Abstract
Context:
Subclinical hypothyroidism (SCH) has been associated with an increased risk for cardiovascular disease. However, few studies have specifically examined the association between SCH and myocardial infarction (MI), and the relationship is poorly understood.
Objectives:
The purpose of this study was to evaluate incident MI risk in relation to SCH and severities of SCH among postmenopausal women.
Methods:
We used a population-based nested case-cohort design within the Women's Health Initiative observational study to examine the association between SCH and incident first-time MI risk among postmenopausal women in the United States. SCH was assessed using blood specimens collected at baseline. Participants presenting with normal free T4 levels and with thyrotropin levels of greater than 4.68–6.99 mU/L or 7.00 mU/L or greater were defined as having mild SCH or moderate/severe SCH, respectively. MI cases were centrally adjudicated by trained Women's Health Initiative staff. The primary analysis included 736 incident MI cases and 2927 randomly selected subcohort members. Multivariable adjusted Cox-proportional hazard models were used to assess MI risk in relation to SCH.
Results:
Compared with euthyroid participants, the multivariable adjusted hazard ratio (HR) for participants with any SCH was 1.05 [95% confidence interval (CI) 0.77–1.44]. HRs for participants with mild SCH, moderate/severe SCH, and moderate/severe SCH and the presence of antithyroid peroxidase antibodies (TPOAb) were 0.99 (95% CI 0.67–1.46), 1.19 (95% CI 0.72–1.96), and 0.90 (95% CI 0.47–1.74), respectively.
Conclusion:
We did not find evidence to suggest that SCH is associated with increased MI risk among a population of predominantly older postmenopausal women with no prior history of MI.
Diminished thyroid function is more common among women and with advancing age (1, 2). Diagnosis of overt hypothyroidism is based on decreased free T4 (fT4) levels and increased TSH. Laboratory testing also identifies individuals with elevated TSH and normal thyroid hormone levels who may or may not be symptomatic. Although fT4 levels are within the population reference range in these individuals, TSH elevation suggests the fT4 concentration is not normal for them (3). This circumstance, presumed to lie along the continuum of decreased thyroid function, has been termed subclinical or mild hypothyroidism (SCH) and can be further subclassified by the presence of antithyroid peroxidase antibodies (TPOAb). High TSH and high TPOAb levels are so strongly predictive of overt disease that this subgroup has been described as having impending overt disease (4). Five percent to 20% of postmenopausal women fall into this category, with prevalence increasing in each decade of age, compared with 1%–3% who have overt hypothyroidism (5–8).
SCH is of special interest in the context of cardiac disease. Like overt hypothyroidism, SCH has been associated with increased risk for heart failure (9–12) and atherosclerotic disease (13, 14). Studies suggest increased risk of incident coronary heart disease (CHD) events (15, 16) and deaths (15, 17) in relation to SCH but not consistently (18–21). These studies are informative, but comparison between studies is often difficult because of the broadly defined outcome statuses including CHD, ischemic heart disease (IHD), and cardiovascular disease. Definitions for these outcomes are overlapping yet not always consistent. Although composite outcomes may provide greater statistical efficiency, a key assumption is that it applies equal weight and importance to all of its component events, an assumption that is not always justified for mechanisms leading to each outcome. Against the backdrop of the US Preventative Services Task Force judgment that there was insufficient evidence to support screening for thyroid disease, we focused on examining noncomposite putative targets such as myocardial infarction (MI) or ischemic stroke, the leading causes of death in women to generate evidence for or against the screening of thyroid disease.
The relationship between SCH and MI as a noncomposite outcome has been reported by only 1 large cross-sectional study (22) and 3 follow-up studies with inconclusive results (8, 9, 23). In this study we examined the association between SCH and subsequent risk for MI in a large prospective cohort of postmenopausal women. Specifically our objective was to determine whether SCH at baseline is independently associated with risk for incident MI in the 7 years after enrollment in the Women's Health Initiative Observational Study (WHI-OS).
Materials and Methods
Study population
Participants were part of the WHI-OS enrolled at 40 clinical centers across the United States (24). Enrollment occurred between October 1993 and December 1998 and included 93 676 women who had a minimum of 5 years of follow-up at the time the data were prepared for this ancillary analysis. Inclusion criteria for this study were postmenopausal status, age 50–79 years at screening for enrollment, anticipated residence in the local study area for at least 3 years, and available baseline serum in the specimen bank. Exclusion criteria were a self-reported history of current or prior thyroid disease, the use of medications with a potential to alter TSH or fT4 levels (thyroid medications, amiodarone, oral corticosteroids, dopamine, lithium, immunomodulators and immunosuppressive agents, hydantoins, and carbamazepine), a history of MI, a history of coronary artery bypass grafting or carotid endarterectomy, unknown race/ethnicity, and no outcome forms after baseline.
MI cases
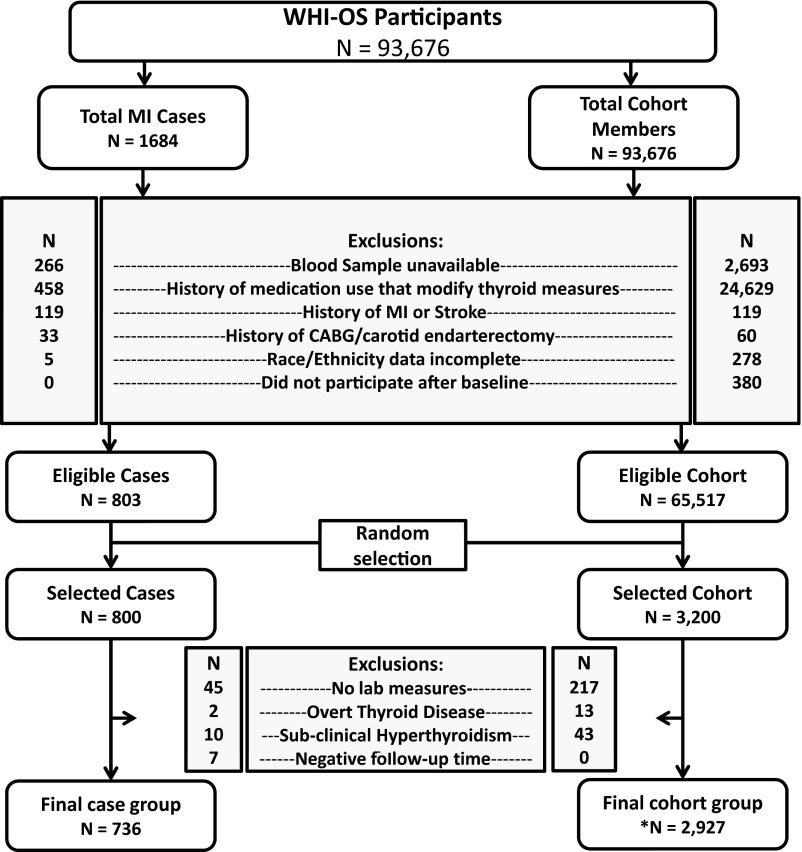
During follow-up, MI ascertainment was conducted every 12 months. All diagnoses were adjudicated by trained WHI staff, which had no knowledge of participants' thyroid status. MI was diagnosed using an algorithm and adjudication process that included clinical data, electrocardiogram readings, and cardiac enzymes; the methods have been fully described (25). All incident-fatal and nonfatal MIs reported as of December 15, 2004, were selected as potential cases for this study and subject to the baseline criteria described above. During the follow-up period, there were 1684 MIs; Figure 1 illustrates the step down in available cases based on each inclusion/exclusion criterion, resulting in 803 available and eligible cases. At the approval of this ancillary analysis, we had permission to use 800 MI case specimens; 3 cases were chosen by random sample for exclusion. We further excluded participants who had negative follow-up time (n = 7) and participants whose laboratory measures of thyroid hormones could not be determined (n = 45) or whose laboratory results indicated the presence of overt thyroid disease (n = 2) or subclinical hyperthyroidism (n = 10).
Figure 1.
Flow diagram representing the selection process for cases and representative cohort from the Women's Health Initiative Observational Study Participants. *, Includes 60 participants who were chosen as cases and were again selected in the randomly chosen comparison cohort.
Cohort sampling
The inclusion/exclusion criteria were applied to the full cohort to generate a sampling frame for the subcohort portion of this case-cohort study (Figure 1). We had permission to use specimens from 3200 cohort members from the 65 517 who were potentially eligible. This subcohort was designed to serve as a comparison group for 2 analyses: 1) the present analysis assessing SCH and MI risk and 2) another assessing SCH and stroke risk. For the subcohort, we used random sampling within age strata (5 year brackets), race, and recruitment location to density-match to the distribution of the 800 MI cases and 750 stroke cases. As with cases, we excluded observations whose laboratory measures were unavailable (n = 217) or indicated overt thyroid disease (n = 13) or subclinical hyperthyroidism (n = 43). Our primary analysis included 736 and 2927 observations in the case group and subcohort, respectively.
Thyroid hormone measurement
Fasting blood specimens were collected at baseline, aliquoted, and stored frozen at −70°C at the WHI central repository. Specimens were shipped on dry ice and stored at −20°C before analysis. Laboratory personnel conducting the assays were blind to participant characteristics. We used a highly specific, third-generation immunometric assay with a chemiluminescent detection on a Vitros ECI analyzer (Ortho Diagnostics, North Raritan, New Jersey) to measure TSH. The assay had a sensitivity to 0.01 mU/L with a TSH analytic range from 0.01 to 100.00 mU/L. The controls (BioRad Laboratories, Hercules, California) were assayed across the range of clinical laboratory quality-control concentrations every 24 hours.
Specimens with TSH not within the reference range (0.46–4.68 mU/L) were tested for fT4 and TPOAb. fT4 was measured by a competitive immunoassay using direct chemiluminescent detection on the Bayer ADVIA Centaur analyzer (Tarrytown, New York). The analytical measurement range for the fT4 assay is 0.1–12.0 ng/dL and the adult reference range is 0.70–1.85 ng/dL. Two levels of controls (BioRad Laboratories) were assayed every 24 hours. TPOAb was measured using a solid-phase, sequential chemiluminescent immunometric assay on the Immulite 2000 analyzer (Diagnostic Products Corp, Los Angeles, California). The analytical reportable range for the TPOAb assay is 10–1000 IU/mL. Specimens with concentrations above 1000 IU/mL were diluted and repeated. The adult reference range is less than 35 IU/mL. Controls (BioRad Laboratories) were assayed every 24 hours. Specimens were analyzed between March 2005 and December 2006. Between-batch coefficients of variance were less than 6.3%, less than 8%, and less than 9.5% for TSH, fT4, and TPOAb measurements, respectively. Across each of the 3 types of assays, 5% of the specimens received were processed as blind duplicates and results returned to the WHI Laboratory Working Group. All met quality control criteria.
Statistical analysis
Population characteristics were compared by thyroid status (euthyroid vs SCH) measured at baseline using a t test or a Fisher's exact test as applicable. We aimed to obtain an unbiased estimate of the main effects of SCH on MI. We used weighted cox proportional hazard models to assess the marginal influence of SCH alone, a parsimonious model to account for confounding variables, and a fully adjusted model to account for additional risk factors for MI. Briefly, the sampling strategy of a case-cohort design allows cases to be included in the subcohort portion of the study population. Because of the nonindependent score contributions that result from such strategies, the design requires the use of a pseudolikelihood method, specifically a weighted cox-regression model, instead of a partial-likelihood function as done with a regular Cox regression model. Furthermore, the correlation between the nonindependent score contributions need to be accounted for in estimating the variance.
We utilized the weighting method proposed by Barlow et al (26) and a jack-knife variance estimation strategy (26, 27) because these have been shown to produce reliable results for case-cohort analyses. For all analyses, euthyroid served as the reference. Candidate covariates for the full-model were selected a priori and included age, race/ethnicity, WHI site, years since menopause, gravidity, parity, hypertension, current diabetes, hormone therapy, smoking history, alcohol consumption, and body mass index. The parsimonious model comparing SCH with euthyroid was developed using directed acyclic graphs to include only known or suspected confounders in the relation between SCH and MI. These included age, race/ethnicity, gravidity, smoking status, hormone therapy, and alcohol consumption. Causal intermediates were not included in the parsimonious model. All additional models retain the same covariates. We compared MI risk stratified by severity of SCH: mild SCH (TSH 4.69–6.99 mU/L), moderate/severe SCH (TSH ≥ 7.00 mU/L) and moderate/severe SCH with the presence of TPOAb, all compared with euthyroid women. Hazard ratios (HRs) were similar whether we used the full (additional adjustment for MI risk factors and causal intermediates) or parsimonious models; we present the results of the latter. The proportional hazards assumption in all models was tested by including an interaction term with time for SCH variables; no statistically significant violations of assumptions were noted (P > .55). The likelihood ratio test was used to formally test for interaction between the 6 retained covariates and SCH in relation to MI.
In sensitivity analyses we limited our assessment to individuals who did not have impending thyroid disease (TSH levels ≥ 20 mU/L). Antihyperlipidemic medication use was used as a proxy for baseline cholesterol and lipid levels because biomarker information on these causal intermediates was unavailable. Medication reviews were not conducted annually; therefore, to censor those who reported clinically diagnosed or treated thyroid disease, we constructed separate models using self-reported thyroid disease and medication status in the third year of follow-up. Most analyses were done in STATA 12 (STATA Corp, College Station, Texas). Application of robust variance in Cox proportional hazard models was done in SAS 9.3 (SAS Institute, Cary, North Carolina). This study was reviewed and determined to be exempt from further review by the Institutional Review Board at the University of North Carolina, Chapel Hill.
Results
The study population of 3663 women had a mean age of 67.5 years at baseline, and the majority was white race/ethnicity by self-report (85%). Eight percent (n = 282) met criteria for SCH. Among that group 193 (68%) had TSH between 4.69 and 6.99 mU/L and were classified as mild SCH; 89 women (32%) had TSH 7.00 mU/L or greater and were classified as moderate/severe SCH; all had normal levels of fT4. In bivariate analysis, factors associated with having SCH at baseline compared with being euthyroid included age at baseline, race/ethnicity (with white participants more likely than others to have SCH), and years from menopause, which maps closely with age (Table 1).
Table 1.
Distribution of Baseline Characteristics and Cardiovascular Risk Factors According to Baseline Thyroid Function Status Among 3663 Postmenopausal Women From the Women's Health Initiative Observational Study
| Characteristics | Subclinical Hypothyroidism |
P Valuea | |||
|---|---|---|---|---|---|
| Euthyroid (n = 3381) | Any SCH (n = 282) | Mild SCH (n = 193) | Mod/Sev SCH (n = 89) | ||
| Thyroid measures | |||||
| Median TSH, mU/L (IQR) | 1.91 (1.31–2.69) | 5.85 (5.12–7.70) | 5.38 (4.95–5.90) | 9.14 (7.79–12.70) | |
| Median fT4, ng/dL (IQR) | — | 0.98 (0.88–1.10) | 1.02 (0.92–1.13) | 0.92 (0.81–1.00) | |
| TPOAb present, n (%) | — | 133 (47.2) | 71 (36.8) | 62 (69.3) | |
| Age at baseline, y | |||||
| 50–64 | 1107 (32.8) | 66 (23.4) | 40 (20.7) | 26 (29.2) | <.001 |
| 65–70 | 999 (29.5) | 78 (27.7) | 55 (28.5) | 23 (25.8) | |
| 71–79 | 1275 (37.7) | 138 (48.9) | 98 (50.8) | 40 (44.9) | |
| Race or ethnic group | |||||
| White | 2828 (83.8) | 264 (94.0) | 182 (94.8) | 82 (92.1) | <.001 |
| Black | 286 (8.5) | 6 (2.1) | 5 (2.6) | 1 (1.1) | |
| Hispanic | 90 (2.7) | 8 (2.8) | 3 (1.6) | 5 (5.6) | |
| Asian or Pacific Islander | 123 (3.7) | 1 (0.4) | 0 (0.0) | 1 (1.1) | |
| American Indian | 21 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Unknown | 24 (0.7) | 2 (0.7) | 2 (1.0) | 0 (0.0) | |
| Smoking | |||||
| None | 1714 (51.5) | 143 (51.6) | 102 (53.7) | 41 (47.1) | .93 |
| Prior | 1408 (42.4) | 119 (43.0) | 81 (42.6) | 38 (43.7) | |
| Current | 202 (6.1) | 15 (5.4) | 7 (3.7) | 8 (9.2) | |
| Diabetes | |||||
| No | 3150 (93.8) | 265 (94.6) | 179 (93.7) | 86 (96.6) | .70 |
| Yes | 207 (6.2) | 15 (5.4) | 12 (6.3) | 3 (3.4) | |
| Waist to hip ratio | |||||
| ≤0.80 | 1581 (47.0) | 142 (50.5) | 101 (52.6) | 41 (46.1) | .35 |
| >0.80–0.85 | 856 (25.5) | 61 (21.7) | 40 (20.8) | 21 (23.6) | |
| >0.85 | 924 (27.5) | 78 (27.8) | 51 (26.6) | 27 (30.3) | |
| Body mass index, kg/m2 | .73 | ||||
| <25.00 | 1345 (40.2) | 106 (37.9) | 73 (37.8) | 33 (37.9) | |
| 25.00 to < 30.00 | 1194 (35.7) | 103 (36.8) | 72 (37.3) | 31 (35.6) | |
| ≥30 | 806 (24.1) | 71 (25.3) | 48 (24.9) | 23 (26.4) | |
| Hormone therapy use | |||||
| Never | 1532 (45.4) | 126 (44.7) | 83 (43.0) | 43 (48.3) | .95 |
| Past | 580 (17.2) | 50 (17.7) | 38 (19.7) | 12 (13.5) | |
| Current | 1264 (37.4) | 106 (37.6) | 72 (37.3) | 34 (38.2) | |
| Years from menopause, y | |||||
| 0–15 | 966 (31.6) | 59 (22.2) | 39 (21.2) | 20 (24.4) | .002 |
| 16–23 | 1087 (35.5) | 99 (37.2) | 69 (37.5) | 30 (36.6) | |
| 24–26 | 1005 (32.9) | 108 (40.6) | 76 (41.3) | 32 (39.0) | |
| Hypertension | |||||
| Never | 1670 (49.6) | 142 (50.4) | 90 (46.6) | 52 (57.8) | .89 |
| Past or controlled | 698 (20.8) | 55 (19.5) | 42 (21.8) | 13 (14.4) | |
| Current | 995 (29.6) | 85 (30.1) | 61 (31.6) | 25 (27.8) | |
| Antihyperlipidemic useb | |||||
| No | 2672 (96.6) | 233 (98.3) | 155 (98.7) | 78 (97.5) | .18 |
| Yes | 95 (3.4) | 4 (1.7) | 2 (1.3) | 2 (2.5) | |
Abbreviations: IQR, interquartile range; Mod/Sev, moderate/severe. Mild is TSH 4.7 to 6.99 mU/L; moderate or severe is TSH 7.00 mU/L or greater; —, data not collected.
Comparison of euthyroid with all with subclinical hypothyroidism using a t test with unequal variance for continuous variables and Fisher's exact test for categorical variables.
Antihyperlipidemic medication use is defined by the WHI-OS as use of bile sequestrants, fibric acid derivatives, intestinal cholesterol absorption inhibitors, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, nicotinic acid derivatives, or combinations. Information at baseline was missing for 614 observations and 45 observations for participants with normal thyroid and with any subclinical hypothyroidism, respectively.
The hazard ratio for incident MI in the average of seven years after baseline for those with SCH compared with those who were euthyroid was 1.14 (95% CI 0.84–1.55) in unadjusted models and 1.05 (95% CI 0.87–1.44) in the parsimoniously adjusted model (Table 2).
Table 2.
Myocardial Infarction Hazard Ratios (95% CI) in Relation to Any SCH, Severity of SCH, and Presence of TPOAb Among Postmenopausal Women From the Women's Health Initiative Observational Study
| Thyroid Condition | Unadjusted Model |
Adjusted Model |
|||
|---|---|---|---|---|---|
| n (Case/Cohort) | HR (Unadjusted)a | n (Case/Cohort) | HR (Unadjusted)b | HR (Adjusted)c | |
| Euthyroid | 677/2704 | 1.00 (Referent) | 650/2926 | 1.00 (Referent) | 1.00 (Ref) |
| SCHd | 59/223 | 1.14 (0.84–1.55) | 58/217 | 1.18 (0.87–1.60) | 1.05 (0.77–1.44) |
| Milde | 38/155 | 1.08 (0.74–1.56) | 37/151 | 1.10 (0.75–1.60) | 0.99 (0.67–1.46) |
| Moderate/Severef | 21/68 | 1.28 (0.78–2.11) | 21/66 | 1.35 (0.82–2.22) | 1.19 (0.72–1.96) |
| Moderate/Severe and TPOAb+g | 11/51 | 0.99 (0.51–1.92) | 11/49 | 1.06 (0.55–2.04) | 0.90 (0.47–1.74) |
SCH is TSH 4.7 mU/L or greater; mild, TSH 4.7 to 6.99 mU/L; moderate/severe, TSH 7.00 mU/L or greater; and TPOAb+, anti-TPOAb are present.
Sample size is based on entire sample until the respective follow-up time.
Sample size is based on same observations used in the adjusted model.
Adjusted for age (50–64 years, 65–70 years, and 71–79 years), ethnicity (white, black, Hispanic, Asian/Pacific Islander, American Indian, or other), gravidity (never pregnant, 1, 2–4, or 5+), smoking status (never, prior, or current), hormone therapy (never, prior, or current), and alcohol consumption (1 or fewer drinks/wk, 2–6 drinks/wk, or 7+ drinks/wk); covariates were measured at baseline only.
Any subclinical hypothyroid vs euthyroid.
Mild subclinical hypothyroid vs euthyroid (severe category omitted).
Moderate/severe subclinical hypothyroid vs euthyroid (mild category omitted).
Moderate/severe and TPOAb positive vs euthyroid (mild and TPOAb negative omitted).
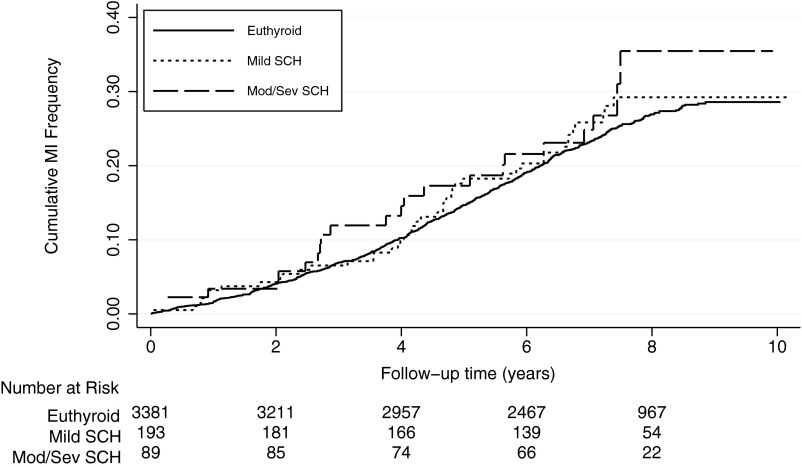
Stratification by severity of SCH (mild, grouping those with moderate and severe SCH, and then those with moderate and severe SCH as well as TPOAb+, all compared with euthyroid) did not substantively change our findings with multivariable-adjusted HRs of 0.99 (95% CI 0.67–1.46), 1.19 (95% CI 0.72–1.96), and 0.90 (95% CI 0.47–1.74), respectively (Table 2). The cumulative hazard curves as estimated by the Nelson-Aalen method are shown by strata of severity of SCH (Figure 2). We investigated the effect modification of the relationship between SCH and MI by age, ethnicity, gravidity, smoking status, hormone replacement, and alcohol consumption; we did not identify any substantive interactions (Table 3).
Figure 2.
Nelson-Aalen cumulative hazard estimates for acute MI by severity of SCH among women from the Women's Health Initiative Observational Study. Mod/Sev, Moderate/severe. Euthyroid is a TSH 0.46–4.68 mU/L; mild SCH, TSH 4.69–6.99 mU/L; and moderate/sev SCH, TSH 7.00 mU/L or greater.
Table 3.
Multivariable Adjusted Hazard Ratios for Myocardial Infarction in Relation to Any SCH, Moderate/Severe SCH, and Presence of TPOAb by Strata of Important Covariates Among Women from the Women's Health Initiative Observational Study
| Covariate | Stratum-Specific, Multivariable-Adjusted Hazard Ratios (95% CI)a |
||
|---|---|---|---|
| SCH | Mod/Sev SCH | Mod/Sev SCH and TPOAb+ | |
| Age, y | |||
| 50–64 | 0.98 (0.52, 1.83) | 1.74 (0.82, 3.70) | 1.37 (0.57, 3.30) |
| 65–70 | 0.99 (0.42, 2.35) | 0.53 (0.13, 2.26) | 0.23 (0.02, 2.07) |
| 71–79 | 1.11 (0.51, 2.39) | 1.27 (0.44, 3.69) | 1.04 (0.27, 4.02) |
| P for interaction | .9 | .16 | .12 |
| Smoking | |||
| Never | 1.11 (0.71, 1.73) | 1.16 (0.55, 2.44) | 0.94 (0.36, 2.46) |
| Past | 1.07 (0.56, 2.03) | 1.12 (0.38, 3.34) | 0.78 (0.17, 3.50) |
| Current | 0.74 (0.21, 2.53) | 1.47 (0.36, 6.07) | 1.11 (0.22, 5.69) |
| P for interaction | .69 | .9 | .89 |
| Ethnicityb | |||
| White | 1.07 (0.78, 1.48) | 1.28 (0.77, 2.13) | 0.93 (0.47, 1.84) |
| Black | 1.88 (0.32, 10.88) | — | — |
| Other | 0.7 (0.14, 3.50) | 0.56 (0.06, 5.11) | 1.02 (0.09, 11.07) |
| P for interactionb | .72 | — | — |
| Alcoholb | |||
| 0 to < 1 drink/wk | 1.00 (0.70, 1.45) | 1.36 (0.79, 2.33) | 0.9 (0.44, 1.83) |
| 1–6 drinks/wk | 1.27 (0.57, 2.84) | 1.15 (0.21, 6.29) | 2.04 (0.33, 12.66) |
| 7+ drinks/wk | 0.97 (0.33, 2.87) | — | — |
| P for interactionb | .9 | — | — |
| Gravidity | |||
| Never pregnant | 1.13 (0.38, 3.39) | 2.47 (0.61, 9.98) | 2.82 (0.57, 13.81) |
| 1 | 1.50 (0.31, 7.27) | 2.15 (0.22, 20.87) | 3.32 (0.28, 39.52) |
| 2–4 | 1.12 (0.35, 3.62) | 1.3 (0.27, 6.11) | 0.78 (0.12, 4.89) |
| 5+ | 0.84 (0.24, 2.98) | 0.73 (0.13, 4.13) | 0.61 (0.08, 4.78) |
| P for interaction | .68 | .33 | .18 |
| Hormone therapy | |||
| Never | 0.94 (0.59, 1.51) | 0.76 (0.34, 1.71) | 0.62 (0.22, 1.73) |
| Past | 1.19 (0.49, 2.90) | 1.21 (0.24, 6.06) | 0.58 (0.05, 6.54) |
| Current | 1.12 (0.56, 2.22) | 1.98 (0.66, 5.92) | 1.66 (0.41, 6.60) |
| P for interaction | .76 | .1 | .2 |
Abbreviations: Mod/Sev, moderate/severe; —, data not collected. SCH is TSH 4.70 mU/L or greater; moderate/severe is TSH 7.00 mU/L or greater; TPOAb+ is anti-TPOAb present.
Hazard ratios for each interaction covariate category was mutually adjusted for all other covariates as listed and categorized in the table.
Effect estimates and P value for interaction are left blank for certain ethnicity and alcohol categories for the Mod/Sev SCH and Mod/Sev SCH and TPOAb+ groups due to unstable effect estimates.
To eliminate the potential effect of impending overt thyroid disease on the association between SCH and MI, we performed analyses by excluding individuals (n = 7) who had TSH levels greater than 20 mU/L. HRs were similar for those with any SCH 1.08 (95% CI 0.79–1.48) or moderate/severe SCH 1.29 (95% CI 0.78–2.16), both compared with euthyroid individuals. Data on thyroid medication use at the 3-year visit were missing for 768 euthyroid women (22.72%), and 57 women with SCH (20.21%) at baseline. A larger proportion of women with SCH at baseline (17.38%) had initiated thyroid medication use at the 3-year visit compared with euthyroid women (1.60%). Additionally, medication use increased with increasing severity of SCH status at baseline: 13%, 25%, and 27% for women with mild SCH, moderate SCH, and severe SCH, respectively. To examine the potential influence of intercurrent diagnosis and treatment of thyroid disease, we then restricted the analysis to only women for whom we had complete 3-year follow-up responses about medication use and self-report of being told they had a thyroid condition. Women who reported thyroid disease or use of thyroid medications during the 3-year follow-up visit were censored. The association remained similar, with adjusted HRs of 1.02 (95% CI 0.76–1.51) for any SCH, 0.96 (95% CI 0.68–1.79) for mild SCH, and 1.13 (95% CI 0.66–2.05) for moderate/severe SCH.
Discussion
In this large case-cohort investigation of US postmenopausal women aged 50–79 years, we did not find evidence for an association between SCH and risk for MI. The lack of association between SCH and MI was not changed by the presence or absence of TPOAb and also did not vary by the severity of SCH [mild SCH (TSH 4.69–6.99 mU/L) or moderate/severe SCH (TSH ≥ 7.00 mU/L)].
To our knowledge, 3 prospective studies have specifically reported the association between SCH and MI as a noncomposite outcome. Hak et al (8) reported a relative risk of MI: 2.5 (0.7–9.1) for TSH greater than 4.0 mU/L. Rodondi et al (9), in a subgroup analysis, reported an increased risk for MI, HR 4.73 (1.0–22.1), only among those with TSH 10 mU/L or greater. However, the associations observed in both these studies were based on a small number of incident MI cases (n = 16 and n < 98, respectively) and therefore lack precision in their estimates. In a large Norwegian prospective cohort (n women = 18 030; MI cases = 474), Åsvold et al (23) found no association between women with SCH and incident MI hospitalizations (HR 1.05; 95% CI 0.82–1.59) compared with euthyroid women with TSH values of 0.5–1.4 mU/L. They reported similar results for males. As part of a continuum of disease progression, SCH likely follows a gradient ranging from mild subclinical to severe subclinical dysfunction. Individuals with severe SCH are more likely to be similar to those with overt hypothyroidism than those with euthyroid status and thus may have higher MI risk. Although Åsvold et al did not report the association between SCH and MI by severity of SCH, the results from our study are in agreement with their findings.
Other prospective studies have evaluated the association between SCH and broadly defined outcome statuses such as CHD, IHD, and death due to CHD and have yielded heterogeneous results. The Whickham study showed a nonsignificant elevated risk of IHD, 1.7 (95% CI 0.9–3.2) among women with elevated TSH and positive TPOAb (28). A reanalysis of the study in 2010 showed a statistically significant association between SCH and IHD risk, 1.8 (95% CI 1.2–2.7); however, the statistical significance did not remain after excluding subsequent levothyroxine treatment from their model (20). Of the other prospective studies that examined the association between SCH and CHD, 4 showed no evidence of association (18, 19, 29, 30), 2 showed statistically significant increased risk (15–17), and another demonstrated some evidence of association among men but no evidence among women (31). A recent individual-level pooled data analysis of prospective cohort studies suggested that SCH may be associated with marginally increased risk for CHD [HR 1.2 (95% CI 1.0–1.4)] and notably higher risk [HR 1.9 (95% CI 1.3–2.8)] among those with TSH concentrations of 10 mU/L or greater (21).
Recognizing the heterogeneity of composite outcomes assessed in the current literature and the difficulty of comparison that follows, we examined only confirmed incident first-time MI cases. The present study is one of the largest studies to date to have examined the association between SCH and MI specifically among postmenopausal women; our study had 736 MI cases, which is severalfold larger than previous studies that showed a positive association. Despite the larger sample size, we did not find an association. A priori, assuming 800 MI cases, 4:1 cohort to case ratio, and a type I error of 5%, we approximated a minimum detectable effect estimate of 1.43 with 90% power if the prevalence of SCH was 10% and an effect estimate of 1.81 with 80% power if the prevalence of SCH was 2%. Even though the prevalence of SCH was below 10% and we had slightly fewer MI cases than originally planned, our study was still equipped to detect an effect estimate of 1.47 with 80% power. However, the effect estimates we observed were much lower and closer to the null. It can also be argued that MI alone as an outcome may be too restrictive in providing information on general CHD risk. In an effort to address this, we examined the association between SCH and composite CHD (composed of first occurrence of clinical MI, definite silent MI, death due to any CHD, and other definite or possible CHD events) in the randomly selected subcohort participants, who are representative of the WHI-OS population. The effect estimates that we observed for any CHD event were similar to those that we observed in our primary case-cohort investigation of MI; corresponding adjusted HRs for any SCH, mild SCH, and moderate/severe SCH were 1.14 (95% CI 0.66–1.99), 1.05 (95% CI 0.52–2.15), and 1.37 (95% CI 0.66–2.87), respectively.
We did not have information on lipid levels and thus are unable to directly assess the relationship between SCH, lipids, and MI. However, we examined antihyperlipidemic medication use at the 3-year follow-up visit and found the proportion of antihyperlipidemic medication use to be similar, regardless of SCH status at baseline (data not shown). Interestingly, lipid levels are strong and graded predictors of cardiovascular disease including MI among middle-aged adults (32, 33); however, the association among older adults remains controversial (34, 35). Psaty et al (36) showed only marginal increases in MI risk among men and women 65 years of age or older in relation to elevated total cholesterol and low-density lipid cholesterol (LDL-C). Approximately 70% of our study population was 65 years of age or older at baseline. If the primary mechanism of MI progression in SCH is through increased LDL-C, then the predominantly older population in our study may explain the lack of association. By extension of this rationale, upon excluding individuals with history of MI, we may have effectively eliminated MI cases induced at least in part by SCH. On the other hand, only a few studies have shown elevated total- and LDL-C in relation to SCH (37). Evidence regarding altered cardiometabolic profiles in relation to SCH has not been consistent (37, 38), which may reflect the lack of association between SCH and MI in our study. Similar to results of this study, a recent study by Hyland et al (30) found no association between persistent SCH and CHD risk in an older population of men and women 65 years of age or older. However, opposing this hypothesis, the pooled analysis by Rodondi et al (21) found no such heterogeneity in results by age strata. It has also been suggested that TSH levels in otherwise healthy older adults may be higher than their younger counterparts. Changing the TSH threshold of SCH classification (decreasing it to 4.5 mU/L or increasing it to 5.0 mU/L) yielded very similar results (data not shown).
We measured thyroid biomarkers only at baseline and were unable to determine whether thyroid dysfunctions we detected persisted at a constant level, deteriorated, or improved over the study period. Somwaru et al (39) reported approximately 46% of participants with SCH (TSH 4.5–6.9 mU/L) reverted to euthyroid status during a 4-year period. Subsequent development of SCH among euthyroid participants, reversion to euthyroid status among SCH participants after baseline, or initiation of thyroid medications past the 3-year visit could have potentially biased our results toward the null. Information on thyroid medication use was available only for the 3-year visit and was missing for 23% of the study population. Due to the retrospective nature of this study, participants were not aware of their thyroid status and the study would be predicted to have lower rates of thyroid medication use during follow-up than participants in other studies. However, this advantage did not change the probable scenario in which rates of thyroid medication use at the 3-year follow-up visit were higher for women with SCH at baseline than for women with normal thyroid status at baseline.
Censoring women at the time of medication use did not materially alter the results of our study. Furthermore, our 1-time assessment of thyroid levels does not account for transient changes in TSH values, potentially leading to misclassification of SCH, especially for individuals with borderline threshold TSH values. This misclassification is likely nondifferential and may partly explain the null association between any SCH and MI. Additionally, we were unable to assess MI risk among participants with severe SCH because only 36 participants presented with TSH levels 10 mU/L or greater. Selection bias is a highly unlikely explanation for the results presented here because our cases and the subcohort came from the same, well-defined population. However, results from our study may not generalize for men of a similar age range, middle-aged women, or those with a previous history of CHD.
In summary, our study did not find evidence for an association between SCH and the risk for incident MI among a population of postmenopausal, predominantly older women with no prior history of CHD. Although not investigated in our study, the effect of SCH on MI may still be of relevance among individuals who are exposed to TSH levels 10 mU/L or greater, in whom the onset of SCH occurs at a younger age or persists for a longer period of time than that in our study.
Acknowledgments
We thank Alicia Curtis and Lindsay Martin for their assistance in the laboratory analysis of thyroid measures. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. The long list of WHI investigators include the following: Program Office (National Heart, Lung, and Blood Institute, Bethesda, Maryland), Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. The clinical coordinating centers include the following: Fred Hutchinson Cancer Research Center, Seattle, Washington, Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg, Barbara Cochrane, Julie Hunt, Marian Neuhouser, Lesley Tinker, Susan Heckbert, and Alex Reiner. The regional centers include the following: Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, and Maria Bueche; MedStar Health Research Institute/Howard University, Washington, DC, Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Brian Walitt, and Amy Park; The Ohio State University, Columbus, Ohio, Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, and Michael Blumenfeld; Stanford Prevention Research Center, Stanford, California, Marcia L. Stefanick, Mark A. Hlatky, Manisha Desai, Jean Tang, and Stacy T. Sims; University of Arizona, Tucson/Phoenix, Arizona, Cynthia A. Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, and Marcia Ko; University at Buffalo, Buffalo, New York, Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Amy Millen, and Michael LaMonte; University of Florida, Gainesville/Jacksonville, Florida, Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, and Yvonne Brinson; University of Iowa, Iowa/Davenport, Iowa, Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, and Jennifer Robinson; University of Pittsburgh, Pittsburgh, Pennsylvania, Lewis Kuller, Jane Cauley, and N. Carole Milas; University of Tennessee Health Science Center, Memphis, Tennessee, Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, and Fran Tylavsky; Wake Forest University School of Medicine, Winston-Salem, North Carolina, Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, and Michelle Naughton. The Women's Health Initiative Memory Study include the following: Wake Forest University School of Medicine, Winston-Salem, North Carolina, Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, and Michelle Naughton. The Former Principal Investigators and Project Officers include the following: Albert Einstein College of Medicine, Bronx, New York, Sylvia Wassertheil-Smoller; Baylor College of Medicine, Houston, Texas, Haleh Sangi-Haghpeykar, Aleksandar Rajkovic, Jennifer Hays, and John Foreyt; Brown University, Providence, Rhode Island, Charles B. Eaton and Annlouise R. Assaf; Emory University, Atlanta, Georgia, Lawrence S. Phillips, Nelson Watts, Sally McNagny, and Dallas Hall; Fred Hutchinson Cancer Research Center, Seattle, Washington, Shirley A.A. Beresford, and Maureen Henderson; George Washington University, Washington, DC, Lisa Martin, Judith Hsia, and Valery Miller; Harbor-UCLA Research and Education Institute, Torrance, California, Rowan Chlebowski; Kaiser Permanente Center for Health Research, Portland, Oregon, Erin LeBlanc, Yvonne Michael, Evelyn Whitlock, Cheryl Ritenbaugh, and Barbara Valanis; Kaiser Permanente Division of Research, Oakland, California, Bette Caan and Robert Hiatt; National Cancer Institute, Bethesda, Maryland, Carolyn Clifford; Medical College of Wisconsin, Milwaukee, Wisconsin, Jane Morley Kotchen; National Heart, Lung, and Blood Institute, Bethesda, Maryland, Linda Pottern; Northwestern University, Chicago/Evanston, Illinois, Linda Van Horn and Philip Greenland; Rush University Medical Center, Chicago, Illinois, Lynda Powell, William Elliott, and Henry Black; State University of New York at Stony Brook, Stony Brook, New York, Dorothy Lane and Iris Granek; University at Buffalo, Buffalo, New York, Maurizio Trevisan; University of Alabama at Birmingham, Birmingham, Alabama, Cora E. Lewis and Albert Oberman; University of Arizona, Tucson/Phoenix, Arizona, Tamsen Bassford, Cheryl Ritenbaugh, and Tom Moon; University of California at Davis, Sacramento, California, John Robbins; University of California at Irvine, Irvine, California, F. Allan Hubbell and Frank Meyskens Jr; University of California at Los Angeles, Los Angeles, California, Lauren Nathan and Howard Judd; University of California at San Diego, LaJolla/Chula Vista, California, Robert D. Langer; University of Cincinnati, Cincinnati, Ohio, Michael Thomas, Margery Gass, and James Liu; University of Hawaii, Honolulu, Hawaii, J. David Curb; University of Massachusetts/Fallon Clinic, Worcester, Massachusetts, Judith Ockene; University of Medicine and Dentistry of New Jersey, Newark, New Jersey, Norman Lasser; University of Miami, Miami, Florida, Mary Jo O'Sullivan and Marianna Baum; University of Minnesota, Minneapolis, Minnesota, Karen L. Margolis and Richard Grimm; University of Nevada, Reno, Nevada, Robert Brunner and Sandra Daugherty; University of North Carolina, Chapel Hill, North Carolina, Gerardo Heiss, Barbara Hulka, and David Sheps; University of Tennessee Health Science Center, Memphis, Tennessee, Karen Johnson and William Applegate; University of Texas Health Science Center, San Antonio, Texas, Robert Brzyski and Robert Schenken; University of Wisconsin, Madison, Wisconsin, Gloria E. Sarto and Catherine Allen; Wake Forest University School of Medicine, Winston-Salem, North Carolina, Mara Vitolins, Denise Bonds, Electra Paskett, and Greg Burke; and Wayne State University School of Medicine/Karmanos Cancer Institute, Detroit, Michigan, Michael S. Simon and Susan Hendrix.
This nested case-cohort study within the Women's Health Initiative Observational Study was supported by Grant RO1 HL076645 funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. The NHLBI had no influence on the study design, data collection, analysis and interpretation, or approval of this manuscript. The WHI program is funded by the NHLBI, National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2267
- CHD
- coronary heart disease
- fT4
- free T4
- HR
- hazard ratio
- IHD
- ischemic heart disease
- LDL-C
- low-density lipid cholesterol
- MI
- myocardial infarction
- SCH
- subclinical hypothyroidism
- TPOAb
- thyroid peroxidase antibody
- WHI-OS
- Women's Health Initiative Observational Study.
References
- 1. Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid. 2007;17[1050–7256 (Print)]:1211–1223 [DOI] [PubMed] [Google Scholar]
- 2. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87[0021–972X (Print)]:489–499 [DOI] [PubMed] [Google Scholar]
- 3. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87[0021–972X (Print)]:1068–1072 [DOI] [PubMed] [Google Scholar]
- 4. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf). 1991;34(1):77–84 [DOI] [PubMed] [Google Scholar]
- 5. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534 [DOI] [PubMed] [Google Scholar]
- 6. Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68 [DOI] [PubMed] [Google Scholar]
- 7. Bagchi N, Brown TR, Parish RF. Thyroid dysfunction in adults over age 55 years: a study in an urban US community. Arch Intern Med. 1990;150(4):785–787 [PubMed] [Google Scholar]
- 8. Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A, Witteman JCM. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270–278 [DOI] [PubMed] [Google Scholar]
- 9. Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165(21):2460–2466 [DOI] [PubMed] [Google Scholar]
- 10. Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol. 2008;52(14):1152–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nanchen D, Gussekloo J, Westendorp RG, et al. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J Clin Endocrinol Metab. 2012;97(3):852–861 [DOI] [PubMed] [Google Scholar]
- 12. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from six prospective cohorts. Circulation. 2012;126(9):1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bastenie PA, Vanhaelst L, Golstein J, Smets P. Asymptomatic autoimmune thyroiditis and coronary heart-disease. Cross-sectional and prospective studies. Lancet. 1977;2[0140–6736 (Print)]:155–158 [DOI] [PubMed] [Google Scholar]
- 14. Morris MS, Bostom AG, Jacques PF, Selhub J, Rosenberg IH. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis. 2001;155[0021–9150 (Print)]:195–200 [DOI] [PubMed] [Google Scholar]
- 15. Iervasi G, Molinaro S, Landi P, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167(14):1526–1532 [DOI] [PubMed] [Google Scholar]
- 16. Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165(21):2467–2472 [DOI] [PubMed] [Google Scholar]
- 17. Tseng FY, Lin WY, Lin CC, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60(8):730–737 [DOI] [PubMed] [Google Scholar]
- 18. Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol. 2010;72(3):404–410 [DOI] [PubMed] [Google Scholar]
- 19. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Razvi S, Weaver JU, Vanderpump MP, Pearce SHS. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab. 2010;95(4):1734–1740 [DOI] [PubMed] [Google Scholar]
- 21. Rodondi N, den Elzen WPJ, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tunbridge WM, Evered DC, Hall R, et al. Lipid profiles and cardiovascular disease in the Whickham area with particular reference to thyroid failure. Clin Endocrinol (Oxf). 1977;7(6):495–508 [DOI] [PubMed] [Google Scholar]
- 23. Åsvold BO, Bjøro T, Platou C, Vatten LJ. Thyroid function and the risk of coronary heart disease: 12-year follow-up of the HUNT Study in Norway. Clin Endocrinol (Oxf). 2012;77(6):911–917 [DOI] [PubMed] [Google Scholar]
- 24. The Women's Health Initiative Study Group Design of the Women's Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials. 1998;109:61–109 [DOI] [PubMed] [Google Scholar]
- 25. Curb JD, Mctiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9):122–128 [DOI] [PubMed] [Google Scholar]
- 26. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172 [DOI] [PubMed] [Google Scholar]
- 27. Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078 [Google Scholar]
- 28. Vanderpump MP, Tunbridge WM, French JM, et al. The development of ischemic heart disease in relation to autoimmune thyroid disease in a 20-year follow-up study of an English community. Thyroid. 1996;6(3):155–160 [DOI] [PubMed] [Google Scholar]
- 29. Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358(9285):861–865 [DOI] [PubMed] [Google Scholar]
- 30. Hyland KA, Arnold AM, Lee JS, Cappola AR. Persistent subclinical hypothyroidism and cardiovascular risk in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2013;98(2):533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89(7):3365–3370 [DOI] [PubMed] [Google Scholar]
- 32. Stamler J, Wentworth D, Neaton JD. Is the relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356 222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256(20):2823–2828 [PubMed] [Google Scholar]
- 33. Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284(3):311–318 [DOI] [PubMed] [Google Scholar]
- 34. Psaty BM, Koepsell TD, Manolio TA, et al. Risk ratios and risk differences in estimating the effect of risk factors for cardiovascular disease in the elderly. J Clin Epidemiol. 1990;43(9):961–970 [DOI] [PubMed] [Google Scholar]
- 35. Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153(9):1065–1073 [PubMed] [Google Scholar]
- 36. Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(10):1639–1647 [DOI] [PubMed] [Google Scholar]
- 37. Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97(2):326–333 [DOI] [PubMed] [Google Scholar]
- 38. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131 [DOI] [PubMed] [Google Scholar]
- 39. Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2012;97(6):1962–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]