Abstract
Context:
Bone marrow fat (BMF) and bone mineral density (BMD) by dual x-ray energy absorptiometry (DXA) are negatively correlated. However, little is known about the association of BMF with fracture or with separate trabecular and cortical bone compartments.
Objective:
Our objective was to assess the relationships between vertebral BMF, BMD by quantitative computed tomography, and fracture in older adults.
Design, Setting, and Participants:
We conducted a cross-sectional study in the Age Gene/Environment Susceptibility-Reykjavik cohort.
Main Outcome Measures:
Outcomes measures included vertebral BMF (L1–L4) measured with magnetic resonance spectroscopy, quantitative computed tomography and DXA scans of the hip and spine, and DXA vertebral fracture assessments. Previous clinical fracture was determined from medical records.
Results:
In 257 participants without recent bone-active medication use, mean age was 79 (SD 3.1) years. Mean BMF was 53.5% ± 8.1% in men and 55.0% ± 8.4% in women. Those with prevalent vertebral fracture (21 men, 32 women) had higher mean BMF in models adjusted for BMD. In separate models by sex, the difference was statistically significant only in men (57.3% vs 52.8%, P = 0.02). BMF was associated with lower trabecular volumetric BMD (vBMD) at the spine (−10.5% difference for each 1 SD increase in BMF, P < 0.01), total hip, and femoral neck, but not with cortical vBMD, in women. In men, BMF was marginally associated with trabecular spine vBMD (−6.1%, P = 0.05). Total hip and spine areal BMD (aBMD) were negatively correlated with BMF in women only.
Conclusion:
Higher marrow fat correlated with lower trabecular, but not cortical, BMD in older women but not men. Higher marrow fat was associated with prevalent vertebral fracture in men, even after adjustment for BMD.
Although it has been understood for many years that marrow adiposity increases with age, this has historically been viewed as a neutral process, with the adipose tissue serving as a space filler in the bone marrow. However, recent studies indicate that marrow fat accumulation is part of dynamic processes that also affect bone density. A shift in stem cell lineage allocation toward adipogenesis and away from osteoblastogenesis may contribute to age-related bone loss (1). In vitro studies have demonstrated that marrow fat produces factors that affect osteoblast and osteoclast activity (2, 3). Consistent with these observations, clinical studies using different methods to assess marrow fat have found a negative correlation with bone density (4). Most of these studies have used dual x-ray energy absorptiometry (DXA) for measurement of bone density. Data are not available in older adults on the associations of marrow fat with the separate compartments of trabecular and cortical bone, measured with quantitative computed tomography (QCT). Limited data are available on the relationship between marrow fat and fracture. Previous studies have reported an association between prevalent vertebral fracture and higher vertebral marrow fat content measured by biopsy (5) and with magnetic resonance spectroscopy (MRS) (6). Additional research is needed to confirm these findings with vertebral fracture and to determine whether marrow fat is associated with other fractures. To assess the relationships among vertebral marrow fat, volumetric bone mineral density (vBMD), and fracture in older men and women, we used data from the Age Gene/Environment Susceptibility (AGES)-Reykjavik cohort.
Subjects and Methods
Study participants
Participants attending a follow-up visit for the AGES-Reykjavik study were invited to participate in this ancillary study of vertebral bone marrow fat (BMF). AGES-Reykjavik is a longitudinal observational study of older men and women in Iceland, previously described in detail (7). Briefly, the AGES-Reykjavik Study is an extension of the original Reykjavik Study cohort (n = 30 795) that started in 1967 (8, 9). Participants in the AGES-Reykjavik Study were selected randomly from survivors of the Reykjavik Study. The baseline visit for the AGES-Reykjavik Study included 5764 men and women aged 67 to 93 years. Of these, 3411 attended the follow-up visit approximately 5 years later. Beginning in June 2010, eligible participants attending a follow-up visit were invited to participate in the BMF ancillary study. Eligibility criteria included completion of QCT scans at the AGES follow-up visit and no contraindications for magnetic resonance imaging (MRI) testing (metal in or near vertebral bodies L1–L4, other implants that will cause safety issues, or claustrophobia). Participants who were too large for the size and weight limitations of the MRI scanner (body mass index [BMI] >38 kg/m2, weight >113.4 kg) were excluded. Of 403 eligible participants, 336 agreed to participate in the ancillary study, and 304 attended an ancillary study visit. The ancillary study was approved by the institutional review boards of the National Bioethics Committee in Iceland, the National Institute on Aging, and the University of California, San Francisco (UCSF). All participants provided written informed consent.
At the main study visit, height and weight were measured. An interviewer administered a questionnaire that included demographics and history of medical conditions. Participants were asked to bring any medications and supplements used in the past 2 weeks to the study visit. These were recorded and then coded according to the Anatomical Therapeutic Chemical classification system. A fasting blood specimen was drawn at the visit and analyzed for glucose. Diabetes status was based on self-report, use of diabetes medications, or an elevated fasting glucose (≥7 mmol/L). Participants reporting current use of medications known to affect bone density, including thiazolidinediones, oral glucocorticoids, treatments for osteoporosis (bisphosphonates, raloxifene, calcitonin, or PTH), hormone therapy, tibolone, and antiepileptics (n = 44) were excluded from these analyses.
CT measures of bone
QCT scans were obtained for the lumbar spine and hip using a 4-detector CT system (Sensation; Siemens Medical Systems, Erlangen, Germany), as previously described (10). Briefly, to relate the QCT image units to equivalent concentration of calcium hydroxyapatite and to provide simultaneous calibration, a bone mineral reference standard (3-sample calibration phantom; Image Analysis, Columbia, Kentucky) was placed under the participant's spine and hips and scanned simultaneously. The lumbar spine scanning included a helical study of the L1 and L2 vertebrae (120 kilovolt peak [kVp], 150 milliamp seconds [mAs] 1-mm slice thickness, pitch = 1). A helical study of the hip (120 kVp, 140 mAs, 1-mm slice thickness, pitch = 1) included the proximal femur from a point 1 cm superior to the acetabulum to a point 3 to 5 mm inferior to the lesser trochanter.
QCT images were transferred to a network of computer workstations and processed to extract measures of vBMD and bone size using analysis techniques described previously (11). For each trabecular, cortical, and integral region of interest, vBMD (grams per cubic centimeter), bone mineral content (grams), and bone volume (cubic centimeters) were computed. Spine trabecular bone mineral density (BMD) was calculated from an elliptical region in the anterior midvertebra. Integral BMD of the spine used the entire midvertebra excluding transverse elements.
Vertebral BMF
BMF (ratio of fat to water plus fat, percent) was measured with a 1.5-T scanner (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel cervical-thoracic-lumbar spine coil (using the lower 3 elements; GE Healthcare). The imaging protocol included a standard clinical sagittal T2-weighted Fast Spin Echo (FSE) sequence (Repetition [TR]/Echo Time [TE] = 5000/87 msec, echo train length = 32, field of view = 22 cm, slice thickness = 6 mm), which was used for visual assessment of lumbar vertebrae and for prescription of the spectral acquisition box. Single-voxel MRS was acquired in vertebral bodies from L1 to L4 using the point-resolved spectroscopy (PRESS) sequence with the following parameters: TR/TE = 2000/37 msec, 64 averages without water suppression, sweep width = 5000 Hz, data point = 2048, voxel size = 12 × 12 × 20 mm3 = 2.88 cm3. The PRESS box was positioned in the middle of the vertebral body and the PRESS box size was kept the same for each vertebral level and for all subjects. Outer volume saturation bands were used to eliminate potential contamination of outside signals.
The spectral data were analyzed using GE SAGE software. After phase, baseline, and frequency shift correction, 2 peaks were fitted using Marquardt Fit: water peak at 4.67 ppm and fat peak at 1.3 ppm (the bulk CH2 methylene protons). The area under each peak was calculated, and the BMF was defined as fat/(fat + water) × 100%. The mean BMF of all 4 levels was used in data analyses.
Five healthy controls were scanned (from L1–L4) twice with repositioning between 2 scans to examine reproducibility. The root mean square coefficient of variation of BMF was 5.87%, indicating excellent in vivo reproducibility. The spectral data from 67 subjects were transferred to UCSF Radiology for postprocessing quality control. The root mean square coefficient of variation of BMF quantified by the 2 different sites, Iceland AGES and UCSF Radiology, was less than 1%.
Of the 304 participants attending the ancillary study visit, 1 participant experienced acute claustrophobia and could not complete the MRS measurement. The MRS measurement was not successful in an additional participant because of a corrupted file. One participant had a mean MRS value for BMF, based on only 2 evaluable levels, of 18.9%, below the range considered physiologically plausible in older age. This participant was excluded. MRS was completed successfully on 301 participants. As noted earlier, 44 participants using a bone-active medication were excluded; a total of 257 participants were included in these analyses.
DXA measures of bone and vertebral fracture
Participants in the ancillary study also had DXA scans of the whole body, hip, anterior-posterior spine, and lateral spine for assessment of vertebral fracture. Scans were obtained with a GE Healthcare Lunar iDXA scanner, running software version 11.4. The vertebral fracture assessment by DXA is based on vertebral height measured at each evaluable level using 6-point morphometry and then assigned a grade of none, mild, moderate, or severe deformity (12). A grade of moderate or severe was considered evidence of a vertebral fracture for these analyses. Levels T4 through L4 are included in the image, but visualization is better for levels T7 through L4 (12).
Clinical fractures
History of clinical fractures was assessed using the Reykjavik Study fracture registry. As previously described, medical records for AGES-Reykjavik participants from 1966 through 2009 were searched to identify all clinical fractures since entry into the original Reykjavik Study to the time of the second clinic visit that coincided with the measurements of BMF (13, 14). All outpatient and inpatient medical records in Iceland are linked through a personal identification number. All fractures were verified by medical record review. If radiographs were not available for rib or vertebral fracture, medical records for the case were reviewed by 1 orthopedic surgeon who classified the event as a fracture or not. Stress fractures, malignancy-related fractures, and avulsion detachments less than 5 × 6 mm2 were excluded. Fractures were not excluded based on trauma because traumatic fractures are associated with lower BMD (15). These analyses were restricted to reported fractures that occurred within 10 years of the BMF measurement. Frailty fractures, also restricted to the previous 10 years, were defined to include fractures that increase exponentially with older age: hip, proximal humerus, and clinical spine.
Statistical analyses
Mean BMF (L1–L4) was compared in those with and without fracture, using linear regression models adjusted for age and gender and, for vertebral fracture, trabecular spine vBMD. Bone outcomes were skewed, and log transformation was used to achieve a normal distribution. The relationships between mean BMF (L1–L4) and log-transformed bone outcomes were assessed using linear regression models, adjusted for age, BMI, and diabetes status. Interactions between BMF and gender were evaluated by including cross-product terms in regression models. We found no evidence of interaction for fracture outcomes. However, statistically significant (P < .10) interactions were found for BMF and gender and several bone outcomes, and these results are reported separately for men and women.
Results
Baseline characteristics of this cohort of 118 men and 139 women with mean age of 79 (SD 3.1) years are provided in Table 1. Twenty-one men (18%) and 32 women (23%) had a prevalent vertebral fracture, measured by DXA. Ten men (8%) and 33 women (24%) had a previous clinical fracture; 3 men (2.5%) and 14 women (10%) had a previous frailty (hip, proximal humerus, or clinical spine) fracture. Mean BMF (L1–L4) was 53.5% (SD 8.1%) in men and 55.0% (SD 8.4%) in women (P = .14).
Table 1.
Baseline Characteristics by Gender and Vertebral Fracture Statusa
| Menb |
Women |
|||||
|---|---|---|---|---|---|---|
| All | Prevalent Vertebral Fracture |
All | Prevalent Vertebral Fracture |
|||
| None | ≥1 | None | ≥1 | |||
| n | 118 | 96 | 21 | 139 | 107 | 32 |
| Age, y | 80.0 ± 3.1 | 80.1 ± 3.2 | 79.6 ± 2.6 | 78.6 ± 3.0 | 78.6 ± 3.0 | 78.5 ± 3.1 |
| Diabetes, n (%) | 7 (5.9) | 5 (5.2) | 2 (9.5) | 10 (7.2) | 6 (5.6) | 4 (12.5) |
| History of alcoholism, n (%) | 17 (14.5) | 12 (12.5) | 5 (23.8) | 5 (3.6) | 4 (3.8) | 1 (3.2) |
| Clinical fracture in previous 10 y, n (%) | 10 (8) | 9 (9.4) | 1 (4.8) | 33 (24) | 22 (20.6) | 11 (34.4) |
| Frailty fracturec in previous 10 y, n (%) | 3 (2.5) | 3 (3.1) | 0 (0.0) | 14 (10) | 8 (7.5) | 6 (18.8) |
| BMI, kg/m2 | 27.2 ± 3.6 | 27.1 ± 3.5 | 27.9 ± 4.0 | 28.0 ± 4.0 | 27.7 ± 4.2 | 29.07 ± 3.0 |
| Trabecular spine vBMD, g/cm3 | 0.084 ± 0.029 | 0.085 ± 0.026 | 0.081 ± 0.040 | 0.075 ± 0.032 | 0.078 ± 0.032 | 0.065 ± 0.029 |
| Vertebral marrow fat, % | 53.5 ± 8.1 | 52.8 ± 7.4 | 57.1 ± 9.8 | 55.0 ± 8.4 | 54.2 ± 8.4 | 57.7 ± 8.0 |
Results are shown as n (%) or mean ± SD. Vertebral fracture was identified by DXA vertebral fracture assessment.
Vertebral fracture assessment was not available on 1 of 118 men.
Frailty fracture was defined as a hip, proximal humerus, or clinical spine fracture.
Mean BMF increased from L1 to L4. Mean values were 51.9%, 53.4%, 55.4%, and 56.4% for L1 through L4, respectively. For L1, 15 participants could not be evaluated for marrow fat. At L2 to L4, there were 7, 9, and 11 participants who did not have an evaluable level, respectively.
In women, BMF was negatively associated with QCT-derived trabecular and integral BMD and compressive strength of the spine, but not with cross-sectional area (Table 2). For a 1-SD (about 8%) difference in marrow fat, women had 10.5% (95% confidence interval [CI] = −17.2% to −3.2%) lower trabecular spine BMD. In men, BMF was also negatively associated with trabecular and integral spine BMD and compressive strength, but none of the associations were statistically significant. At the total hip and femoral neck, BMF was negatively associated with trabecular but not cortical BMD in women. In men, there were no statistically significant associations between BMF and hip bone density measured by QCT.
Table 2.
Percent Difference in Bone Outcome for Each 1-SD Increase in Vertebral Marrow Fata
| Men (n = 118) |
Women (n = 139) |
|||
|---|---|---|---|---|
| % Difference | (95% CI) | % Difference | (95% CI) | |
| QCT measurement | ||||
| Spine | ||||
| Trabecular BMD | −6.14 | −11.90 to 0.00 | −10.49 | −17.21 to −3.23 |
| Integral BMD | −1.00 | −4.09 to 2.19 | −4.83 | −7.93 to −1.63 |
| Vertebral compressive strength | −6.99 | −15.90 to 2.86 | −15.57 | −23.06 to −7.35 |
| Cross-sectional area | −0.30 | −2.79 to 2.26 | −0.23 | −2.36 to 1.95 |
| Femoral neck | ||||
| Trabecular BMD | 5.19 | −1.52 to 12.36 | −7.08b | −12.51 to −1.31 |
| Cortical BMD | 0.01 | −1.66 to 1.71 | −0.30 | −1.69 to 1.11 |
| Integral BMD | 0.73 | −2.63 to 4.20 | −2.54 | −5.54 to 0.55 |
| Total hip | ||||
| Trabecular BMD | 0.97 | −2.80 to 4.88 | −3.95 | −7.64 to −0.12 |
| Cortical BMD | 0.13 | −1.15 to 1.42 | −0.53 | −1.71 to 0.67 |
| Integral BMD | 0.53 | −2.47 to 3.62 | −2.64 | −5.53 to 0.34 |
| DXA measurement | ||||
| Spine BMD | 0.78 | −2.25 to 3.91 | −2.91b | −5.45 to −0.30 |
| Total hip BMD | 0.29 | −2.10 to 2.73 | −2.33 | −4.47 to −0.14 |
| Femoral neck BMD | 0.85 | −1.63 to 3.38 | −2.12 | −4.39 to 0.19 |
Adjusted for age, BMI, and diabetes status.
P value for interaction between BMF and gender < 0.10.
The unadjusted correlation between BMF and trabecular spine BMD in women did not vary substantially when each vertebral level was considered separately. The correlations with trabecular spine BMD (L1–L2) were: −0.23 for BMF measured at L1, −0.20 for L2, −0.23 for L3, −0.28 at L4, and −0.26 for the mean L1 through L4.
In women only, BMF was negatively associated with lumbar spine areal BMD (aBMD) (−2.9% for 1-SD increase in BMF, 95% CI = −5.4% to −0.3%) and total hip aBMD (−2.3% for 1-SD increase in BMF, 95% CI = −4.5% to −0.1%) measured by DXA (Table 2). The negative association with femoral neck aBMD was of similar magnitude in women but was not statistically significant. No statistically significant associations were observed in men between BMF and spine or hip bone density by DXA.
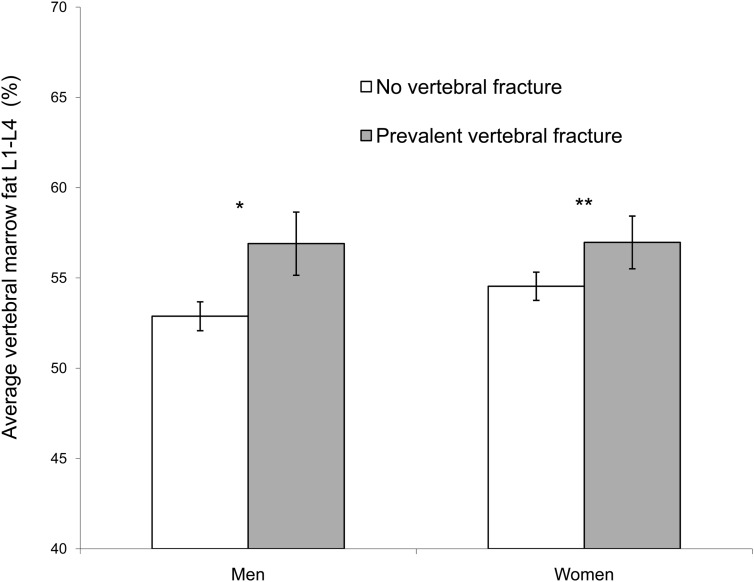
Those with prevalent vertebral fracture had higher mean BMF (57.3% vs 53.6%, P = .003) in models adjusted for age and gender. The difference in mean BMF, comparing those with and without fracture, was −4.1% (P = .03) in men and −3.5% (P = .04) in women. With additional adjustment for trabecular spine vBMD, the difference remained similar in men (−4.0%, P = .03) but was attenuated in women and no longer statistically significant (−2.4%, P = .14) (Figure 1). A history of alcoholism was associated with prevalent vertebral fracture in men but was not associated with BMF in men or women. Additional adjustment for history of alcoholism did not substantially alter the results for men, comparing BMF in those with and without vertebral fracture (−4.0%, P = .04). Results for men and women were not statistically different (P for interaction = .60). For men and women together, those with prevalent vertebral fracture had higher mean BMF (56.9% vs 53.7%, P = .010) in models adjusted for age, gender, and trabecular spine vBMD. Mean BMF was not statistically different between those with and without a previous clinical fracture (53.3% vs 54.4%, P = .41) or frailty fracture (56.7% vs 54.1%, P = .22) in models adjusted for age and gender.
Figure. 1.
Mean vertebral BMF (L1–L4) was assessed by the presence of vertebral fracture (no vertebral fracture or 1 or more prevalent vertebral fractures), adjusted for age and trabecular spine vBMD. Error bars represent SEM. *, P = .03; **, P = .14.
Discussion
In a cohort of older adults, higher vertebral marrow fat was associated with prevalent vertebral fractures, independent of bone density. Higher marrow fat was also associated with lower trabecular bone density at the spine and hip in women only. Marrow fat was not associated with cortical bone density in men or women.
Our findings confirm earlier reports of higher marrow fat with prevalent vertebral fractures. Justesen et al (5) reported higher marrow fat, measured in iliac crest biopsies, in 26 women with prevalent vertebral fracture compared with age-matched controls (63% vs 54%, P = .02). Wehrli et al (6) also found higher marrow fat in those with prevalent vertebral deformities in a study of older adults (106 women, 33 men). Mean BMF at the spine was 55% and 45% (P < .001) in those with and without a baseline vertebral fracture, respectively, and remained statistically different after adjustment for spine BMD by DXA.
We found an increased prevalence of vertebral fractures associated with a history of alcoholism in men, consistent with a previous report (16). Animal and in vitro studies indicate that alcohol reduces osteoblastogenesis and increases adipogenesis in bone marrow (17), but we did not find evidence of an association between alcoholism and marrow fat in our cohort. Adjustment for history of alcoholism did not affect the observed association between marrow fat and prevalent vertebral fracture in men.
We found that marrow fat was higher in those with prevalent vertebral fracture even after adjustment for trabecular spine vBMD. This difference was greater in men (−4.0%) than in women (−2.4%), although a test for interaction did not find evidence of statistical difference in these results. Our data suggest that marrow fat is associated with vertebral fracture in women largely through reduced BMD but may be associated with vertebral fracture in men through other reductions in bone strength that are not captured with QCT or DXA scans. A larger study is needed to determine whether there are indeed gender differences in the associations among marrow fat, bone, and vertebral fracture.
There are no previous reports of the relationship between BMF and clinical fractures. We did not find an association between BMF and history of clinical fractures when we considered all fractures or in analyses limited to frailty (hip, proximal humerus, and clinical spine) fractures. However, our ability to address this question was limited by a small number of participants with a prevalent frailty fracture.
Previous studies have found a negative correlation between BMF assessed by MRS and bone density measured by DXA in older women (18) and men (19). This relationship has also been reported for pelvic BMF measured using MRI and whole-body BMD in older adults (20). To our knowledge, there are no previous studies of marrow fat and bone in older adults that have used hip and spine QCT to measure bone density. However, a previous study in younger premenopausal women reported a negative correlation between vertebral BMF (by MRS) and trabecular spine vBMD (21). A study in young adults measured bone marrow in the femoral shaft and bone parameters in the spine and femoral shaft using dual-energy QCT (22). In both men and women, higher BMF was associated with lower trabecular spine vBMD and lower femoral shaft cortical bone area but not with vertebral cross-sectional area or femoral cross-sectional area.
A limitation of QCT measurements of trabecular bone is that higher marrow fat tends to artificially reduce the observed BMD (23). A similar effect is seen with DXA (24). This might account for some of the observed negative correlations of marrow fat with trabecular vBMD and with aBMD at the spine and total hip. However, we observed gender and skeletal site differences in the relationship between BMF and BMD, suggesting that measurement artifacts do not fully account for the observed correlations. The cross-sectional design is also a limitation of this study that prevents us from determining the temporal relationships between marrow fat and the outcomes of bone density and fracture. In addition, these results are limited to older adults and may not apply to younger age groups.
Increased marrow fat may be linked to lower bone density and vertebral fracture risk as a marker of a shift in mesenchymal stem cell lineage allocation that favors adipocytes over osteoblasts. This pathway contributes to age-related bone loss (1, 25) and may also weaken bone through changes in microarchitecture that are not captured by DXA or QCT. Factors that control mesenchymal stem cell differentiation include peroxisome proliferator-activated receptor gamma (PPARγ) and runt-related transcription factor 2 (Runx2). For example, thiazolidinediones, a class of antidiabetic medications, are PPARγ ligands that cause increased marrow fat, reduced bone density, and higher fracture risk (26, 27). Another possible mechanism linking marrow fat and bone fragility is through a direct effect of factors secreted by marrow fat on bone cells. As with other fat depots, marrow fat produces adipokines and fatty acids that may produce a lipotoxic environment for bone cells (28). There is evidence that adipocytes inhibit osteoblast proliferation (29) and promote osteoclast differentiation (30).
Our results indicate that there are gender differences in the relationship between marrow fat and bone density and possibly in the relationship between marrow fat and fracture. Higher estrogen levels have a negative influence on levels of marrow fat and a positive influence on bone density (31). It is possible that the higher estrogen levels in older men compared with postmenopausal women may attenuate the relationship between BMF and BMD.
In conclusion, we observed correlations between marrow fat and trabecular, but not cortical, bone in older women. Higher marrow fat was associated with prevalent vertebral fracture in men, even after adjustment for trabecular spine BMD. Longitudinal studies are needed to determine whether marrow fat is an independent risk factor for incident fracture.
Acknowledgments
This ancillary study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR057819). The AGES Reykjavik Study is supported by funding from the National Institutes of Health (Contract N01-AG-12100), the National Institute on Aging Intramural Research Program, Hjartavernd (The Icelandic Heart Association), and the Althingi (The Icelandic Parliament). C.R. received support from the National Institute of Diabetes and Digestive and Kidney Diseases (R24DK092759).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- areal BMD
- AGES
- Age Gene/Environment Susceptibility
- BMD
- bone mineral density
- BMF
- bone marrow fat
- BMI
- body mass index
- CI
- confidence interval
- DXA
- dual x-ray energy absorptiometry
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- QCT
- quantitative computed tomography
- vBMD
- volumetric bone mineral density.
References
- 1. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004;3:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26:485–489 [DOI] [PubMed] [Google Scholar]
- 3. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteopor Rep. 2011;9:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171 [DOI] [PubMed] [Google Scholar]
- 6. Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–538 [DOI] [PubMed] [Google Scholar]
- 7. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjornsson O, Davidsson D, Olafsson H. Report ABC XVIII Health Survey in the Reykjavik Area: Men Stages I–III 1967–1969, 1970–1971, and 1974–1976. Participants Invitation Response etc. Reykjavik, Iceland: Icelandic Heart Association; 1979 [Google Scholar]
- 9. Bjornsson G, Bjornnsson O, Davidsson D. Report ABC XXIV Health Survey in the Reykjavid Area: Women Stages I–III 1968–1969, 1971–1972, and 1976–1978. Participants Invitation Response etc. Reykjavik, Iceland: Icelandic Heart Association; 1982 [Google Scholar]
- 10. Sigurdsson G, Aspelund T, Chang M, et al. Increasing sex difference in bone strength in old age: the Age, Gene/Environment Susceptibility-Reykjavik study (AGES-Reykjavik). Bone. 2006;39:644–651 [DOI] [PubMed] [Google Scholar]
- 11. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012 [DOI] [PubMed] [Google Scholar]
- 12. Binkley N, Krueger D, Gangnon R, Genant HK, Drezner MK. Lateral vertebral assessment: a valuable technique to detect clinically significant vertebral fractures. Osteoporos Int. 2005;16:1513–1518 [DOI] [PubMed] [Google Scholar]
- 13. Siggeirsdottir K, Aspelund T, Sigurdsson G, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol. 2007;22:631–639 [DOI] [PubMed] [Google Scholar]
- 14. Siggeirsdottir K, Aspelund T, Jonsson BY, et al. Effect of vertebral fractures on function, quality of life and hospitalisation the AGES-Reykjavik study. Age Ageing. 2012;41:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388 [DOI] [PubMed] [Google Scholar]
- 16. Samelson EJ, Hannan MT, Zhang Y, Genant HK, Felson DT, Kiel DP. Incidence and risk factors for vertebral fracture in women and men: 25-year follow-up results from the population-based Framingham study. J Bone Miner Res. 2006;21:1207–1214 [DOI] [PubMed] [Google Scholar]
- 17. Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16 [DOI] [PubMed] [Google Scholar]
- 18. Griffith JF, Yeung DK, Antonio GE, et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–838 [DOI] [PubMed] [Google Scholar]
- 19. Griffith JF, Yeung DK, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951 [DOI] [PubMed] [Google Scholar]
- 20. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring). 2010;19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glüer CC, Reiser UJ, Davis CA, Rutt BK, Genant HK. Vertebral mineral determination by quantitative computed tomography (QCT): Accuracy of single and dual energy measurements. J Comput Assist Tomogr. 1988;12:242–258 [DOI] [PubMed] [Google Scholar]
- 24. Blake GM, Griffith JF, Yeung DK, Leung PC, Fogelman I. Effect of increasing vertebral marrow fat content on BMD measurement, T-score status and fracture risk prediction by DXA. Bone. 2009;44:495–501 [DOI] [PubMed] [Google Scholar]
- 25. Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266 [DOI] [PubMed] [Google Scholar]
- 26. Grey A, Beckley V, Doyle A, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. 2012;166:1087–1091 [DOI] [PubMed] [Google Scholar]
- 27. Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4:61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maurin AC, Chavassieux PM, Vericel E, Meunier PJ. Role of polyunsaturated fatty acids in the inhibitory effect of human adipocytes on osteoblastic proliferation. Bone. 2002;31:260–266 [DOI] [PubMed] [Google Scholar]
- 30. Hozumi A, Osaki M, Goto H, Sakamoto K, Inokuchi S, Shindo H. Bone marrow adipocytes support dexamethasone-induced osteoclast differentiation. Biochem Biophys Res Commun. 2009;382:780–784 [DOI] [PubMed] [Google Scholar]
- 31. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]