Abstract
Purpose:
A phase 1 study was initiated to determine the safety, potential effectiveness, and maximal tolerated dose and recommended phase 2 dose of efatutazone and paclitaxel in anaplastic thyroid cancer.
Experimental Design:
Patients received efatutazone (0.15, 0.3, or 0.5 mg) orally twice daily and then paclitaxel every 3 weeks. Patient tolerance and outcomes were assessed, as were serum efatutazone pharmacokinetics.
Results:
Ten of 15 patients were women. Median age was 59 years. Seven patients received 0.15 mg of efatutazone, 6 patients received 0.3 mg, and 2 patients received 0.5 mg. One patient receiving 0.3 mg of efatutazone had a partial response from day 69 to day 175; 7 patients attained stable disease. Median times to progression were 48 and 68 days in patients receiving 0.15 mg of efatutazone and 0.3 mg of efatutazone, respectively; corresponding median survival was 98 vs 138 days. The median peak efatutazone blood level was 8.6 ng/mL for 0.15-mg dosing vs 22.0 ng/mL for 0.3-mg twice daily dosing. Ten patients had grade 3 or greater adverse events (Common Terminology Criteria for Adverse Events), with 2 of these (anemia and edema) related to efatutazone. Thirteen events of edema were reported in 8 patients, with 2 of grade 3 or greater. Eight patients had ≥1 serious adverse event, with 1 of these (anemia) attributed to efatutazone and 1 (anaphylactic reaction) related to paclitaxel. The maximal tolerated dose was not achieved. Angiopoietin-like 4 was induced by efatutazone in tissue biopsy samples of 2 patients.
Conclusions:
Efatutazone and paclitaxel in combination were safe and tolerated and had biologic activity.
Thyroid cancer incidence has steadily increased during the past decade in the United States; the estimate for new cases in 2012 was 56 000, with 1780 deaths, and it is now the fifth most common cancer in women (1). Although anaplastic thyroid cancer (ATC) comprises only a small fraction of all thyroid cancers (1.7% in the United States) (2), it has a dire prognosis with median survival of 3.9 months for stage IVB and 1.7 months for stage IVC (metastatic) (Ref. 3 and McIver, B., personal communication).
Traditional chemotherapeutics have had limited success in patients with ATC, with taxanes showing some promise (4–6). Several newer “targeted” therapies have also been tried, including an epidermal growth factor receptor antagonist (7), a multitargeted tyrosine kinase inhibitor (8), and a vascular-disrupting agent (9), again with limited efficacy. Additional agents specifically targeting dysregulated molecular ATC pathways warrant attention (10), with one attractive candidate group in particular being peroxisome proliferator–activated receptor (PPAR)-γ agonists.
PPAR-γ is a DNA-binding nuclear hormone receptor that regulates cellular energy metabolism through transcriptional effects. Laboratory studies suggest that PPAR-γ agonists may inhibit tumor growth through the induction of terminal cell differentiation, cell cycle arrest, and/or apoptosis induction, as well as via inhibition of angiogenesis (11, 12). Notably, PPAR-γ agonists have shown promising antitumor activity against a variety of cancers both in vitro and in vivo (13).
Efatutazone (eg, RS5444 and CS-7017) is a selective and highly potent orally bioavailable agonist of PPAR-γ of the thiazolidinedione class of synthetic PPAR ligands. The chemical structure of efatutazone has been reported previously (13). This third-generation thiazolidinedione is the most potent with a 50% inhibition of transcriptional response (EC50) of 1 nM and an inhibitory concentration at which 50% of cell proliferation is inhibited (IC50) of 0.8 nM (14). Efatutazone is selective in its activation of the PPAR-γ subtype, and its effect may be enhanced in combination with other cytotoxic drugs (14). In preclinical studies, we previously showed that efatutazone inhibits ATC cell proliferation by up-regulation of RhoB and p21 and also synergistically potentiates apoptosis when combined with paclitaxel (15, 16), providing the basis for the present phase 1 trial of the combination in ATC.
The study hypotheses were that at least one dose level of the combination of efatutazone and paclitaxel would be safe and well tolerated by subjects with advanced ATC and that a dose-response relationship would exist between the dose of efatutazone and biomarker expression. The primary objective was to determine the recommended phase 2 dose for efatutazone administered twice daily (bid) in combination with paclitaxel once every 3 weeks to subjects with advanced ATC. Secondary objectives were to evaluate the safety profile of the combination of efatutazone and paclitaxel and to determine the pharmacokinetics of efatutazone.
Patients and Methods
Patients
Subjects >18 years of age with histologically or cytologically diagnosed advanced ATC with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) and at least 1 lesion (primary or metastatic) accessible for biopsy were enrolled from February 7, 2008, to December 1, 2009. Eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤2, adequate bone marrow and organ function (hemoglobin ≥9 g/dL, absolute neutrophil count ≥1.5 × 109/L, and platelet count ≥100 × 109/L), serum creatinine ≤1.5 upper limit of normal (ULN) or creatinine clearance >60 mL/min, aspartate aminotransferase and alkaline phosphatase <2.5 × ULN if without liver metastases and ≤5.0 × ULN with liver metastases, total bilirubin ≤2.0 × ULN, and prothrombin time/international normalized ratio within normal limits unless therapeutically anticoagulated. A central pathology review was not undertaken, but all treatment sites were referral centers experienced in pathologic assessment of thyroid cancers.
Men and women of childbearing age were required to use effective contraception while receiving treatment and for 3 months thereafter. All female patients of childbearing potential had a negative pregnancy test within 7 days of starting the study, and baseline laboratory tests and tumor assessments were performed within 2 weeks before study initiation. The trial was approved by all involved institutional review boards, and all subjects were counseled and thereafter provided written informed consent. Exclusion criteria included an anticipated need for a major surgical procedure or radiotherapy during the study; treatment with chemotherapy, hormonal therapy, other thiazolidinediones, minor surgery, or any investigational agent within 4 weeks before study enrollment; treatment with nitrosoureas or mitomycin C, immunotherapy, biological therapy, or major surgery within 6 weeks before study enrollment; or treatment with radiotherapy within 1 week before study enrollment.
Patients could not have a history of any of the following conditions: diabetes mellitus requiring treatment with insulin or oral agents; myocardial infarction with significant impairment of cardiac function (eg, ejection fraction ≤50%); severe/unstable angina pectoris; coronary/peripheral artery bypass graft; New York Heart Association (NYHA) class III or IV congestive heart failure; cerebrovascular accident or transient ischemic attack, pulmonary embolism, or other clinically significant thromboembolic event; clinically significant pulmonary disease (eg, severe chronic obstructive pulmonary disease or asthma); or significant pleural or pericardial effusions. Further exclusions were clinically active brain metastasis (untreated or with progression within 4 weeks after completion of radiotherapy), uncontrolled seizure disorder, spinal cord compression, or carcinomatous meningitis. Patients were also excluded if they had a clinically significant active infection requiring antibiotic therapy, were HIV-positive and receiving antiretroviral therapy, were pregnant or breast feeding, had a known history of severe hypersensitivity reactions to efatutazone or paclitaxel formulations, or had other serious intercurrent medical or psychiatric illnesses.
Study design
This represents an investigator-initiated and designed multicenter, open-label, phase 1 study, sponsored by Daiichi Sankyo (Edison, New Jersey). All study primary data were freely available to the authors, with study data and interpretation unencumbered by the sponsor in any way. Objectives were to establish the safety and tolerability of efatutazone in combination with paclitaxel to determine the recommended phase 2 doses and to define efatutazone pharmacokinetics. Additional objectives were to examine blood and tissue biomarkers. Sequential cohorts of 3 to 6 subjects per dose level were treated with efatutazone and paclitaxel in accordance with prospectively defined dose levels (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.), dose escalation rules, and definitions of treatment-related dose-limiting toxicities (DLTs). All subjects began with a 1-week run-in period of efatutazone monotherapy, after which there were 3-week treatment cycles combining efatutazone with iv administered paclitaxel.
Each 3-week cycle consisted of combination treatment with continuously administered bid oral efatutazone, with iv paclitaxel given only on the first day of each 21-day cycle. Disease assessments were performed after the first 4 weeks of study participation and then every 3 to 6 weeks (1–2 cycles) at the discretion of the treating investigators. Treatment continued until disease progression, unacceptable toxicity, or consent withdrawal; there was no preset limit to the number of allowed treatment cycles. After discontinuation of study treatment, subjects had an end-of-treatment visit 30 to 45 days after the last dose; subsequently, subjects were followed at 3-month intervals to obtain information about subsequent treatment and survival status.
Intrapatient dose escalation was permitted to allow subjects to go from dose level 1a to 1b. In addition, dosage in subjects could be escalated to the highest completed dose level once the safety and tolerability of that dose level were demonstrated after 2 cycles of combination treatment at their initially assigned lower dose levels at the discretion of the investigator (patient consent required).
Safety
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, with DLT assessment based on the most severe toxicities. DLTs occurring during the first cycle of combination therapy deemed clearly related to paclitaxel were not considered as DLTs for the efatutazone-paclitaxel combination.
The maximal tolerated dose was defined as the highest dose below the dose at which ≥2 of 6 subjects experienced DLTs during the run-in segment (1 week of efatutazone monotherapy) and the first treatment cycle (3 weeks of combination treatment) or, in the case of dose levels 1a and 1b, 1 cycle at dose level 1a, and 1 cycle at dose level 1b.
Pharmacokinetics
Pharmacokinetic (PK) analysis was performed on day 7 (final day of the 1 week run-in segment in which efatutazone was administered as monotherapy) and on day 1 of cycle 2. On day 7, PK samples were collected at −5 minutes (predose of the morning dose of efatutazone) and 1, 2, 4, 6, and 8 to 10 hours after dosing with efatutazone; on day 1 of cycle 2, PK samples were collected at −5 minutes (before efatutazone) and immediately after a tumor biopsy specimen was obtained (before the start of the paclitaxel infusion). Plasma concentrations of the free form of efatutazone and key metabolites were measured using validated assays (14).
Biomarker studies (blood)
Exploratory biomarker variables included TGF-β1, thyroglobulin, and CA 19.9 in serum and adiponectin in plasma. Adiponectin, an indicator of PPAR-γ activation, was measured as described previously (14). Patients were seen at baseline and at follow-up visits at 7, 14, 21, 28, and 49 days and sporadically after that.
Tumor biopsy samples
Biopsy tissue samples were collected in 10% formalin before treatment, after the 1 week run-in with efatutazone treatment alone, and at 4 weeks of treatment with the combinatorial therapy of efatutazone plus paclitaxel. Once tissues were embedded in paraffin, they were cut into 5-μm sections, deparaffinized, hydrated, and blocked with diluent that contained background-reducing components (Dakocytomation, Glostrup, Denmark), and probed for specified antibodies that included retinoid X receptor-α (RXR-α), RhoB (Santa Cruz Biotechnology, Santa Cruz, California), p21 (Dako, Carpinteria, California), angiopoietin-like 4 (ANGPTL4) (Sigma-Aldrich, St Louis, Missouri), and PPAR-γ (kind gift from E. A. Thompson, Mayo Clinic, Jacksonville, Florida). Negative control sections were performed according to College of American Pathologists guidelines, which consisted of incubation of the slides in the absence of the primary antibody followed by secondary anti-rabbit and anti-mouse antibodies. Images were obtained at ×20 using a Scanscope XT (Aperio Technologies, Vista, California), and the staining was scored using an algorithm in Imagescope software (Aperio Technologies) created by a histologist based on signal intensity (0, 1+, 2+, and 3+) and percentage of positive cells (0, <5%; 1+, 5%–20%; 2+, 20%–50%; and 3+, >50%). H scores were calculated using the formula [(1+% × 1) + (2+% × 2) + (3+% × 3)]. Subjects were excluded from analyses if there was insufficient tumor tissue.
Statistics
Separately in each dose group, changes in CA 19.9, thyroglobulin, TGF-β1, and adiponectin were evaluated using mixed-effects linear regression models, including a random effect for each patient, and considering thyroglobulin, TGF-β1, and adiponectin on the logarithm scale because of their skewed distributions. Changes in these measures were not formally assessed with statistical tests in patients receiving a 0.50-mg dose because there were only 2 of these patients. Progression-free survival (time to progressive disease) and overall survival after the date of the first efatutazone dose were estimated using the Kaplan-Meier method; censoring occurred at the date of last follow-up for overall survival because not all patients died during the follow-up period. Median time to progressive disease and time to death were estimated. All statistical analyses were performed using R Statistical Software (version 2.11.0; R Foundation for Statistical Computing, Vienna, Austria). All raw data were provided by the sponsor to the investigators for independent review.
Results
Nineteen patients provided informed consent, but 4 did not receive therapy (3 who had disease progression and 1 who was found ineligible). Clinical characteristics of the 15 patients who received the study drug are given in Table 1. Two thirds of the patients were women, and most had at least partial tumor resection. Eight had received radiotherapy, and 4 had received cytotoxic chemotherapy. Pathologic analysis showed coexisting papillary thyroid cancer in 3 patients. (In 1 patient, ATC was confirmed from an endobronchial biopsy containing no differentiated tumor, in a second patient, ATC was diagnosed on a right neck mass excisional biopsy sample, which was noted to have “a component of well-differentiated papillary thyroid carcinoma, classic variant” within the anaplastic thyroid tumor, squamoid variant, and 1 patient had primarily a Warthin variant of papillary cancer with foci of tall cell variant and anaplastic lesions and lymph node metastases of ATC; this patient died during the run-in phase.) Hürthle cell thyroid cancer was present in 1 patient (the tumor was predominantly anaplastic with ATC confirmed in lymph node and lung).
Table 1.
Clinical characteristics
| Patients (n = 15)a | |
|---|---|
| Median age (range), y | 59 (43–82) |
| Men | 5 |
| Women | 10 |
| Surgery | |
| Biopsy | 4 |
| Resection | 11 |
| Radiotherapy | 8 |
| Prior chemotherapy | 4 |
| TNM stage | |
| IVB | 4 |
| IVC | 11 |
| Concomitant DTC | 4 |
Abbreviation: TNM, tumor, node, metastasis.
Four additional patients provided consent but did not receive any therapy.
Cohort 1 (n = 7) received 0.15 mg bid efatutazone with either 135 or 175 mg/m2 paclitaxel every 3 weeks. Three of these 7 had stage IVB disease at the time of study entry; the others had stage IVC disease. Cohort 2 (n = 6) received 0.3 mg bid efatutazone with 175 mg/m2 paclitaxel, and only 1 patient had stage IVB disease. Cohort 3 (n = 2) received only 0.5 mg bid efatutazone, and both of these patients with stage IVC ATC incurred disease progression during the run-in phase, suggesting that the drug is not useful as a single agent.
The median number of treatment cycles in cohort 1 was week 3 of cycle 2 (range, cycle 1/week 2 to cycle 3/week 3) and in cohort 2 was week 3 of cycle 3 (range, cycle 1/week 2 to cycle 8/week 3). No patient encountered DLT, and there were no dose de-escalations. The study was ultimately discontinued by the sponsor because of slow accrual.
Adverse events (AEs)
Table 2 summarizes all treatment-emergent AEs. Table 3 lists individually all AEs with a CTCAE grade of 3 or greater, and treatment attribution and outcomes of all serious AEs are given in Supplemental Table 2. Because fluid retention was a prominent side effect of efatutazone, all instances of edema and/or swelling are given in Supplemental Table 3.
Table 2.
Summary of Treatment-Emergent AEs by Class
| Dose of Efatutazone (bid) |
Overall (n = 15) | |||
|---|---|---|---|---|
| 0.15 mg (n = 7) | 0.30 mg (n = 6) | 0.50 mg (n = 2) | ||
| Any AEa | 6 | 6 | 2 | 14 |
| Any AE of grade 3 or greater | 3 | 5 | 2 | 10 |
| Any AE attributed to efatutazone | 4 | 4 | 2 | 10 |
| AE of grade 3 or greater attributed to efatutazone | 0 | 2 | 0 | 2 |
| SAE leading to treatment discontinuationb | 1 | 0 | 1 | 2 |
| Patient deathsc | 2 | 0 | 0 | 2 |
| DLTs | 0 | 0 | 0 | 0 |
AEs that began or worsened after dispensation of study drug.
No patients discontinued therapy due to events attributed to efatutazone.
Two subjects died because of disease progression; there were no therapy-related deaths.
Table 3.
Subjects with SAEs (Enrolled Analysis Set)
| Subject Identification | Dose Level at Time of Event Onset |
Event (Preferred Term) | CTCAE Grade | Relationship (Yes/No, Specify) | Treatment Discontinued (Yes/No) | Outcome | |
|---|---|---|---|---|---|---|---|
| Efatutazone (mg bid) | Paclitaxel (mg/m2) | ||||||
| 01 | 0.15 | 0 | Confusional state | 1 | No | No | Resolved |
| 0.15 | 135 | Septic shock | 4 | No | Yes, fatutazone | Resolved | |
| 05 | 0.15 | 175 | Dysphagia | 3 | No | Interrupted, efatutazone | Resolved |
| 08 | 0.30 | 175 | Clostridial infection | 2 | No | No | Resolved |
| 12 | 0.30 | 175 | Dyspnea | 4 | No | No | Not resolved |
| 0.30 | 0 | Anemia | 4 | Yes, efatutazone and paclitaxel | No | Resolved | |
| 15 | 0.30 | 175 | Pneumonia | 3 | No | Interrupted, efatutazone | Resolved |
| 18 | 0.50 | 175 | Loss of consciousness | 3 | No | No | Resolved |
| 19 | 0.50 | 175 | Anaphylactic reaction | 4 | Yes, paclitaxel | Paclitaxel discontinued permanently; no efatutazone action was taken | Resolved |
Subject 11 was enrolled in the study but did not receive treatment and therefore was not included in the safety analysis set.
Ten patients had AEs of grade 3 or greater, with 2 (anemia and localized edema) attributed to efatutazone. Thirteen events of fluid retention/edema were reported in 8 patients, but only 2 were grade 3 or greater. Diuretics were used to manage the edema. Eight patients had ≥1 serious adverse event (SAE), with 1 of these (anemia) deemed due to efatutazone. One SAE (anaphylactic reaction) was related to paclitaxel. No DLT was observed.
Efficacy
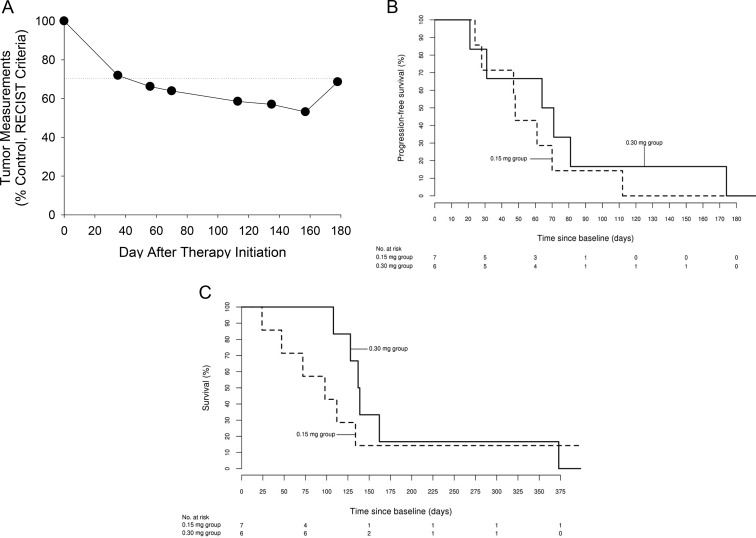
Of the 7 patients in the 0.15-mg bid efatutazone group, 4 had stable disease and 2 had progressive disease as their best response; 1 patient was withdrawn from study due to an SAE. In the 0.3-mg bid group, 3 had stable disease, 1 had a confirmed partial response (from day 69 to day 175) (Figure 1A), and 2 had progressive disease as their best response. Median time to progression in 7 patients at 0.15 mg bid (which included 3 of the 4 patients with stage IVB disease) was 48 days but was 68 days in the 0.3-mg bid group (42% prolongation) (Figure 1B); the corresponding median survival was 98 days (0.15-mg bid) vs 138 days (0.3-mg bid), 41% greater with the 0.3-mg bid group (Figure 1C). Median peak efatutazone blood levels were 8.6 ng/mL (range, 5.1–13.7 ng/mL) for the 0.15-mg bid group and 22.0 ng/mL (range, 17.0–31.5 ng/mL) for the 0.3-mg bid group, 155% greater.
Figure 1.
A, A 74-year-old woman with metastases to lung and hilar lymph nodes received the combination of 0.3 mg bid efatutazone and 175 mg/m2 paclitaxel iv every 3 weeks. She did not receive prior radiotherapy. and her tumor did not contain differentiated features. She had a RECIST-confirmed partial response that persisted from day 69 until day 175. B, Kaplan-Meier estimated proportion of patients free of progressive disease in the 0.15-mg (n = 7) and 0.3-mg (n = 6) efatutazone groups. Median time to progression was 48 vs 68 days, respectively. C, Kaplan-Meier estimated survival in the 0.15-mg (n = 7) and 0.3-mg (n = 6) efatutazone groups. Median survival was 98 vs 138 days, respectively.
Immunohistochemistry
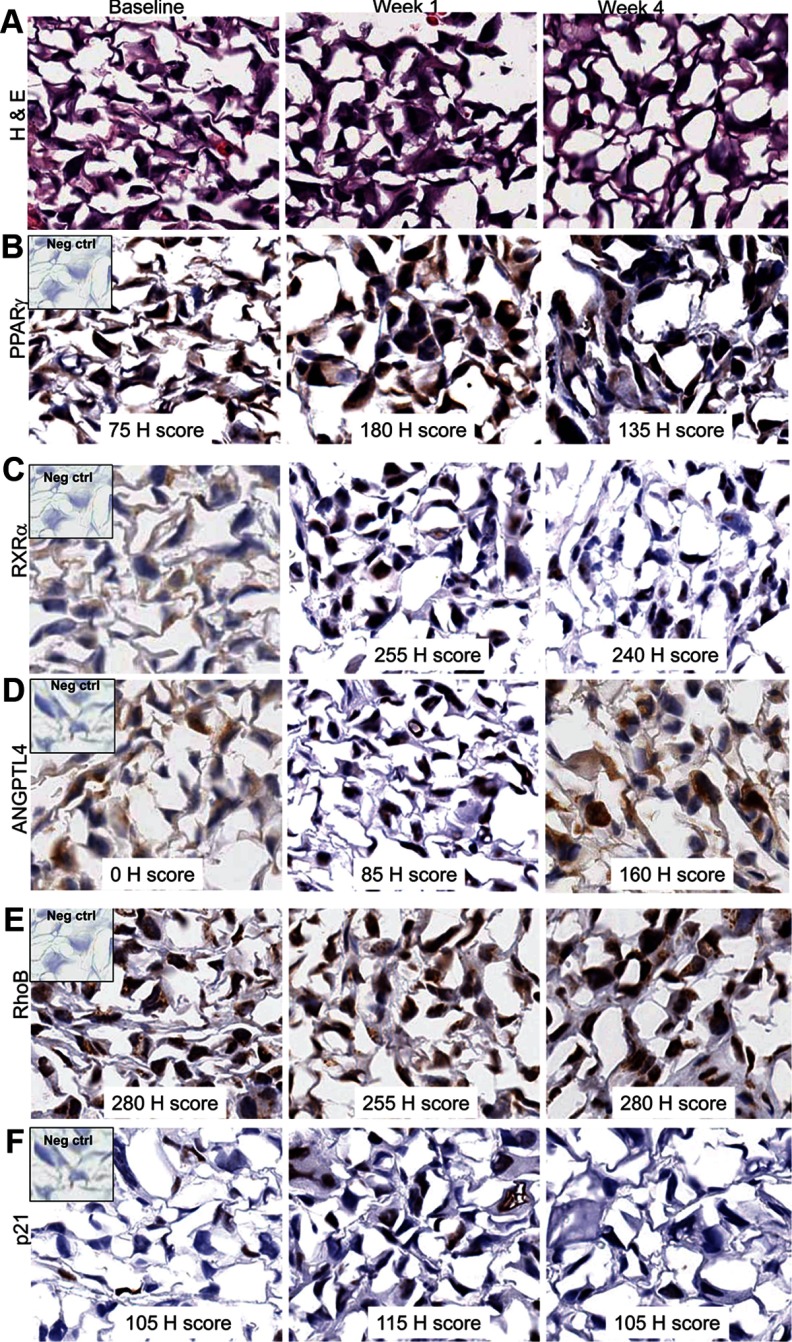
Whenever feasible, serial tumor biopsy samples were collected before and during the treatment phase of the study for the purpose of examining PPAR-γ expression and determining whether PPAR-γ–regulated protein levels were elevated in efatutazone-treated patients. Of the 19 study patients, 7 had biopsies at baseline, 5 had biopsies at the end of the 1-week PPAR-γ run-in phase, and 2 had biopsies at week 4. Of the 7 who had baseline biopsies, 4 patients had usable samples at baseline, 4 had usable samples at the end of the 1-week efatutazone run-in, and 2 had samples obtained during combinatorial therapy. Immunohistochemical analysis was performed on paraffin-embedded tissues for PPAR-γ, RXR-α, RhoB, p21, and angiopoietin-like 4 (ANGPTL4). At baseline, PPAR-γ was detectable in 4 of 4 patients, RXR-α in 3 of 4 patients, RhoB in 4 of 4 patients, p21 in 2 of 4 patients, and ANGPTL4 in 2 or 4 patients. At the end of week 1, PPAR-γ was detectable in 3 of 4 patients, RXR-α in 3 of 4 patients, RhoB in 4 of 4 patients, p21 in 3 of 4 patients, and ANGPTL4 in 4 of 4 patients. At 4 weeks, all 5 biomarkers were detected in both patients. PPAR-γ and RXR-α were present in the nucleus and increased during treatment in the 1 analyzed patient with complete serial data (Figure 2), providing proof of principle that efatutazone could bind the PPAR-γ-RXR-α nuclear complex in patients, thereby stimulating transcription of PPAR-γ–responsive genes. ANGPTL4, a well-known, highly sensitive PPAR-γ–responsive gene, was undetectable at baseline and highly elevated at day 7 and at the end of cycle 1 (Figure 2). This result indicates that efatutazone is biologically active in the ATC tumor. RhoB, a newly identified PPAR-γ–responsive gene noted to mediate antitumor activity in ATC cell lines (16), was elevated at baseline and found in the nucleus (Figure 2). The typical location for RhoB is cytoplasmic/endosomal (17). p21, like RhoB, did not change with treatment. Thus, it appears that the highly responsive PPAR-γ gene ANGPTL4 increases in response to efatutazone treatment when PPAR-γ and RXR-α are present in tumor tissues. Another clinical trial with a larger patient cohort, however, is necessary to confirm this initial finding and allow correlation with the survival benefit.
Figure 2.
Results of immunohistochemical analysis of tumor biopsy tissues from 1 patient with usable samples at baseline, week 1 (run-in phase), and week 4: A, hematoxylin and eosin (H & E); B, PPAR-γ; C, RXR-α; D, ANGPTL4; E, RhoB; and F, p21.
Serum/plasma biomarkers
Plasma adiponectin increased from a median of 5958 ng/mL at baseline to 23 810 ng/mL at 7 days in the 0.15-mg bid efatutazone group and from a median of 4007 to 26 810 ng/mL at 7 days in the 0.3-bid efatutazone group. Plasma adiponectin remained elevated at day 21, with median values of 41 450 and 44 820 ng/mL, respectively, in both patient groups. This increase was significant in both groups (P < .001). All other serum biomarkers showed no consistent changes.
Discussion
ATC is one of the most aggressive tumors known, with life expectancy measured in weeks to a few months. Data from prospective clinical trials are limited, and survival data from pilot therapeutic studies are scant. Although up to 15% to 20% of patients have been reported to be long-term survivors, the series reporting these results included patients with stage IVA (resectable) disease. In the current study, most patients had stage IVC disease (distant metastases), whereas a few had unresectable locoregional (stage IVB) disease. In patients with stage IVC disease, the median survival was 2, 2.5, 3.3, and 5.5 months in other studies (4, 18–20). This patient population best represents the patients who participated in our clinical trial.
The decision to use paclitaxel in the current study was based on the results of a report by Ain et al (4) using single-agent paclitaxel. In one of the few prospective trials, they reported a median survival of 5.5 months (median survival for responders was 32 weeks, but only 7 weeks for nonresponders). Subsequently, the taxane docetaxel was used in 2 recent studies. Kawada et al (5) treated 7 patients with ATC; 1 had a complete response and 2 had stable disease, with median time to progression and survival of 6 and 13 weeks, respectively. Troch et al (6) treated 6 patients with ATC (4 patients with stage IVB and 2 patients with stage IVC) with radiation (40–60 Gy) and docetaxel and achieved complete responses in 4 patients and 1 partial response at 6 months, with median survival of 15 months and 5 of 6 patients still alive. Mooney et al (9) treated 26 patients with ATC with fosbretabulin (also a microtubule poison like taxanes). Seven patients had IVB disease, and 19 patients had stage IVC disease. Although 7 patients had stable disease as their best response, and the 3 longest survivors (15.9–37.9 months) had distant metastases, median survival was only 4.7 months.
PPAR-γ is a nuclear transcription factor that heterodimerizes with RXR receptors. The rationale for examining a potent PPAR-γ agonist for oncologic activity is supported by preclinical studies demonstrating G1 cell cycle arrest with up-regulation of p18, p21, p27, phosphatase and tensin homolog, and decreased expression of cyclin D1, as well as inhibition of angiogenesis, cyclooxygenase-2, and prostaglandin E2 (11, 12, 14, 15). In addition, PPAR-γ enhances apoptosis by activating caspases, up-regulating Bax, and down-regulating bcl-2, survivin, and c-myc while also inhibiting β-catenin/T cell factor signaling and up-regulating E-cadherin (11, 12, 14). PPAR-γ agonists in preclinical studies have inhibited tumor growth in multiple malignancies including colon, stomach, prostate, breast, pancreas, lung, liver, and brain (12), although in 1 study PPAR-γ depletion arrested the cell cycle and reduced proliferation (21).
We have shown the benefits of combining efatutazone and paclitaxel in anaplastic thyroid cell lines and xenograft models (15, 16). In humans, efatutazone was tested in a phase 1 report in patients with advanced malignancies (14), and preliminary results of a phase 2 randomized study in combination with carboplatin and paclitaxel in patients with non–small cell lung cancer was reported at the American Society of Clinical Oncology (22).
This phase 1 study of combinatorial therapy with the PPAR-γ agonist efatutazone and paclitaxel in advanced ATC is of potential importance in several respects. First, we demonstrated safety and feasibility of the efatutazone + paclitaxel combination, observing no DLTs when the combination of efatutazone (up to 0.3 mg bid) and paclitaxel (175 mg/m2) was administered every 3 weeks. Future studies may consider weekly paclitaxel dosing, given the rapid doubling time of ATC or the use of nanoparticle albumin–bound paclitaxel. As expected, fluid retention was a common AE associated with efatutazone administration, with 13 events noted in 8 subjects. However, only 2 patients experienced grade 3 edema. Peripheral neuropathy was not a significant side effect. There were 2 SAEs (grade 4), one each attributed to efatutazone (anemia) and paclitaxel (anaphylaxis). All deaths were attributed to disease progression. Unfortunately, early study closure due to slow accrual prohibited definition of whether an even higher dosage of efatutazone (eg, >0.3 mg twice daily) might also have been acceptably tolerated when combined with paclitaxel.
Second, although the primary objectives of this study involved establishing safety and dosages of the efatutazone-paclitaxel combination for subsequent phase 2 trials, 1 of 15 patients achieved a durable RECIST response (Figure 1A). Such a profound and durable response is rare in ATC. Moreover, in exploratory analyses, patients receiving dose level 2 had a 42% and 41% improvement in median time to progression and median survival compared with patients receiving dose level 1, respectively, reinforcing the hypothesis that this drug combination might have meaningful clinical effects. This possibility is bolstered by the knowledge that 3 of the 4 patients with stage IVB disease (who would have been expected to have had better, rather than worse, prognosis) were in the worse outcome dose level 1 cohort. Given that 3 of the 7 dose level 1 patients received only the 135 mg/m2 dose of paclitaxel, we cannot conclude that there is a dose-dependent benefit of efatutazone alone. A larger study randomized to paclitaxel with or without efatutazone will be required. In addition to the single durable RECIST partial response, further exploratory analyses revealed that 8 additional patients attained periods of disease stabilization, with the duration of disease stability being longer in patients receiving the higher efatutazone dosage (68 vs 48 days). Overall, we believe that these outcomes are encouraging in the setting of a disease with historically miserable outcomes.
Third, as hypothesized, serum efatutazone concentrations increased with increasing efatutazone doses, with evidence of dose-dependent biologic activity also observed. In particular, exploratory serum biomarker analyses demonstrated that adiponectin increased within 7 days and remained elevated for the 7 weeks it was monitored. Exploratory assessment of tissue biomarkers also showed that PPAR-γ, RXR-α, RhoB, and p21 were present at baseline, with PPAR-γ and RXR-α increasing further during drug treatment. In contrast, ANGPTL4 was substantially induced after therapy in 3 patients, indicating that efatutazone was producing the hypothesized biologic effects in assessed patient tumors.
Taken together, results from the present trial provide encouraging pilot data in support of further development of the efatutazone-paclitaxel combination in advanced ATC. Efforts are therefore currently underway to extend these preliminary results to develop a more definitive randomized trial to assess the incremental effects of the addition of efatutazone to paclitaxel monotherapy in patients with advanced ATC.
Supplementary Material
Acknowledgments
The clinical trial was funded by Daiichi Sankyo.
The immunohistochemistry analyses were funded in part by National Institutes of Health/National Cancer Institute Grant R01 CA136665 (to J.A.C. and R.C.S.).
Disclosure Summary: J.A.C. has received research funding from Daiichi Sankyo. M.S.B. has received research funding and honoraria from Bayer Healthcare, Onyx, and Exelixis, research funding from Novartis and Genentech/Roche, and consulting fees from AstraZeneca, Bristol-Myers Squibb, and OXiGENE. Y.H. has received consulting fees from Exelixis. M.H.S. has received research funding from Daiichi Sankyo. R.V.R. is an employee of Daiichi Sankyo. The other authors have nothing to disclose.
Footnotes
- ANGPTL4
- angiopoietin-like 4
- ATC
- anaplastic thyroid cancer
- bid
- twice daily
- CTCAE
- Common Terminology Criteria for Adverse Events
- DLT
- dose-limiting toxicity
- PK
- pharmacokinetic
- PPAR
- peroxisome proliferator–activated receptor
- RECIST
- Response Evaluation Criteria in Solid Tumors
- RXR-α
- retinoid X receptor-α
- UNL
- upper limit of normal.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29 [DOI] [PubMed] [Google Scholar]
- 2. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22:486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034 [DOI] [PubMed] [Google Scholar]
- 4. Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587–594 [DOI] [PubMed] [Google Scholar]
- 5. Kawada K, Kitagawa K, Kamei S, et al. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol. 2010;40:596–599 [DOI] [PubMed] [Google Scholar]
- 6. Troch M, Koperek O, Scheuba C, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95:E54–E57 [DOI] [PubMed] [Google Scholar]
- 7. Hogan T, Yu JJ, Williams HJ, Altaha R, Liang X, He Q. Oncocytic, focally anaplastic, thyroid cancer responding to erlotinib. J Oncol Pharm Pract. 2009;15:111–117 [DOI] [PubMed] [Google Scholar]
- 8. Ha HT, Lee JS, Urba S, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–980 [DOI] [PubMed] [Google Scholar]
- 9. Mooney CJ, Nagaiah G, Fu P, et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid. 2009;19:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Theocharis S, Margeli A, Vielh P, Kouraklis G. Peroxisome proliferator-activated receptor-γ ligands as cell-cycle modulators. Cancer Treat Rev. 2004;30:545–554 [DOI] [PubMed] [Google Scholar]
- 12. Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419–429 [DOI] [PubMed] [Google Scholar]
- 13. Shimazaki N, Togashi N, Hanai M, et al. Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor γ agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer. 2008;44:1734–1743 [DOI] [PubMed] [Google Scholar]
- 14. Pishvaian MJ, Marshall JL, Wagner AJ, et al. A phase 1 study of efatutazone, an oral peroxisome proliferator-activated receptor γ agonist, administered to patients with advanced malignancies. Cancer. 2012;118:5403–5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25:2304–2317 [DOI] [PubMed] [Google Scholar]
- 16. Marlow LA, Reynolds LA, Cleland AS, et al. Reactivation of suppressed RhoB is a critical step for the inhibition of anaplastic thyroid cancer growth. Cancer Res. 2009;69:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prendergast GC. Actin' up: RhoB in cancer and apoptosis. Nat Rev Cancer. 2001;1:162–168 [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi T, Asakawa H, Umeshita K, et al. Treatment of 37 patients with anaplastic carcinoma of the thyroid. Head Neck. 1996;18:36–41 [DOI] [PubMed] [Google Scholar]
- 19. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321–330 [DOI] [PubMed] [Google Scholar]
- 20. Voutilainen PE, Multanen M, Haapiainen RK, Leppäniemi AK, Sivula AH. Anaplastic thyroid carcinoma survival. World J Surg. 1999;23:975–978; discussion 978–979 [DOI] [PubMed] [Google Scholar]
- 21. Wood WM, Sharma V, Bauerle KT, et al. PPARγ promotes growth and invasion of thyroid cancer cells. PPAR Res. 2011;2011:171765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaw A, Ghizdavescu D, Jain M, et al. Randomized phase 2 study of efatutazone in combination with carboplatin and paclitaxel as first-line therapy for metastatic non-small cell lung cancer (NSCLC). Presented at American Association for Cancer Research; March 31–April 4, 2012, Chicago, IL [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.