Abstract
Background:
Infusion of ghrelin to supraphysiologic levels inhibits glucose-stimulated insulin secretion, reduces insulin sensitivity, and worsens glucose tolerance in humans.
Objective:
The purpose of this study was to determine the effects of lower doses of ghrelin on insulin secretion and insulin sensitivity in healthy men and women.
Methods:
Acyl ghrelin (0.2 and 0.6 nmol kg−1 h−1) or saline was infused for 225 minutes in 16 healthy subjects on 3 separate occasions in randomized order. An iv glucose tolerance test was performed, and the insulin sensitivity index (SI) was derived from the minimal model. Insulin secretion was measured as the acute insulin response to glucose (AIRg) and the disposition index was computed as AIRg × SI.
Results:
Ghrelin infusions at 0.2 and 0.6 nmol kg−1 h−1 raised steady-state plasma total ghrelin levels 2.2- and 6.1-fold above fasting concentrations. Neither dose of ghrelin altered fasting plasma insulin, glucose, or SI, but both doses reduced insulin secretion compared with the saline control, computed either as AIRg (384 ± 75 and 354 ± 65 vs 520 ± 110 pM · min [mean ± SEM], respectively; P < .01 for both low- and high-dose vs saline) or disposition index (2238 ± 421 and 2067 ± 396 vs 3339 ± 705, respectively; P < .02 for both comparisons). The high-dose ghrelin infusion also decreased glucose tolerance.
Conclusions:
Ghrelin infused to levels occurring in physiologic states such as starvation decreases insulin secretion without affecting insulin sensitivity. These findings are consistent with a role for endogenous ghrelin in the regulation of insulin secretion and suggest that ghrelin antagonism could improve β-cell function.
Ghrelin is a gastrointestinal peptide first identified as a stimulus for GH secretion (1, 2) but subsequently demonstrated to be a circulating factor that promotes food intake and increases adiposity (3, 4). Ghrelin is secreted mainly from the stomach and proximal small bowel with a small amount also produced in a novel endocrine islet cell type (ϵ cells) in the pancreas (5). The peptide backbone of ghrelin is acylated at the serine-3 residue (2), a modification required for the binding and activation of the GH secretagogue receptor (GHSR) 1a, the only known target for ghrelin (6). GHSR is expressed in specific nuclei of the central nervous system (7) but has also been found on pancreatic islet α- and β-cells (8, 9). Preclinical studies suggest a direct effect of ghrelin to suppress insulin secretion and cause glucose intolerance (10, 11).
We recently demonstrated that infusion of exogenous ghrelin suppressed glucose-stimulated insulin secretion and caused glucose intolerance in healthy humans (12). In these studies, the doses of exogenous ghrelin raised plasma concentrations to supraphysiologic levels, greatly exceeding those occurring even in pathologic states such as severe weight loss, anorexia nervosa, liver failure, and cancer cachexia in which endogenous plasma ghrelin is elevated 3- to 4-fold (13, 14). Thus, although our initial observations served to demonstrate a potential new regulatory effect of ghrelin, the large doses of peptide used in this study raised the question of whether the effects of circulating ghrelin on islet function also occurred at levels comparable to physiologic states (15). This article describes experiments using a dose of acyl ghrelin that was half of the lowest dose used in our previous study, with a goal of raising circulating ghrelin within a range occurring during physiologic states such as prolonged fasting (eg, 2–3 times the normal preprandial level) or ∼3500 pg/mL as seen in patients with anorexia nervosa (16, 17). In these settings, typically associated with significantly enhanced sensitivity to insulin, a brake on insulin secretion would allow maintenance of glucose homeostasis. Prior studies in humans using supraphysiologic doses of ghrelin suggested that ghrelin impairs insulin sensitivity, possibly by increasing circulating cortisol and fatty acid levels (18, 19), findings that are not consistent with the states of negative energy balance that have been associated with elevated circulating ghrelin. Therefore, we performed frequently sampled intravenous glucose tolerance tests (FSIGTs) in a group of healthy volunteers to determine the role of physiologic levels of ghrelin on both insulin secretion and insulin sensitivity.
Patients and Methods
Subjects
Healthy men and women between the ages of 18 and 39 years with a body mass index between 18 and 29 kg/m2 were recruited from the greater Cincinnati area. Subjects with a history or clinical evidence of impaired fasting glucose or diabetes mellitus, recent myocardial infarction, congestive heart failure, active liver or kidney disease, GH deficiency or excess, neuroendocrine tumor, or anemia or who were taking medications known to alter insulin sensitivity along with subjects who had fasting blood glucose concentrations of >5.5 mM were excluded from the study. Subjects were instructed to consume their usual diet for the 3 days before the study and to refrain from strenuous physical activity.
All study procedures were conducted at the Cincinnati Children's Medical Center Clinical and Translational Research Center. All study participants gave informed consent for the study by signing a form approved by University of Cincinnati and Cincinnati Children's Medical Center institutional review boards.
Experimental protocol
Subjects arrived at the Clinical Translational Research Center between 7:30 and 8:00 am after a 10- to 12-hour fast on 3 occasions separated by at least 5 days. Intravenous catheters were placed in the veins of both forearms for blood sampling and infusion of test substances. The arm with the sampling catheter was placed in a 55°C chamber to arterialize venous blood.
Synthetic human acyl ghrelin was obtained from Bachem AG (Rubendorf, Switzerland) as detailed in our previous publication (12). On the morning of the 3 study days, either saline (as a control) or acyl ghrelin was infused at doses of 0.2 or 0.6 nmol kg−1 h−1 (equivalent to 0.5 or 2.0 μg kg−1 h−1) for a total of 225 min. The 0.2 nmol kg−1 h−1 dose was determined using information on acyl ghrelin pharmacokinetics collected from our previous investigation with the goal of elevating steady-state ghrelin concentrations to periphysiologic levels. The 0.6 nmol kg−1 h−1 dose was included to investigate dose-response characteristics and as a positive control because our previous experience suggested that this would be sufficient to inhibit insulin release. Study visits were separated by at least 5 days and the order of the studies was randomized. The women participants in this study were taking oral contraceptives.
Ghrelin was infused for 45 minutes to obtain a steady-state plasma level (12), followed by an iv bolus of glucose (11.4 g/m2 body surface area) at time = 0 and continued over 60 seconds. Thereafter, a total of 29 blood samples at prespecified intervals were collected through 180 minutes according to a standard protocol (20). At time 20 minutes during the FSIGT, subjects received an iv infusion of insulin (0.025 U/kg) over 5 min. Blood samples were placed on ice, and plasma was separated by centrifugation within 1 hour; plasma or serum was stored at −80°C until assay. Blood pressure, respiration, heart rate, and body surface temperature were monitored every 15 minutes during the study procedure, given the known effects of ghrelin on the cardiovascular system (21).
Assays
Details of biochemical assays were described previously (12). In brief, blood glucose concentrations were determined by the glucose oxidase method using a glucose analyzer (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, Ohio). Plasma immunoreactive insulin levels were measured using a double-antibody RIA as reported previously (22). Total immunoreactive ghrelin was measured by RIA (Millipore, Billerica, Massachusetts) according to the manufacturer's recommendations (12). The intra-assay and interassay coefficients of variation were 6.4% and 16.3%, respectively. The ghrelin antibody used in the assay was directed toward the C terminus of the molecule and binds both acyl and desacyl ghrelin, as well as truncated ghrelin species. Serum concentrations of human GH were measured by a sandwich immunoassay using the automated Immulite 2000 chemiluminescent assay system (Siemens, Bad Nauheim, Germany) as described previously (12, 23). Cortisol levels were measured using the Corti-Cote RIA kit (MP Biomedicals, Orangeburg, New York). Adiponectin, an adipokine that is related to body fat distribution and insulin sensitivity and thus potentially related to ghrelin action (24), was quantified by an ELISA (Multimeric kit; ALPCO, Salem, New Hampshire). All samples were assayed in duplicate, and all specimens from a given participant were measured in the same assay.
Calculations
Fasting values of insulin and glucose were taken as the mean of samples drawn before ghrelin infusion and baseline levels from −15 to 0 minutes before the FSIGT. The acute insulin response to glucose (AIRg) was calculated as the average of plasma insulin increments above basal at 2, 3, 4, 5, 6, 8, and 10 minutes after iv glucose administration. Insulin sensitivity was quantified as the insulin sensitivity index (SI) using the minimal model of glucose kinetics (25). The disposition index (DI), which provides a measure of β-cell function adjusted for insulin sensitivity, was calculated as SI × AIRg based on the hyperbolic relationship of the 2 measures (26). The rate of glucose disappearance after the iv glucose infusion (Kg) provides an estimate of iv glucose tolerance (27) and was computed for each FSIGT as the slope of the natural logarithm of glucose from 10 to 19 minutes.
Statistical analysis
Plasma concentrations of ghrelin, GH, cortisol, and adiponectin during the study were analyzed by ANOVA for repeated measures using the 3 treatments (infusions of saline and ghrelin at 0.2 and 0.6 nmol kg−1 h−1) with time of sampling as the repeated measure. Post hoc analysis to compare the control with the ghrelin treatments was performed using the Dunnett test. AIRg, SI, DI, and Kg were compared among the 3 treatments using the Friedman test, a nonparametric ANOVA, with post hoc comparisons made with the Wilcoxon test (28); a nonparametric approach was taken because these measures did not follow a normal distribution. The median values of AIRg, SI, DI, and Kg were in descending order from saline to low-dose to high-dose ghrelin infusion (data not shown). The Page L test was used to test for significant dose-response characteristics for saline, low-dose, and high-dose studies. Page L is an ordinal, nonparametric test (29); therefore, a significant result indicates that the results decrease with increased dose. Data were analyzed using SPSS (version 12.0; SPSS, Chicago, Illinois). All results are expressed as means ± SEM unless otherwise noted.
Results
Subject characteristics
Sixteen healthy subjects (8 male and 8 female) aged 27.7 ± 6.3 years with a body mass index of 22.0 ± 2.7 kg/m2 were enrolled in the study. The mean fasting blood glucose for the group was 5.3 ± 0.4 mM, and the mean fasting plasma insulin was 60.8 ± 5.9 pM.
Ghrelin pharmacokinetics
Steady-state levels of plasma ghrelin were reached after approximately 45 minutes for both doses of ghrelin infusion. The 0.2 and 0.6 nmol kg−1 h−1 infusions raised the steady-state plasma ghrelin levels to 2629 ± 534 and 7311 ± 826 pg/mL, 2.2- and 6.1-fold above the baseline concentrations, respectively, as shown in Table 1 and Figure 1. The intra- and intersubject coefficients of variation for plasma ghrelin during the saline and 0.2 and 0.6 nmol kg−1 h−1 ghrelin infusions were 8.7%, 11.2%, and 12.3% and 82.2%, 64.2%, and 35.7%, respectively.
Table 1.
Baseline and Steady-State Plasma Concentrations of Total Ghrelin, Glucose, and Insulin During Continuous Intravenous Infusions of Saline or 0.2 or 0.6 nmol kg−1 h−1 Acyl Ghrelin (0–45 Minutes) Before a FSIGT
| Infusion Rate | Plasma Total Ghrelin Concentration, pg/mL |
Plasma Glucose, mg/dL |
Plasma Insulin, pM |
|||
|---|---|---|---|---|---|---|
| Baseline | Ghrelin steady state (t = 44 min) | Baseline | Ghrelin steady state (t = 44 min) | Baseline | Ghrelin steady state (t = 44 min) | |
| Saline | 1267 ± 337 | 1307 ± 340 | 94.2 ± 2.0 | 94.6 ± 2.1 | 63.1 ± 8.3 | 55.9 ± 8.2 |
| Ghrelin (0.2 nmol kg−1 h−1) | 1213 ± 305 | 2629 ± 534 | 94.5 ± 1.7 | 95.4 ± 1.9 | 51.5 ± 7.1 | 46.4 ± 5.8 |
| Ghrelin (0.6 nmol kg−1 h−1) | 1195 ± 291 | 7311 ± 826 | 95.0 ± 1.5 | 97.5 ± 1.5 | 67.9 ± 14.1 | 46.8 ± 6.2 |
Baseline plasma ghrelin, glucose, and insulin concentrations were calculated as the average of the −15- and −1-minute values.
Figure 1.
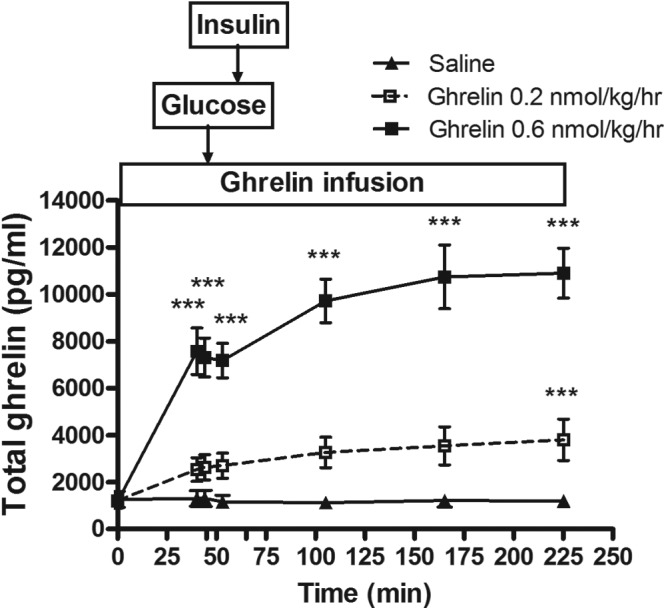
Plasma total ghrelin levels during continuous iv infusions (−15 to 225 minutes) of saline and 0.2 or 0.6 nmol kg−1 h−1 acyl ghrelin in healthy men and women. A bolus iv dose of glucose (11.4 g/m2 body surface area) was infused over 1 minute after plasma ghrelin had reached a steady state (at 45 minutes). The acyl ghrelin infusions resulted in a dose-dependent increase in plasma total ghrelin concentrations. ***, P < .001, ghrelin vs saline.
Effects of exogenous ghrelin on plasma insulin and glucose
The average fasting plasma glucose and insulin values at baseline and at 44 minutes, a time when the ghrelin concentration reached steady state are shown in Table 1. There were no significant changes in either fasting plasma glucose or insulin concentrations during the first 45 minutes of exogenous ghrelin administration from baseline (P > .05 for all comparisons) (see Supplemental Figure 1 published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
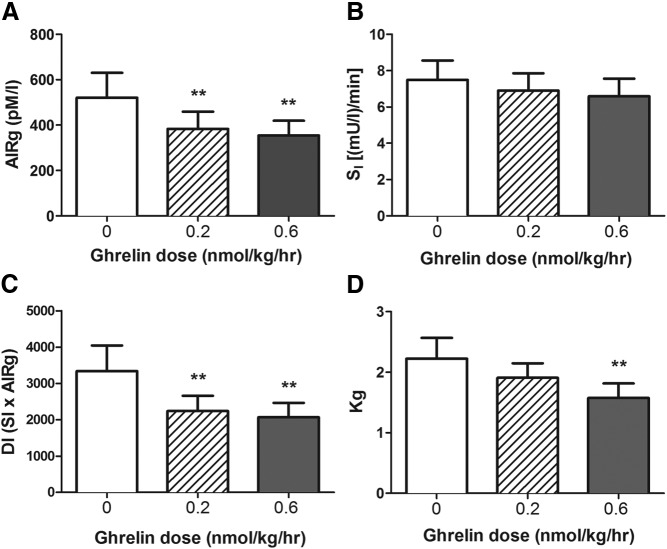
Administration of ghrelin during the FSIGT at both 0.2 and 0.6 nmol kg−1 h−1 caused a significant reduction of AIRg compared with that for the saline control (384 ± 75 and 354 ± 65 vs 520 ± 110 pM · min, respectively; P = .009). Post hoc comparisons of the 3 treatments in each subject demonstrated that both doses of ghrelin suppressed AIRg (P = .008 and 0.003, for low- and high-dose ghrelin vs saline) (Figure 2A). SI among the saline and ghrelin infusion studies did not differ significantly (6.9 ± 0.9 and 6.6 ± 1.0 vs 7.5 ± 1.1 mU l−1 min−1, ghrelin vs saline; P = .83) (Figure 2B). The DI (insulin secretion adjusted for insulin sensitivity) was also reduced during treatment with exogenous ghrelin (2238 ± 421 and 2067 ± 396 vs 3339 ± 705, respectively, P = .007 for the ANOVA, and < 0.02 for low- and high-dose ghrelin vs saline) (Figure 2C). Ghrelin infusion at 0.6 nmol kg−1 h−1 slowed Kg, but this was not observed with the lower dose (1.9 ± 0.3 and 1.6 ± 0.2 vs 2.1 ± 0.3, P = .01 for the ANOVA, and 0.63 and 0.001 for low- and high-dose ghrelin vs saline) (Figure 2D). The test for dose response (Page L test) was significant for AIRg (P = .002), DI (P = .001), and Kg (P = .001), but not for SI (P = .3). When the data were analyzed separately by sex, similar effects of ghrelin on insulin secretion and insulin sensitivity were seen in men and women (data not shown).
Figure 2.
Effects of ghrelin infusion on insulin secretion, insulin sensitivity, and glucose tolerance during a FSIGT in 16 healthy subjects. A, Both the 0.2 and 0.6 nmol kg−1 h−1 dose acyl ghrelin infusions decreased the AIRg. B, Ghrelin administration did not alter SI. C, After adjustment for insulin sensitivity, insulin secretion computed as DI remained lower with ghrelin administration compared with saline. D, The 0.6 nmol kg−1 h−1 dose ghrelin infusion also decreased iv Kg. No effect was seen with the low dose. A FSIGT was performed between 45 and 225 minutes. Intravenous glucose was administered after 45 minutes of ghrelin or saline infusion. Insulin at 0.025 U/kg was given as a short iv infusion between 65 and 70 minutes. **, P ≤ .01.
Effects of exogenous ghrelin on GH and cortisol secretion
As expected, GH levels were significantly increased by both doses of ghrelin administration compared with those for saline (Figure 3). Serum cortisol levels were significantly increased at 53, 105, 165, and 225 minutes after the start of the ghrelin infusion only when subjects received the high-dose (0.6 nmol kg−1 h−1) ghrelin infusion (P < .05 for all comparisons) (Figure 4). No significant change in the cortisol level was seen with the low-dose ghrelin infusion.
Figure 3.
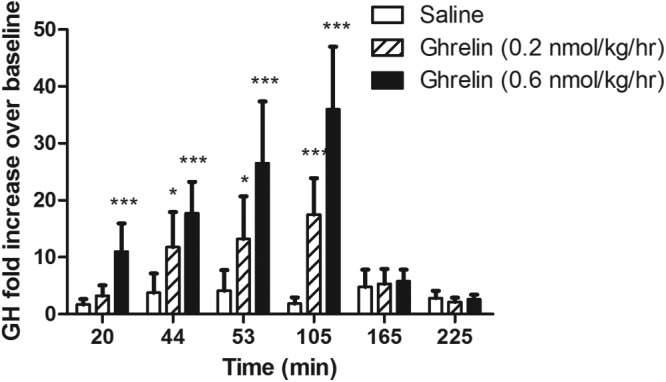
Plasma GH concentrations (displayed as fold increase over baseline) at 20, 44, 53, 105, 165, and 225 minutes during an infusion of acyl ghrelin at a 0.2 or 0.6 nmol kg−1 h−1 dose or saline. An FSIGT was performed between 45 and 225 minutes. Intravenous glucose was administered after 45 minutes of ghrelin or saline infusion. Insulin at 0.025 U/kg was given as a short iv infusion between 65 and 70 minutes. *, P < .05; ***P < .001.
Figure 4.
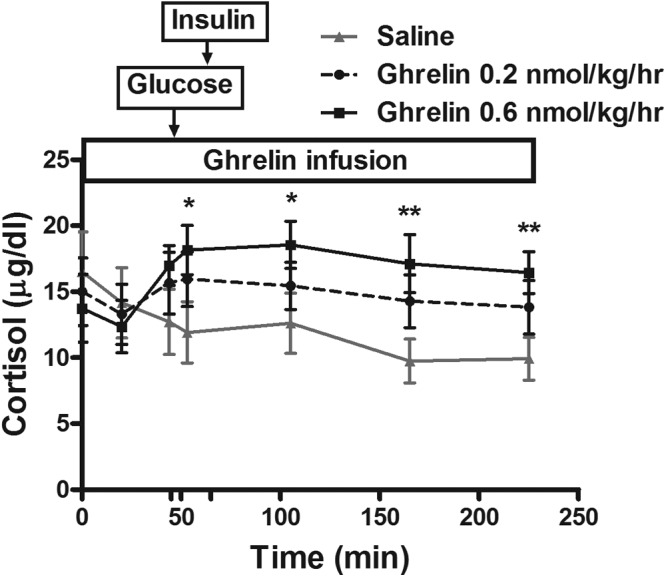
Serum cortisol concentrations during a 225-minute infusion of acyl ghrelin at a 0.2 or 0.6 nmol kg−1 h−1 dose or saline. An FSIGT was performed between 45 and 225 minutes. Intravenous glucose was administered after 45 minutes of ghrelin or saline infusion. Insulin at 0.025 U/kg was given as a short iv infusion between 65 and 70 minutes. *, P < .05; **, P < .01.
Effects of exogenous ghrelin on adiponectin level
Total adiponectin levels were measure at 165 and 225 minutes after ghrelin infusion began. No effect of ghrelin administration was observed on adiponectin level changes (P = .35) (Supplemental Figure 2).
Side effects
Ghrelin infusions were well tolerated. No adverse events were reported except for dizziness for 1 subject during the 0.6 nmol kg−1 h−1 dose ghrelin infusion. The symptom was mild and resolved spontaneously without intervention.
Discussion
High doses of acyl ghrelin suppress insulin secretion, reduce insulin sensitivity, and impair glucose tolerance in humans (12, 18, 30). In the present study we found that even at circulating total ghrelin levels within the range described during physiologic conditions, ghrelin attenuated glucose-stimulated insulin secretion, both in absolute terms and when insulin sensitivity was accounted for. Interestingly, although the effects on β-cell function were evident at both doses of ghrelin, even the high dose had no affect on insulin sensitivity. These findings confirm our previous observation and support the possibility that the levels of circulating ghrelin found in some physiologic states regulate insulin secretion and glucose homeostasis.
In the current study, infusions of 0.2 and 0.6 nmol kg−1 h−1 ghrelin raised plasma total ghrelin by 2- and 6-fold above baseline and suppressed AIRg by 26% and 32%, respectively. This magnitude of suppression is similar to our previous observation that doses of 0.3, 0.9, and 1.5 nmol kg−1 h−1 attenuated first-phase glucose-stimulated insulin secretion by 30% to 45% (12). Plasma ghrelin levels peak before meals and fall to a nadir approximately 1 hour after eating, with changes varying 2- to 4-fold across the fasting-feeding cycle in healthy subjects (16). Under states of negative energy balance, such as prolonged fasting, active weight loss, anorexia nervosa (17, 31), cancer, or cardiac cachexia (14, 32), plasma total ghrelin levels can be elevated 3-fold compared with those in healthy persons. In the current study, the low-dose ghrelin infusion raised steady-state plasma total ghrelin levels 2.2-fold above the baseline, a change within the range seen in states of chronic energy deficiency (17, 31); moreover, the increment in plasma ghrelin was comparable to what can occur in the shift from the fed to the fasted state (16). The significant reduction of stimulated insulin secretion by ghrelin at these plasma levels raises the possibility that endogenous, circulating ghrelin plays a role in modulating islet function.
Consistent with the effect of ghrelin on AIRg was the significant reduction in DI of 30% to 40%. The slightly greater impact on β-cell function when expressed as DI was due to the combination of reduced AIRg and a nonsignificant 8% to 12% decrease in SI with ghrelin treatment. Insulin secretion from the β-cell and tissue insulin sensitivity interact in a tightly regulated relationship best represented by a hyperbolic function (26). Among subjects with normal glucose tolerance, insulin secretion is related to insulin sensitivity in a proportionate manner. Thus, correcting for insulin sensitivity is thought to better identify the appropriate β-cell response to a given stimulus. Computation of DI is particularly well suited to the FSIGT because insulin sensitivity and insulin secretion are measured simultaneously, and the effect of both doses of ghrelin on this parameter is the best evidence to date that this peptide regulates stimulated insulin secretion.
Ghrelin administered in higher doses than we used here caused acute insulin resistance in healthy or GH-deficient individuals studied with hyperinsulinemic glucose clamps (18, 33). The lack of effect of ghrelin on insulin sensitivity in our study may be due to the lower doses of ghrelin used or differences in methodology. We think the former possibility is more likely because SI determined by the minimal model correlates tightly with insulin sensitivity derived from a glucose clamp (34). Regardless, based on the findings reported herein, it appears that ghrelin has a relatively greater effect on insulin secretion than the dynamic insulin sensitivity measured as SI.
We have previously reported that ghrelin administration worsens iv glucose tolerance (Kg), but only at higher doses (0.9 and 1.5 nmol kg−1 h−1) (12). Our current finding is consistent with this report because only the 0.6 nmol kg−1 h−1 dose but not the 0.2 nmol kg−1 h−1 dose lowered Kg. Thus, both glucose clearance and insulin secretion appear to be dose-dependent within the periphysiologic to pharmacologic range of exogenous ghrelin used in our studies.
The role of ghrelin in the regulation of insulin secretion has been debated. Consistent with our previous publication (12) and the results reported by Gutierrez et al (36), we did not observe any changes in fasting plasma insulin or glucose levels. Thus, in healthy humans it appears that ghrelin specifically regulates stimulated insulin secretion. We have only tested glucose as the stimulus, but it is plausible that ghrelin would impair the response to other secretagogues as well. The mechanism(s) by which ghrelin suppresses insulin secretion remains unknown. Both ghrelin O-acyltransferase (GOAT), the only known enzyme that acylates ghrelin (35, 36), and the ghrelin receptor, GHSR, are expressed in rat and mouse pancreatic islets (8, 9, 37). Ghrelin may exert its insulinostatic effect through a direct endocrine effect on the β-cell by activating the GHSR. However, it is also possible that β-cells are inhibited by ghrelin produced in the islet through a paracrine mechanism that is simply mimicked by iv administration of ghrelin.
It is worth considering whether the effect of ghrelin on insulin secretion is mediated indirectly by GH because stimulation of GH release is one of the more predictable ghrelin actions. The effect of GH on insulin secretion is complex. A triphasic response to exogenous GH in insulin secretion has been described with an initial increase in insulin release within 5 minutes of administration (38) followed by a slight inhibition of insulin secretion during the subsequent 1 to 5 hours (39). A more profound and persistent rise in plasma insulin is observed with long periods of exposure. Although GH was significantly elevated by ghrelin in our study, there is reason to doubt that this explains the acute effects on insulin secretion. Previous work has demonstrated that acute administration of a physiologic dose of GH does not alter the insulin response to hyperglycemia (40) and the normal nocturnal rise in GH does not appear to regulate morning meal–stimulated insulin secretion (41). Whereas the possible role of GH in ghrelin suppression of insulin secretion will require studies in GH-deficient people, evidence from previous work suggests that this is not likely to be the major mechanism explaining this phenomenon.
The physiologic relevance of the suppressive effect of ghrelin on insulin secretion is unclear but may be related to glucose homeostasis during prolonged fasting or starvation. Emerging evidence suggests that ghrelin, especially acyl ghrelin, may exert different effects on glucose homeostasis during fasting and fed states. Mice that lack GOAT and thus also lack acyl ghrelin have improved glucose tolerance and increased insulin secretion after a 16-hour fast (42) but not after a 6-hour fast (43) compared with wild-type mice. This result is consistent with the established patterns of ghrelin secretion with plasma levels elevated before meals and during sleep when insulin levels are low (16). We propose that the effects of ghrelin on insulin secretion protect against hypoglycemia in states of negative energy balance. A corollary to this hypothesis is that in states of plentiful caloric availability, an abundance of acyl ghrelin may be deleterious to glucose tolerance by constraining the insulin response. Consistent with this, ip administration of a GOAT inhibitor that blocks acyl ghrelin synthesis improved glucose tolerance in wild-type mice, suggesting negative metabolic effects of ghrelin in the fed state (44). Thus, our findings add to emerging evidence suggesting potential benefits of compounds that reduce acyl ghrelin signaling on energy and glucose metabolism.
There are several important limitations to the current study. First, the effects reported here are from short-term ghrelin administration, and we cannot comment on the actions of chronic ghrelin administration. Second, glucose tolerance was determined by the response to iv glucose administration not an oral glucose load or a mixed meal. This approach does not allow evaluation of ghrelin in the context of physiologic insulin secretion during which there are a variety of incretin and neural additions to glycemic stimulation. Third, when acyl ghrelin is given as an iv infusion, the peptide is rapidly degraded into desacyl ghrelin and truncated ghrelin fragments by deacylation enzymes (eg, butyrylcholinesterase in human serum) (45). We did not measure acyl and desacyl ghrelin specifically in the present study, limiting our ability to compare the relative amounts of ghrelin isoforms in the plasma during acyl ghrelin infusion with those occurring from endogenous secretion. The true physiologic range of endogenous acyl and desacyl ghrelin at a population level has not been defined. In a small cohort of 8 young and nonobese men, plasma acyl ghrelin levels measured by the same 2-site sandwich assay as used in our study ranged from ∼10 to ∼50 pg/mL (a 5-fold variation) during the course of a day (46). In our cohort, fasting plasma total ghrelin levels ranged from 393 to 4244 pg/mL. Finally, we studied a young, healthy, lean population that may not be generalizable to subjects with metabolic disease or demonstrate ghrelin effects to the greatest extent.
Findings from this study support a physiologic function of the ghrelin system in the regulation of glucose homeostasis in humans. Specifically, exogenous ghrelin can reduce the first-phase insulin responses to iv glucose and compromise glucose tolerance at both near physiologic and pharmacologic doses in healthy, nonobese individuals. These observations raise the possibility that inhibition of acyl ghrelin or GOAT activity can improve β-cell function and glucose tolerance that might be a clinically relevant strategy to combat diabetes.
Acknowledgments
We thank Brianne Paxton, Yongmei Zhao, and Elizabeth Stambrook for their excellent support for the study.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH) (grant 5K23DK80081 to J.T. and grant R0157900 to D.D.) and supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH (grant 8 UL1 TR000077-04). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
J.T. designed the study, collected and analyzed the data, and was the primary author. R.L.P. assisted with data analysis, contributed to discussion, and reviewed/edited the manuscript. H.W.D. assisted with data collection, contributed to discussion, and reviewed/edited the manuscript. M.B. assisted with data collection and reviewed/edited the manuscript. M.H.T. contributed to discussion and reviewed/edited the manuscript. D.D. contributed to discussion and reviewed/edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIRg
- acute insulin response to glucose
- DI
- disposition index
- FSIGT
- frequently sampled glucose tolerance test
- GHSR
- GH secretagogue receptor
- GOAT
- ghrelin O-acyltransferase
- Kg
- rate of glucose disappearance after the iv glucose infusion
- SI
- insulin sensitivity index.
References
- 1. Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–1545 [DOI] [PubMed] [Google Scholar]
- 2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 3. Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913 [DOI] [PubMed] [Google Scholar]
- 4. Castañeda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60 [DOI] [PubMed] [Google Scholar]
- 5. Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–69 [DOI] [PubMed] [Google Scholar]
- 6. van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457 [DOI] [PubMed] [Google Scholar]
- 7. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kageyama H, Funahashi H, Hirayama M, et al. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul Pept. 2005;126:67–71 [DOI] [PubMed] [Google Scholar]
- 9. Chuang JC, Sakata I, Kohno D, et al. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol Endocrinol. 2011;25:1600–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921 [DOI] [PubMed] [Google Scholar]
- 11. Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55:3486–3493 [DOI] [PubMed] [Google Scholar]
- 12. Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka M, Naruo T, Yasuhara D, et al. Fasting plasma ghrelin levels in subtypes of anorexia nervosa. Psychoneuroendocrinology. 2003;28:829–835 [DOI] [PubMed] [Google Scholar]
- 14. Shimizu Y, Nagaya N, Isobe T, et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778 [PubMed] [Google Scholar]
- 15. Meyer C. Final answer: ghrelin can suppress insulin secretion in humans, but is it clinically relevant? Diabetes. 2010;59:2726–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719 [DOI] [PubMed] [Google Scholar]
- 17. Germain N, Galusca B, Grouselle D, et al. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab. 95:3057–3062 [DOI] [PubMed] [Google Scholar]
- 18. Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57:3205–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Damjanovic SS, Lalic NM, Pesko PM, et al. Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab. 2006;91:2574–2581 [DOI] [PubMed] [Google Scholar]
- 20. Utzschneider KM, Tong J, Montgomery B, et al. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108–113 [DOI] [PubMed] [Google Scholar]
- 21. Nagaya N, Kangawa K. Therapeutic potential of ghrelin in the treatment of heart failure. Drugs. 2006;66:439–448 [DOI] [PubMed] [Google Scholar]
- 22. Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA. β-Cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91:185–191 [DOI] [PubMed] [Google Scholar]
- 23. Tong J, D'Alessio D, Ramisch J, et al. Ghrelin stimulation of growth hormone isoforms: parallel secretion of total and 20-kDa growth hormone and relation to insulin sensitivity in healthy humans. J Clin Endocrinol Metab. 2012;97:3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326 [DOI] [PubMed] [Google Scholar]
- 25. Bergman RN, Cobelli C. Minimal modeling, partition analysis, and the estimation of insulin sensitivity. Fed Proc. 1980;39:110–115 [PubMed] [Google Scholar]
- 26. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 27. Kahn SE, Montgomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:5824–5829 [DOI] [PubMed] [Google Scholar]
- 28. Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. Oxford, UK: Blackwell Science; 2002:597–598 [Google Scholar]
- 29. Page EB. Ordered hypotheses for multiple treatments: a significance test for linear ranks. J Am Stat Assoc. 1963;58:216–230 [Google Scholar]
- 30. Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086 [DOI] [PubMed] [Google Scholar]
- 31. Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673 [PubMed] [Google Scholar]
- 32. Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038 [DOI] [PubMed] [Google Scholar]
- 33. Vestergaard ET, Hansen TK, Gormsen LC, et al. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab. 2007;292:E1829–E1836 [DOI] [PubMed] [Google Scholar]
- 34. Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396 [DOI] [PubMed] [Google Scholar]
- 36. Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. An W, Li Y, Xu G, et al. Modulation of ghrelin O-acyltransferase expression in pancreatic islets. Cell Physiol Biochem. 2010;26:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frohman LA, MacGillivray MH, Aceto T., Jr Acute effects of human growth hormone on insulin secretion and glucose utilization in normal and growth hormone deficient subjects. J Clin Endocrinol Metab. 1967;27:561–567 [DOI] [PubMed] [Google Scholar]
- 39. Adamson U, Cerasi E. Acute effects of exogenous growth hormone in man: time- and dose-bound modification of glucose tolerance and glucose-induced insulin release. Acta Endocrinol. 1975;80:247–261 [DOI] [PubMed] [Google Scholar]
- 40. Bratusch-Marrain PR, Smith D, DeFronzo RA. The effect of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab. 1982;55:973–982 [DOI] [PubMed] [Google Scholar]
- 41. Nielsen MF, Dinneen S, Basu A, Basu R, Alzaid A, Rizza RR. Failure of nocturnal changes in growth hormone to alter carbohydrate tolerance the following morning. Diabetologia. 1998;41:1064–1072 [DOI] [PubMed] [Google Scholar]
- 42. Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barnett BP, Hwang Y, Taylor MS, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005 [DOI] [PubMed] [Google Scholar]
- 46. Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]