection site edema was noted in 10% of VRS-317–treated subjects and 10% of placebo-treated subjects. Injection site pain or tenderness was observed in 15% of VRS-317–treated subjects. In general, for placebo and study drug–treated patients, injection site reactions appeared within 24 hours and were mild (Draize I, barely perceptible) and transient. There were no instances of injection site lipoatrophy or hypersensitivity reported throughout 60 days of posttreatment observation.
There were no reported safety events or clinically meaningful changes related to any glucose metabolism parameter. No patient had a glucose result in the diabetic range (fasting glucose ≥126 mg/dL and postprandial glucose ≥200 mg/dL). All mean and individual values for HbA1c remained within the normal range. No clinically meaningful changes (≥0.2%) were noted in change from baseline HbA1c vs placebo in any treatment group. One patient each from the 0.10 and 0.20 mg/kg dosing group had worsening of previously elevated levels of serum cholesterol, LDL, and triglycerides as possibly related AEs. However, at the highest VRS-317 dose (0.80 mg/kg), there was a temporal pattern of reduction in cholesterol, LDL, and triglycerides, maximal at day 8 and persisting through day 22. The maximal percent decreases from baseline were 11.3 (P = .0026), 14.6 (P = .014), and 14.5% (P = .19) for cholesterol, LDL, and triglycerides, respectively.
Antibody assessments
Nonspecific binding was noted in the anti-hGH antibody assay. As required by the inclusion criteria, no subject had a significant titer (≥1:10) of specific anti-rhGH antibodies at screening, and no subject tested positive at 7 days after daily rhGH withdrawal. A single subcutaneous administration of VRS-317 to adult GHD patients previously treated with daily rhGH resulted in a minority of subjects (4 of 40) generating an anti–VRS-317 antibody response at a low titer (3 of 4 subjects at 1:5 and 1 subject at 1:25). Three of these 4 subjects had nonspecific binding in the anti-hGH antibody assay. Analysis of potential antibody effects on clinical or pharmacological endpoints was precluded by the low number of subjects testing positive for anti–VRS-317; there were no notable differences in IGF-I responses with these 4 subjects.
Discussion
VRS-317 is an investigational long-acting rhGH in development for long-term replacement therapy for adults and children with GHD. VRS-317 was designed to achieve once-monthly dosing with the anticipation that a reduced frequency of administration (12 vs up to 365 injections per year) would increase treatment adherence and thereby potentially improve overall treatment outcomes. VRS-317 is a novel rhGH fusion protein that was designed to minimize receptor-mediated clearance through a reduction in receptor binding without mutations to rhGH by genetically fusing XTEN amino acid sequences to the N and C termini of the native hGH sequence (20). Functionally, the XTEN domains increase the hydrodynamic radius and reduce binding affinity to the GH receptor in vitro. Despite reduced binding affinity, durable PD responses are seen in vivo, possibly relating to reduced rates of receptor-mediated clearance of VRS-317 (20). The reduced rate of clearance significantly prolongs serum residence times of VRS-317, resulting in enhanced ligand time on target and potentially increasing the probability of a successful ligand-receptor interaction. The terminal elimination half-life of VRS-317 at the highest dose was 131 hours; this represents a 30- to 60-fold increase over those reported in package inserts for daily rhGH.
The current study was the first in humans for VRS-317 and extends prior knowledge about long-acting rhGH because it represents the most prolonged duration of action of any rhGH analog in the treatment of adults with GHD. All subjects were adults with GHD diagnosed in accordance with current consensus guidelines of The Endocrine Society (21), the American Association of Clinical Endocrinologists (22), and the Growth Hormone Research Society (26). There was a slight preponderance of male subjects (29 men and 21 women), but the numbers of each sex were adequate to test for sex effects on drug distribution and PD effects. Each subject was initially stabilized with daily rhGH injections and to achieve stable IGF-I SDSs within the normal range had been taking 0.2 to 1.0 mg of hGH/d (1.5–10.5 μg/kg/d) (mean, 0.6 mg/d). After discontinuation of daily rhGH, IGF-I SDSs decreased in all subjects with group mean decrements of 1.7 to 2.4 SD. Subjects requiring daily medication that could alter sensitivity to rhGH (e.g., insulin, oral estrogens, and anti-inflammatory doses of glucocorticoids) were excluded from this first dosing study of VRS-317.
Over the VRS-317 dosing range, drug exposure parameters (Cmax and AUC) were directly and highly proportional to dose. In general, both the amplitude and duration of exposure increases with increased VRS-317 doses. No sex or age effects were detected in the VRS-317 dose-exposure relationship. VRS-317 was safe and well tolerated at all dose levels, suggesting that greater dose and duration of exposures can be explored in future human studies. The PD (IGF-I and IGFBP-3) responses to VRS-317 were also directly proportional to dose, with amplitude and duration increasing with increased dose. At the highest dose, the mean IGF-I SDS was maintained at greater than −1.5 for approximately 3 weeks. Given the demonstrated proportionality between dose and duration, the duration of IGF-I normalization could be extended by increased VRS-317 doses. Over the dose range assessed in this study, the duration of IGF-I normalization does not come at the expense of overexposure to IGF-I because only 1.6% of IGF-I SDSs were ≥2; moreover, these elevations were transient. There were age and sex effects on IGF-I responses to VRS-317. Women and older subjects had lower IGF-I responses than men and younger subjects. Sex differences for IGF-I induction are well known for daily rhGH and are likely to be due to estrogen effects on the liver affecting IGF-I production (27–30). Similar to the effects of daily rhGH, IGF-I induction by VRS-317 in adults may be lower in women than in men.
VRS-317 was administered at doses from 0.05 to 0.80 mg/kg, approximating daily rhGH doses of 0.3 to 5.0 μg/kg/d over 30 days. Over this range, a single dose of VRS-317 was safe and well tolerated. There were no treatment-emergent serious AEs or suspected unexpected serious adverse reactions. No subject withdrew from the study after dosing; all subjects completed the protocol-specified 60-day safety observation period. Minimal transient erythema at the injection site(s) was the most commonly reported AE. Other events considered as possibly, probably, or definitely related to the study drug were typical of those seen when adult patients with GHD receive replacement therapy. These events were transient and were categorized as mild to moderate. No injection site lipoatrophy was observed. Surveillance for VRS-317 alterations in carbohydrate metabolism included serial measurements of fasting glucose and insulin, postprandial glucose, and HbA1c. No clinically meaningful temporal or dose-related changes were observed in any of these parameters, indicating that the prolonged action and delayed clearance of VRS-317 did not confer any additional risk to overall glycemic safety in these patients. These findings are in accordance with previous studies using low doses of daily rhGH (23, 31–34) but in contrast to other studies showing elevated glucose and insulin with decreased insulin sensitivity indices using higher rhGH doses (>0.6 mg/d) (35–37). Although 2 subjects in a lower dose group had increases in previously elevated levels of LDL, total cholesterol, and triglycerides, there was a temporal pattern of decrease in these parameters with the highest VRS-317 dose (0.80 mg/kg). It is thought that rhGH dose and duration effects as well as individual susceptibility will influence glucose, lipid, and insulin responses. Continued surveillance for alterations in lipid and glucose parameters is warranted during subsequent chronic dosing trials (38).
Four of the 40 VRS-317–treated subjects had detectable anti–VRS-317 antibodies appearing at days 30 and/or 60 after VRS-317 dosing. These subjects had received VRS-317 doses of 0.20 mg/kg (1 subject), 0.40 mg/kg (2 subjects), and 0.80 mg/kg (1 subject). Three of these 4 had had nonspecific binding in the anti-rhGH antibody screening assay. Additional testing of adult subjects with GHD who are naive to treatment and testing during repeat dosing will be required to better assess the immunogenicity of VRS-317.
In conclusion, single-dose administration of VRS-317 is safe and well tolerated over the range of doses studied and provides prolonged normalization of IGF-I responses in adults with GHD. The safety and PK/PD profiles indicate that VRS-317 doses may be further increased to prolong IGF-I responses in this population. With monthly VRS-317, IGF-I SDS may be normalized for prolonged periods with minimal risk of exposure to supraphysiological IGF-I levels. Given its delayed clearance, VRS-317 has the potential for monthly dosing in adults with GHD.
Supplementary Material
Acknowledgments
The sponsor gratefully acknowledges Drs. G. Alexander Fleming, David Orloff, Ron Rosenfeld, Christian Strasburger, Gudmundur Johansson, and Pinchas Cohen for thoughtful discussions on the proposed design and conduct of the trial. The investigators serving as coauthors also contributed to the design of the study and enrolled patients in the study. Drs. Jolene Berg (Cetero Research), Mark Christiansen (Diablo Research), Kathleen Colleran (Univ. New Mexico), Anthony Heaney (UCLA), Charlotte Hoybye (Karolinska Institute), Omar Serri (CHUM Notre Dame Hospital), Larry Stonesifer (Seattle, Washington), Peter Trainer (The Christie Hospital), and Jacqueline Weil (Clinical Trial Network of Houston) also contributed patients to the trial. We acknowledge the team of Clinical Study Coordinators whose efforts and expertise were essential to the successful completion of this study.
This work was supported by Versartis, Inc., the National Center for Advancing Translational Sciences at the National Institutes of Health (grant UL1RR024140 to K.C.J.Y.) and the Harvard Clinical and Translational Science Center from the National Center for Research Resources (grant 1 UL1 RR025758-04 to B.M.K.B.).
This study was registered with clinical trial registration number NCT01359488 (www.ClinicalTrials.gov).
Disclosure Summary: E.H., G.M.B., and J.L.C. are employees of Versartis, Inc. B.M.K.B. and M.K. received advisory board consultancy fees from Versartis, Inc. K.C.J.Y., G.S.C., V.P., G.R.M., T.B., A.H.H., B.M.K.B., and M.K. were investigators for the trial. J.A.M. is a pharmacology consultant to Versartis, Inc.
Footnotes
- AE
- adverse event
- ANCOVA
- analysis of covariance
- AUC
- area under the curve
- BMI
- body mass index
- GHD
- GH deficiency
- HbA1c
- hemoglobin A1c
- hGH
- human GH
- IGFBP-3
- IGF binding protein-3
- LDL
- low-density lipoprotein
- PD
- pharmacodynamic
- PK
- pharmacokinetic
- rhGH
- recombinant human GH
- SDS
- standard deviation score.
References
- 1. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143–154 [DOI] [PubMed] [Google Scholar]
- 2. Desrosiers P, O'Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Pediatr Endocrinol Rev. 2005;3(2 suppl):327–331 [PubMed] [Google Scholar]
- 3. Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148 [DOI] [PubMed] [Google Scholar]
- 4. Hunter I, de Vries C, Morris A, MacDonald T, Greene SA. Human growth hormone therapy: poor adherence equals poor growth. Arch Dis Child. 2000;82(suppl 1):A8 [Google Scholar]
- 5. Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6:e16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippe B, Frasier SD, Kaplan SA. Use of growth hormone-gel. Arch Dis Child. 1979;54:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silverman BL, Blethen SL, Reiter EO, Attie KM, Neuwirth RB, Ford KM. A long-acting human growth hormone (Nutropin depot): efficacy and safety following two years of treatment in children with growth hormone deficiency. J Pediatr Endocrinol Metab. 2002;15:715–722 [DOI] [PubMed] [Google Scholar]
- 8. Hoffman AR, Biller BM, Cook D, et al. Efficacy of a long-acting growth hormone (GH) preparation in patients with adult GH deficiency. J Clin Endocrinol Metab. 2005;90:6431–6440 [DOI] [PubMed] [Google Scholar]
- 9. Kemp SF, Fielder PJ, Attie KM, et al. Pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (Nutropin depot) in GH-deficient children. J Clin Endocrinol Metab. 2004;89:3234–3240 [DOI] [PubMed] [Google Scholar]
- 10. Cook DM, Biller BM, Vance ML, et al. The pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (Nutropin depot) in GH-deficient adults. J Clin Endocrinol Metab. 2002;87:4508–4514 [DOI] [PubMed] [Google Scholar]
- 11. Biller BM, Ji HJ, Ahn H, et al. Effects of once-weekly sustained-release growth hormone: a double-blind, placebo-controlled study in adult growth hormone deficiency. J Clin Endocrinol Metab. 2011;96:1718–1726 [DOI] [PubMed] [Google Scholar]
- 12. Péter F, Bidlingmaier M, Savoy C, Ji HJ, Saenger PH. Three-year efficacy and safety of LB03002, a once-weekly sustained-release growth hormone (GH) preparation, in prepubertal children with GH deficiency (GHD). J Clin Endocrinol Metab. 2012;97:400–407 [DOI] [PubMed] [Google Scholar]
- 13. Fares F, Guy R, Bar-Ilan A, Felikman Y, Fima E. Designing a long-acting human growth hormone (hGH) by fusing the carboxyl-terminal peptide of human chorionic gonadotropin beta-subunit to the coding sequence of hGH. Endocrinology. 2010;151:4410–4417 [DOI] [PubMed] [Google Scholar]
- 14. Søndergaard E, Klose M, Hansen M, et al. Pegylated long-acting human growth hormone possesses a promising once-weekly treatment profile, and multiple dosing is well tolerated in adult patients with growth hormone deficiency. J Clin Endocrinol Metab. 2011;96:681–688 [DOI] [PubMed] [Google Scholar]
- 15. de Schepper J, Rasmussen MH, Gucev Z, Eliakim A, Battelino T. Long-acting pegylated human GH in children with GH deficiency: a single-dose, dose-escalation trial investigating safety, tolerability, pharmacokinetics and pharmacodynamics. Eur J Endocrinol. 2011;165:401–409 [DOI] [PubMed] [Google Scholar]
- 16. Ferrandis E, Pradhananga SL, Touvay C, et al. Immunogenicity, toxicology, pharmacokinetics and pharmacodynamics of growth hormone ligand-receptor fusions. Clin Sci (Lond). 2010;119:483–491 [DOI] [PubMed] [Google Scholar]
- 17. Rasmussen MH, Bysted BV, Anderson TW, Klitgaard T, Madsen J. Pegylated long-acting human growth hormone is well-tolerated in healthy subjects and possesses a potential once-weekly pharmacokinetic and pharmacodynamic treatment profile. J Clin Endocrinol Metab. 2010;95:3411–3417 [DOI] [PubMed] [Google Scholar]
- 18. Bidlingmaier M, Kim J, Savoy C, et al. Comparative pharmacokinetics and pharmacodynamics of a new sustained-release growth hormone (GH), LB03002, versus daily GH in adults with GH deficiency. J Clin Endocrinol Metab. 2006;91:2926–2930 [DOI] [PubMed] [Google Scholar]
- 19. Schellenberger V, Wang CW, Geething NC, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27:1186–1190 [DOI] [PubMed] [Google Scholar]
- 20. Cleland JL, Geething NC, Moore JA, et al. A novel long-acting human growth hormone fusion protein (VRS-317): enhanced in vivo potency and half-life. J Pharm Sci. 2012;101:2744–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609 [DOI] [PubMed] [Google Scholar]
- 22. Cook DM, Yuen KC, Biller BM, Kemp SF, Vance ML. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr Pract. 2009;15(suppl 2):1–29 [DOI] [PubMed] [Google Scholar]
- 23. Yuen KC, Biller BM, Molitch ME, Cook DM. Clinical review: Is lack of recombinant growth hormone (GH)-releasing hormone in the United States a setback or time to consider glucagon testing for adult GH deficiency? J Clin Endocrinol Metab. 2009;94:2702–2707 [DOI] [PubMed] [Google Scholar]
- 24. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4. evs.nci.nih.gov/ftp1/CTCAE/About.html Accessed May 1, 2013
- 25. Draize JH, Woodward G, Calvary HO. methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390 [Google Scholar]
- 26. Ho KK, 2007 GH Deficiency Consensus Workshop Participants Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700 [DOI] [PubMed] [Google Scholar]
- 27. Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72:374–381 [DOI] [PubMed] [Google Scholar]
- 28. Drake WM, Coyte D, Camacho-Hübner C, et al. Optimizing growth hormone replacement therapy by dose titration in hypopituitary adults. J Clin Endocrinol Metab. 1998;83:3913–3919 [DOI] [PubMed] [Google Scholar]
- 29. Hoffman AR, Strasburger CJ, Zagar A, et al. Efficacy and tolerability of an individualized dosing regimen for adult growth hormone replacement therapy in comparison with fixed body weight-based dosing. J Clin Endocrinol Metab. 2004;89:3224–3233 [DOI] [PubMed] [Google Scholar]
- 30. Birzniece V, Sutanto S, Ho KK. Gender difference in the neuroendocrine regulation of growth hormone axis by selective estrogen receptor modulators. J Clin Endocrinol Metab. 2012;97:E521–E527 [DOI] [PubMed] [Google Scholar]
- 31. Spina LD, Soares DV, Brasil RR, et al. Glucose metabolism and visceral fat in GH deficient adults: 1 year of GH replacement. Growth Horm IGF Res. 2004;14:45–51 [DOI] [PubMed] [Google Scholar]
- 32. Hana V, Silha JV, Justova V, Lacinova Z, Stepan JJ, Murphy LJ. The effects of GH replacement in adult GH-deficient patients: changes in body composition without concomitant changes in the adipokines and insulin resistance. Clin Endocrinol (Oxf). 2004;60:442–450 [DOI] [PubMed] [Google Scholar]
- 33. Bülow B, Link K, Ahrén B, Nilsson AS, Erfurth EM. Survivors of childhood acute lymphoblastic leukaemia, with radiation-induced GH deficiency, exhibit hyperleptinaemia and impaired insulin sensitivity, unaffected by 12 months of GH treatment. Clin Endocrinol (Oxf). 2004;61:683–691 [DOI] [PubMed] [Google Scholar]
- 34. Yuen KC, Dunger DB. Therapeutic aspects of growth hormone and insulin-like growth factor-I treatment on visceral fat and insulin sensitivity in adults. Diabetes Obes Metab. 2007;9:11–22 [DOI] [PubMed] [Google Scholar]
- 35. Boguszewski CL, Meister LH, Zaninelli DC, Radominski RB. One year of GH replacement therapy with a fixed low-dose regimen improves body composition, bone mineral density and lipid profile of GH-deficient adults. Eur J Endocrinol. 2005;152:67–75 [DOI] [PubMed] [Google Scholar]
- 36. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 37. Christopher M, Hew FL, Oakley M, Rantzau C, Alford F. Defects of insulin action and skeletal muscle glucose metabolism in growth hormone-deficient adults persist after 24 months of recombinant human growth hormone therapy. J Clin Endocrinol Metab. 1998;83:1668–1681 [DOI] [PubMed] [Google Scholar]
- 38. Johannsson G. Long-acting growth hormone for replacement therapy. J Clin Endocrinol Metab. 2011;96:1668–1670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.