Abstract
GH secretion is controlled by hypothalamic as well as intrapituitary and peripheral signals, all of which converge upon the somatotroph, resulting in integrated GH synthesis and secretion. Enabling an accurate diagnosis of idiopathic adult GH deficiency (IAGHD) is challenged by the pulsatility of GH secretion, provocative test result variability, and suboptimal GH assay standardization. The spectrum between attenuated GH secretion associated with the normal aging process and with obesity and truly well-defined IAGHD is not distinct and may mislead the diagnosis. Adult-onset GHD is mainly caused by an acquired pituitary deficiency, commonly including prior head/neck irradiation, or an expanding pituitary mass causing functional somatotroph compression. To what extent rare cryptic causes account for those patients seemingly classified as IAGHD is unclear. About 15% of patients with adult GHD and receiving GH replacement in open-label surveillance studies are reported as being due to an idiopathic cause. These patients may also reflect a pool of subjects with an as yet to be determined occult defect, or those with unclear or incomplete medical histories (including forgotten past sports head injury or motor vehicle accident). Therefore, submaximal diagnostic evaluation likely leads to an inadvertent diagnosis of IAGHD. In these latter cases, adherence to rigorous biochemical diagnostic criteria and etiology exclusion may result in reclassification of a subset of these patients to a distinct known acquired etiology, or as GH-replete. Accordingly, rigorously verified IAGHD likely comprises less than 10% of adult GHD patients, an already rare disorder. Regardless of etiology, patients with adult GHD, including those with IAGHD, exhibit a well-defined clinical phenotype including increased fat mass, loss of lean muscle mass, decreased bone mass, and enhanced cardiac morbidity. Definition of unique efficacy and dosing parameters for GH replacement and resultant therapeutic efficacy markers in true IAGHD requires prospective study.
The biochemical and clinical features of adult-onset GH deficiency (GHD) and successful GH replacement therapy are well established (1–6). Nevertheless, important challenges inherent in diagnosing idiopathic adult GHD (IAGHD) and the role of GH replacement and long-term monitoring of these patients remain controversial and are discussed below.
GH Secretion and Action
GH is required for appropriate linear growth during childhood and adolescence, and the hormone also exerts important non-growth-related metabolic functions throughout the adult life span.
The hGH-N gene, coding the 22-kDa (191 aa) GH molecule, is selectively expressed in pituitary somatotroph cells under cell-specific transcriptional control. The specific POUIFI (Pit-1) transcription factor, as well as ubiquitous proteins (eg, Spl), enable GH synthesis. Both Pit-I recruitment and post-translational RNA polymerase 11 modification are regulated by DNase hypersensitive sites of the hGH locus control region, enabling abundant pituitary-specific hGH-N transcription (7). The single chain 191-aa GH molecule is synthesized, stored, and secreted by somatotroph cells comprising approximately 45% of all anterior pituitary cells. The normal adult pituitary gland contains up to 15 mg GH stored mainly in prominent secretory granules approximately 700 μm in diameter (8). About 10% of secreted GH lacks aa residues 32–46, accounting for a 20-kDa variant (9).
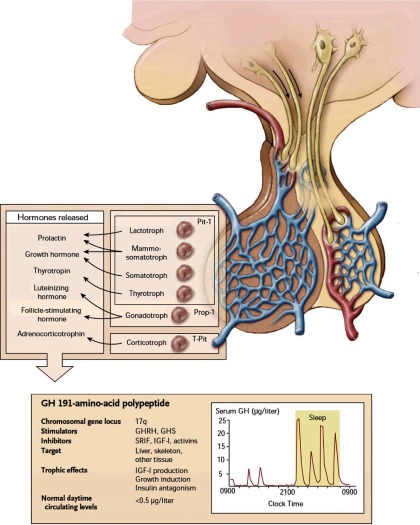
Complex neurogenic and peripheral signals determine GH secretion (Figure 1). GH synthesis and release are induced by hypothalamic GHRH, signaling through the G protein-coupled GHRH receptor to induce somatotroph cAMP, and also by ghrelin, predominantly derived from the gastrointestinal tract (10). Hypothalamic somatostatin is the primary signal attenuating GH release, mediated predominantly by somatotroph SSTR2 signaling to block GH secretion (11). IGF-I, the peripheral target hormone for GH, mediates most growth-promoting GH actions and also participates in negative feedback control, suppressing GH synthesis and release (12). Peripheral hormones including glucocorticoids, estrogen, and thyroid hormone also signal to regulate GH production.
Figure 1.
Hypothalamic pituitary control of GH secretion. Hypothalamic GHRH and somatostatin, which traverse the portal vein, somatotroph-specific transcription factors, and negative feedback control of IGF-I all participate in regulatory GH synthesis and secretion. Accurate measurement of pulsatile GH secretion requires ultrasensitive assays. Prop-1, prophet of Pit-1; GHS, GH secretagogs (eg, ghrelin); SRIF, somatostatin. [Reproduced from S. Melmed: Medical progress: acromegaly. N Engl J Med. 2006;355:2558–2573 (81), with permission. © Massachusetts Medical Society.]
Episodic GH secretion exhibits diurnal rhythmicity, and about 65% of total daily GH production occurs at night, triggered by onset of slow wave sleep. Secretory GH pulses are interspersed with basal trough secretion, during which circulating GH is largely undetectable by routine assays. Accordingly, using conventional immunoassays, serum GH levels are usually undetectable for more than half the 24-hour clock period. Declining GH pulse amplitude, rather than frequency, already starts occurring at the transition to young adulthood (13), and GH continues to decline thereafter with aging. During adulthood, daily GH output is approximately 200–600 μg/d, with women exhibiting higher secretion rates (14).
The somatotroph cell integrates multifactorial signals derived from the hypothalamus, intrapituitary milieu, as well as the periphery in the complex regulation of pituitary GH secretion. The net result of these positive and negative inputs is an episodic release of GH characterized by unique secretory determined by gender (sexual dimorphic patterns), age (attenuation), and sleep (augmentation) patterns (14). These physiological signals are further influenced by nutritional factors, especially obesity leading to blunting of GH secretion and malnutrition augmenting GH secretion.
Peripheral GH signaling is mediated by the ubiquitous GH receptor, a class 1 dimeric cytokine receptor (15) acting predominantly but not exclusively through STAT-5. Growth-promoting GH properties are mediated mainly by IGF-I produced largely in the liver. Several additional mediators, including those required for myoblast fusion (16) and chondrocyte growth (17) have also recently been described.
Diagnosis of IAGHD
The propensity by some practitioners for inappropriate IAGHD diagnosis as a prelude to administering unsafe nonapproved GH supplementation for athletes or the frail elderly underscores the need for a stringent diagnostic approach (18–20). Somatotroph impingement, compression, inflammation, or vascular insult leads to attenuated GH synthesis and secretion, with the resultant clinical sequelae of adult hypo-somatotrophism. Acquired causes of adult GHD are rarely encountered and lead to impaired somatotroph function (Table 1). Nevertheless, confirming an accurate adult GHD diagnosis is imperative to qualify a patient for costly, long-term GH replacement. Thus, the diagnosis of IAGHD in patients with no obvious pituitary lesion represents an important clinical and societal conundrum for the endocrinologist (21).
Table 1.
Etiology of Acquired Adult GHD

Adapted from S. Melmed and K. Ho: Pituitary physiology and diagnostic evaluation. Williams Textbook of Endocrinology (edited by S. Melmed, K. S. Polonsky, P. Reed Larsen, H. M. Kronenberg), Elsevier, Philadelphia, PA, 2010 (83), with permission.
Assessment of “abnormal” GH secretion in the adult requires an awareness of potential pitfalls because, unlike for other pituitary hormones (eg, prolactin, TSH), a single serum GH measurement is not likely to accurately reflect appropriate somatotroph function. Importantly, GH secretion is pulsatile and is integrated over seconds, minutes, and hours (13). The challenges of accurately measuring serum GH levels are further compounded by suboptimal cross-laboratory and cross-assay harmonization, poor assay standardization, and unclear sensitivity cutoff points (22). Measurement of serum IGF-I as a surrogate marker of integrated GH secretion, while intuitively appealing, also does not consistently reflect attenuated GH secretion because normal IGF-I levels may also be encountered in adult patients with validated GHD.
The syndrome of adult GHD has been well-documented since early reports of metabolic and somatic sequelae of attenuated adult pituitary GH secretion (1). However, the diagnosis of the disorder remains challenging but is clinically important because the approval of recombinant GH for adult replacement has led to improved morbidities and quality of life for patients with anterior pituitary somatotroph reserve deficiency. Although most clinical features of adult GHD are nonspecific, including increased fat and decreased lean body mass, low energy, and mood disorders, the costly diagnostic evaluation for GHD should justifiably only be undertaken in patients suspected of disordered hypothalamic-pituitary function (2). In fact, the accuracy of GH testing in patients exhibiting commonly encountered GHD symptoms and signs is strongly determined by appropriate pretesting determination of those patients likely to exhibit compromised somatotroph function.
Rigorous prescreening criteria have been proposed, and these require careful clinical evaluation including obtaining an exhaustive history with careful medical record assessment (Table 2). The currently accepted approach for investigation of adult patients for GH status should be limited to these cohorts (23):
Young adult patients, having received prior GH therapy for childhood GHD to maximize linear growth, now requiring retesting as adults to confirm the GHD diagnosis (24, 25).
Those patients who have undergone surgery or radiation therapy for a pituitary or brain lesion. GH secretion is impaired in approximately 50% of brain tumor patients, likely as a consequence of surgery and radiation (26, 27).
Patients harboring a known hypothalamic-pituitary lesion, including pituitary adenoma, craniopharyngioma, cyst, hypothalamic tumor, or rare mass due to secondary tumor metastasis.
Those patients who may have experienced compromised functional pituitary integrity due to prior motor vehicle accident, with head trauma, contact sports injury, treatment of a brain lesion, or cerebrovascular accident (28).
Those patients with systemic illness known to also impact the hypothalamic-pituitary axis including a granulomatous disorder, viral, bacterial, or fungal infections, or malignancy.
Table 2.
Idiopathic Acquired Adult GHD: Enhancing Diagnostic Precision
| Exhaustive History | Age |
| Childhood health | |
| Brain tumor | |
| Motor vehicle accident or other trauma | |
| Contact sports | |
| Cerebrovascular thrombosis or hemorrhage | |
| Mood disorder | |
| Hyperparathyroidism | |
| Physical examination | Body weight |
| Abdominal obesity | |
| BMI | |
| Pituitary MRI | Subtle changes |
| Partially empty sella | |
| Stalk integrity | |
| New lesion development | |
| Biochemical | 2 GH stimulation tests |
| IGF-I (age-matched) | |
| Pituitary antibodies |
Abbreviation: MRI, magnetic resonance imaging. Listed factors are often subtle clues that may trigger a more precise diagnostic evaluation for GHD.
Unless 1 of the above conditions is met, provocative GH testing should be avoided in adult patients presenting with generalized complaints of weakness, frailty, lethargy, or abdominal obesity. It is likely that adherence to this stringent prescreening approach greatly diminishes the number of patients with a misleading diagnosis of IAGHD and, consequently, leads to reduced numbers of patients receiving inappropriate GH replacement.
Although good clinical practice clearly requires a diagnosis to be considered if deemed appropriate, these well-accepted screening filters for appropriate yet costly diagnostic evaluation may in fact be counterintuitive to ever establishing a diagnosis of truly IAGHD.
Biochemical Testing for IAGHD
Distinguishing IAGHD patients from normal pituitary-replete patients may be difficult, and the diagnosis may entail several clinical and biochemical pitfalls. Because IAGHD may be inadvertently diagnosed after submaximal diagnostic evaluation, rigorous diagnosis requires demonstration of an appropriately blunted GH response to at least 2 provocative tests. Although insulin-induced hypoglycemia (ITT) is the reference “gold standard” for diagnosis of GHD, GHRH + arginine, GHRH + GH-releasing peptide, GH-releasing peptide and glucagon are also commonly employed (2, 4, 29, 30). The ITT is not used in patients with known seizure disorders or those with atherosclerotic coronary artery disease. GHRH-arginine is better tolerated, although supply difficulties in the United States have prompted the use of glucagon as an alternative GH secretagog (31).
Using a sensitive immunochemiluminescent 2-site GH assay, specific and sensitive GH cutoff values after ITT and GHRH-arginine testing for adult GHD were 5.1 and 4.1 μg/L, respectively, with a 96% sensitivity and 92% specificity as assessed by receiver operating characteristic analysis (32). Other proposed cutoffs for GHD diagnosis are < 3 μg/L for the ITT and < 9 μg/L for GHRH-arginine testing, and an evoked GH of > 15 μg/dL accurately discriminated between GHD and healthy adults (33). In a recent multicenter, randomized, open-label study of 69 subjects using a rigorously newly calibrated GH assay, GHRH-arginine stimulation results were highly reproducible, with 95% specificity cutoff at 3.67–4.96 μg/L, as compared with 5.17 to 6.46 μg/L for the ITT (30). Factors that may lead to misleading biochemical diagnosis of attenuated GH secretion, as evidenced by blunted evoked GH responses, include body mass index (BMI), age, and oral estrogen ingestion. In fact, when using the Arg-GHRH stimulation test, abdominal obesity per se appears to be a stronger confounder of elicited GH levels than BMI or age (34–36). For pituitary-replete patients who are obese, or those with relative abdominal obesity, GH responses to stimulation testing are usually blunted, whereas IGF-I levels are usually normal. Importantly, peak GH levels elicited by Arg-GHRH were shown to be blunted by 1 μg/L for each 1-cm increase in waist circumference (36). It is as yet unclear whether these observations reflect impaired GH production and subsequent hyposomatotrophic metabolic sequelae, or whether the blunted stimulation signal is a specific feature of obesity. Accordingly, the presumptive diagnosis of IAGHD requires strong distinguishing evidence in obese patients (29).
Because the somatotroph axis is attenuated with age, especially in men, the use of random GH measurements may lead to an inadvertent diagnosis of IAGHD and subsequent inappropriate GH replacement in patients > 50 years old (29, 37). With aging, spontaneous GH secretion becomes less pulsatile with decreased GH bursts, and peak GH responses to secretagogs are also blunted. Interestingly, although IGF-I levels also decline with aging, liver IGF-I responses to administered GH appear appropriate, suggesting intact age-associated peripheral GH sensitivity (38).
Normal aging is associated with development of abdominal adiposity, decreased lean body mass, loss of bone mineral density, and hyperinsulinemia, and some have ascribed these features to a state of GHD associated with aging. Accordingly, these commonly encountered phenotypic features render the diagnosis of IAGHD difficult in adults aged > 50 years. In addition, age-related abdominal fat mass and hyperinsulinemia are associated with further age-related declines in GH secretion (39). Whether or not decreased GH levels associated with aging are beneficial to overall health and well-being remains controversial (40). In fact, in a unique transgenic mouse model of selective somatotroph ablation, recapitulating the disorder of isolated GHD, insulin sensitivity was actually enhanced independently of diet (41).
To avoid the challenges of false-positive evoked GH response errors measurement of IGF-I levels is also required to rigorously inform adult GHD diagnosis. The finding of a low age-matched IGF-I level increases the likelihood of the diagnosis, especially in the face of obesity (4). However, the finding of a normal age-matched IGF-I level does not necessarily exclude the diagnosis of adult GHD; a low IGF-I level in the presence of 3 or more pituitary hormone deficiencies is highly suggestive of GHD without the requirement to resort to GH reserve testing (42).
In light of the current propensity for inappropriate adult GH prescriptions (19), the diagnosis of IAGHD should only be made in those patients with attenuated GH response to at least 2 stimulation agents. Using 2 provocative tests partially mitigates the significant false-positive error rate in GH levels evoked by a single stimulation test (4).
The description of the adult GHD syndrome was rigorously defined in 1992 (1, 43, 44). In the observational HYPOCCS database, the proportion of adults reported with acquired GHD due to an idiopathic cause increased from 13.9 to 19.3% in the decade between 1996 and 2005 (5) (Figure 2). In the KIMS observational study, about 10% of adult GHD patients were classified as idiopathic (6). Subsequently, the rise of IAGHD to 16% of adult GHD patients was ascribed to regional differences ranging from 2.6% in The Netherlands to 65% in the United Kingdom; 57% of US patients were classified as IAGHD, whereas only 20% harbored a pituitary adenoma (45). In more recent studies, using ITT and GHRH + arginine testing, IAGHD was reported in 7% of patients in the Dutch National Registry (46) and in 13% of adult GHD patients in Sweden (47). In other studies, idiopathic GHD was reported in approximately 13% of subjects (48–50). Results of these heterogeneous global population studies could imply that with increasing awareness of adult GHD and enhanced access to adult GH replacement, the diagnosis of GHD may have been based on drug package insert recommendations, ie, the result of an elicited GH response to a single provocative test, without rigorously prescreening patients for clinical signs of GHD, or for those with known pituitary lesions. Furthermore, patients with increased central adiposity may have exhibited blunted GH responses and been inadvertently included in published observational studies (3, 5, 6, 51, 52). Importantly, adherence to rigorous biochemical diagnostic criteria as proposed by The Endocrine Society Guidelines (4) may not have been universally applied in published reports of earlier open-label surveillance studies (5, 6). Accordingly, neither the diagnosis of IAGHD nor the justification for GH replacement in these adult patients with apparent IAGHD would yet appear to be compellingly documented.
Figure 2.
Proportion of patients with various etiologies of GHD in the HYPOCCS surveillance study. The mean percentages for each etiology are shown by 2-yr intervals across the decade. [Modified from S. M. Webb et al: Changing patterns of the adult growth hormone deficiency diagnosis documented in a decade-long global surveillance database. J Clin Endocrinol Metab. 2009;94:392–399 (5), with permission. © The Endocrine Society.]
Exclusion of Known Acquired GHD Etiologies to Establish the Diagnosis of IAGHD
The causes of acquired adult GHD are rare (Table 1), and an idiopathic etiology for proven adult GHD should be excluded in a subset of these patients (53). Pituitary adenomas and parasellar masses and their attendant therapies, including surgery and radiation, account for approximately 80% of documented acquired GHD causes. Especially, because the peripheral clinical phenotype for all causes of acquired adult-onset GHD is quite similar, no distinctive clinical marker points to an idiopathic etiology, and importantly, adult height will already be attained in most of these patients. To enhance the probability of deriving accurate biochemical test results to enable the very rare diagnosis of IAGHD, exclusion of clinically apparent causes should be undertaken by conducting a comprehensive clinical history to elicit evidence for prior events that may have long been forgotten by the patient (Table 2). Thus, a past motor vehicle accident with head injury, contact sports injury, excessive postpartum hemorrhage, and childhood head or neck irradiation are important conditions to exclude. Patients with traumatic brain injury, as well as those undergoing brain injury rehabilitation, are prone to GHD (28, 54–56). Competitive contact sports participation, especially boxing and football, have been unmasked as important factors contributing to adult GHD (57).
Several causes of acquired adult GHD have more recently been reported, and patients with these newly unmasked causes of adult GHD would heretofore have been classified as IAGHD. For example, GHD has been reported by 1 group in patients with primary hyperparathyroidism (58) and also after subarachnoid hemorrhage (56). High titers of antipituitary antibodies have been observed in patients with autoimmune endocrine disease who also exhibit features of adult GHD. Thus, using an indirect immunofluorescent assay, 4 of 20 adult patients with selective “idiopathic” GHD exhibited high serum antibody titers directed against pituitary membranes (59, 60). More recently, specific circulating autoantibodies to PIT-1 were reported in 3 adults with combined GH, prolactin, and TSH deficiency (61). These adult patients were of normal height, suggesting an acquired etiology for this heretofore cryptic cause of adult pituitary failure. Interestingly, the circulating PIT-1 antibody has been postulated to exhibit cellular toxicity and may actually participate in the pathogenesis of somatotroph failure in these very rarely encountered patients (61). Subtle structural bony or soft tissue parasellar magnetic resonance imaging changes (eg, partially empty sella) associated with GHD may also be overlooked (62, 63). Thus, a category of truly “idiopathic” etiology could reflect a shrinking knowledge gap between newly discovered cryptic GHD causes and those yet to be unmasked.
The Spectrum of Adult GHD
Broadly, adult GHD is usually a consequence of clear-cut hypothalamic-pituitary disease or persistence of a congenital or genetic childhood disorder (64). However, pituitary failure is evidently a dynamic continuum where a spectrum of new hormone deficits may manifest years after an initial diagnosis of purported IAGHD. A state of “partial GHD” has been described with intermediate elicited GH responses ranging between values obtained in normal subjects and those defined as truly GH deficient. The phenotype of this group includes excess abdominal fat mass, reduced lean body mass, altered cardiac function and insulin resistance, all features indistinguishable, except for the degree of severity, from those of adult GHD (53). Thus, the continuum of somatotroph-replete patients may also comprise a subset who are GH “insufficient” and may yet evolve to develop full-fledged adult GHD (53). Importantly, as the cohort studied included those with a history of pituitary damage, these patients cannot be classified as IAGHD.
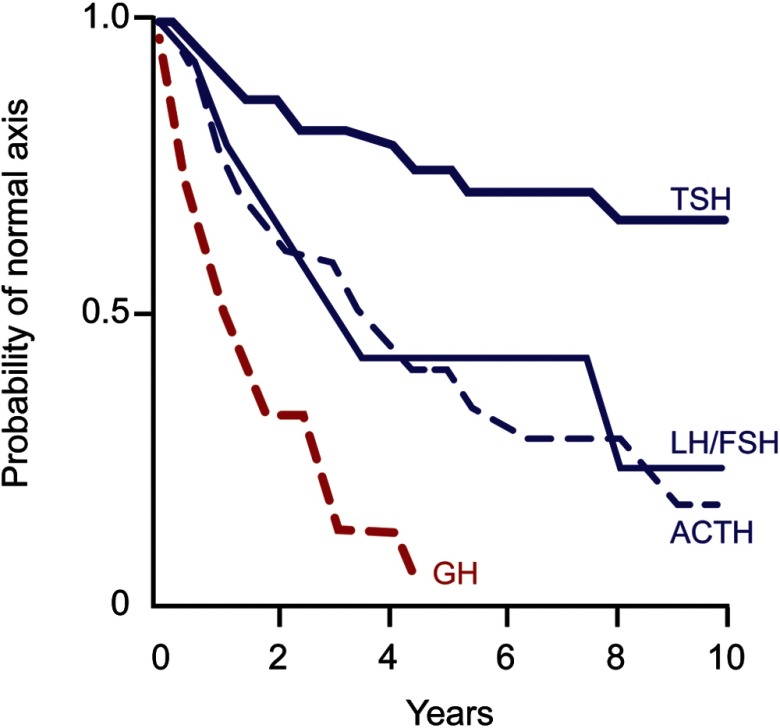
Because the somatotroph cell is most sensitive to early pituitary insults, including trauma, radiation, and gland compression, adult GHD usually precedes development of multiple pituitary hormone deficits, including more readily recognizable gonadal, thyroid, or adrenal failure (65) (Figure 3). These patients may reflect those in whom a pituitary insult is evolving, such as is observed responding to prior radiation. Accordingly, the likelihood of adult GHD already being present in patients with known multiple pituitary hormone failure is high (42). Conversion of one third of patients with isolated GHD to multiple pituitary hormone failure was observed over a period of 6 years after initiating GH therapy. This phenomenon occurred independently of ultimately determined GHD etiology, patient age, or history of pituitary surgery or radiation (52). Nevertheless, because no studies have prospectively documented the timeline for conversion of idiopathic/isolated GHD to multiple pituitary hormone failure, it is unclear how many reported cases of IAGHD are actually precursors to an evolving continuum of multiple pituitary hormone deficits (52). The benefits of GH replacement are also unclear in patients who exhibit clear-cut GHD as established by biochemical testing, but who have normal IGF-I levels and intact gonadal, thyroid, and adrenal anterior pituitary trophic reserves.
Figure 3.
Life-table analysis indicating probabilities of initially normal hypothalamic-pituitary-target gland axes remaining normal after radiotherapy (3750–4250 cGy). GH secretion is the most sensitive of the anterior pituitary hormones to effects of external radiotherapy, and GHD precedes development of ACTH, LH, FSH, and TSH hormone deficiencies. [Modified from M. D. Littley et al: Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160 (82), with permission. © Association of Physicians of Great Britain and Ireland.]
GH Replacement for IAGHD
In the United States, adult GH replacement is approved for acquired pituitary deficiency or for proven prior childhood-onset GHD. GH replacement therapy should only be offered to those patients rigorously diagnosed by evidence-based criteria as being GH-deficient and in whom a clear etiology for the disorder has been established (18, 23). GH is also approved for treating AIDS-related muscle wasting in adults. Adult GH replacement protocols should ideally be individualized, with titration of initial low GH doses determined by clinical responses, IGF-I levels maintained in the mid-normal range, and side-effect frequency and severity. Patients should be monitored every 2 months during dose titration. Thereafter, clinical evaluation and IGF-I measurements should be undertaken every 6 months. Frequency of bone density assessments and cardiac monitoring is determined by clinical judgment.
In patients fulfilling the clinical and diagnostic criteria described above for GHD, physiological GH replacement reverses several related comorbidities, including improving quality of life, enhancing lean body mass, improving cardiovascular function and exercise capacity, and increasing bone density (3, 51, 66, 67) (Table 3). However, neither the benefits nor the safety of GH replacement in adults without a rigorous GHD diagnosis has been effectively evaluated. GH has also been extensively used by athletes in an attempt to enhance performance (68). When administered to healthy athletes, GH may moderately enhance muscle strength and maximal O2 consumption (VO2 max) (69, 70) and enhance sprint capacity by 4% (71), as well as increase anaerobic capacity. These modest effects should be placed in the context of the potential side effects of sustained high-dose GH abuse leading to adverse effects, including ultimately the spectrum of comorbidities associated with acromegaly (72, 73). Thus, GH abuse by athletes or inappropriate GH administration to pituitary-replete subjects entails a significant health risk (74). Dose-dependent side effects of GH replacement to truly GH-deficient adults are reported in up to 30% of patients and include arthralgia, myalgias, edema, paresthesia and carpal tunnel syndrome (3, 75), sleep apnea, insomnia, and dyspnea (76). The long-term safety profile for new cancers and diabetes in GHD adults appears favorable, but it is currently being further assessed in long-term surveillance studies (6, 77, 78). However, in GH-replete subjects, inappropriate GH administration may cause edema, sweating, fatigue, and in higher doses, diabetes and cardiac dysfunction (79, 80) (Figure 4). Because the endogenous endocrine milieu in GH-replete adults likely differs from truly pituitary-deficient patients, the nature of GH-related side effects in patients not fulfilling criteria for GHD is not clearly understood. Given the above risks, the diagnosis of “idiopathic” adult GHD may be inadvertently made to justify unapproved and unsafe GH use in athletes, elderly, or frail patients (20). Because GH administration enhances lean body mass and causes fat loss, the use of GH to reverse, halt, or decelerate age-associated sarcopenia and frailty is tempting, and has been proposed as an unapproved use (19). Because the classification of elderly patients as IAGHD may in fact be misleading because GH and/or IGF-I levels normally are attenuated with aging, adherence to rigorous clinical and biochemical criteria, as well as age-matched IGF-I values, should clearly distinguish truly idiopathic GHD patients from pituitary-replete aged or frail patients.
Table 3.
Adult GHD
| Clinical Consequence | Effect of GH Replacement |
|---|---|
| Body composition | |
| General and central adiposity | Decrease |
| Reduce lean mass | Increase |
| Reduced bone mass | Increase |
| Function | |
| Reduced exercise capacity | Improve |
| Muscle weakness | Improve |
| Impaired cardiac function | Improve |
| Hypohydrosis | Improve |
| Quality of life | |
| Low mood | Improve |
| Fatigue | Improve |
| Low motivation | Improve |
| Reduced satisfaction | Improve |
| Cardiovascular risk profile | |
| Abnormal lipid profile | Improve |
| Insulin resistance | Improves in long term |
| Inflammatory markers | Decrease |
| Intimal media thickening | Decrease |
| Cardiovascular and cerebrovascular events | Unknown |
| Laboratory | |
| Blunted peak GH to stimulation | |
| Low IGF-I | Increase |
| Hyperinsulinemia | Increase |
| High LDL- and low HDL-cholesterol | Improve |
| Longevity | Unknown |
Modified from S. Melmed and K. Ho: Pituitary physiology and diagnostic evaluation. Williams Textbook of Endocrinology (edited by S. Melmed, K. S. Polonsky, P. Reed Larsen, H. M. Kronenberg), Elsevier, Philadelphia, PA, 2010 (83), with permission.
Figure 4.
Summary of side effects reported in double-blind, placebo-controlled studies in healthy subjects of GH administration for 4–12 weeks with median GH dose of 40 μg/kg·/d. Data are presented as a range of percentages of the subjects reporting side effects during GH and placebo administration (20). [Reproduced with permission.]
Identification of IAGHD patients most likely to benefit from GH is as yet unclear. Given the stringent challenges and costs in establishing the diagnosis of IAGHD as outlined above, the justification for replacing GH to these patients requires a measured assessment of both clinical and financial cost–benefit. Evidence supporting cost-effectiveness of long-term adult GH replacement also does not clearly distinguish benefits of GH for established adult GHD vs such patients with milder or idiopathic deficiencies (18). In fact, published clinical efficacy studies for GH replacement do not uniformly address the distinctive “idiopathic” adult GHD patient (3, 51). Of concern is that prospective studies will be difficult to design because rigorously defined treatment efficacy endpoints are challenging to define for IAGHD patients and the condition is rare. Accordingly, the unapproved and illegal (in the United States) use of GH for patients inaccurately diagnosed as IAGHD should not be proposed at this time.
Conclusions
The pathogenesis of true IAGHD as reflected by an unknown cause for compromised somatotroph function remains enigmatic. Understanding acquired mechanisms underlying idiopathic somatotroph failure is also challenged by the heterogeneous pattern of “normal” GH secretion, which reflects a dynamic pulsatility. Attenuation of GH secretion with age further confounds the diagnosis of an idiopathic nonphysiological cause of blunted adult GH secretion. It is also apparent that somatotroph failure may not be an “all or none” phenomenon, but rather a graded spectrum of incipient “subclinical somatotroph failure” (23) eventually followed by total pituitary gland shutdown. Because insights gleaned from careful genetic, biochemical, immunological, pathological, and clinical observations have resulted in an earlier awareness of both presentation and causes of early pituitary failure, it is not inconceivable that with time, the number of patients bearing the stringent diagnosis of IAGHD will continue to shrink as the etiology of more heretofore cryptic acquired causes is unmasked. Application of rigorous criteria for diagnosing true IAGHD is compounded by the need for justifying rational, evidence- and value-driven adult GH replacement regimens.
Acknowledgments
This work was supported by National Institutes of Health Grant CA 75979.
Disclosure Summary: The author has nothing to disclose.
For editorial see page 2270
- BMI
- body mass index
- GHD
- GH deficiency
- IAGHD
- idiopathic adult GHD
- ITT
- insulin-induced hypoglycemia.
References
- 1. Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989;321:1797–1803 [DOI] [PubMed] [Google Scholar]
- 2. Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700 [DOI] [PubMed] [Google Scholar]
- 3. Hoffman AR, Kuntze JE, Baptista J, et al. Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2048–2056 [DOI] [PubMed] [Google Scholar]
- 4. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML; Endocrine Society Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1587–1609 [DOI] [PubMed] [Google Scholar]
- 5. Webb SM, Strasburger CJ, Mo D, et al. Changing patterns of the adult growth hormone deficiency diagnosis documented in a decade-long global surveillance database. J Clin Endocrinol Metab. 2009;94:392–399 [DOI] [PubMed] [Google Scholar]
- 6. Abs R, Bengtsson BA, Hernberg-Stahl E, et al. GH replacement in 1034 growth hormone deficient hypopituitary adults: demographic and clinical characteristics, dosing and safety. Clin Endocrinol (Oxf). 1999;50:703–713 [DOI] [PubMed] [Google Scholar]
- 7. Fleetwood MR, Ho Y, Cooke NE, Liebhaber SA. DNase I hypersensitive site II of the human growth hormone locus control region mediates an essential and distinct long-range enhancer function. J Biol Chem. 2012;287:25454–25465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frohman LA, Burek L, Stachura MA. Characterization of growth hormone of different molecular weights in rat, dog and human pituitaries. Endocrinology. 1972;91:262–269 [DOI] [PubMed] [Google Scholar]
- 9. Ho Y, Elefant F, Liebhaber SA, Cooke NE. Locus control region transcription plays an active role in long-range gene activation. Mol Cell. 2006;23:365–375 [DOI] [PubMed] [Google Scholar]
- 10. Casanueva FF, Camina JP, Carreira MC, Pazos Y, Varga JL, Schally AV. Growth hormone-releasing hormone as an agonist of the ghrelin receptor GHS-R1a. Proc Natl Acad Sci USA. 2008;105:20452–20457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab. 2010;21:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamashita S, Melmed S. Insulinlike growth factor I regulation of growth hormone gene transcription in primary rat pituitary cells. J Clin Invest. 1987;79:449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797 [DOI] [PubMed] [Google Scholar]
- 14. Goldenberg N, Barkan A. Factors regulating growth hormone secretion in humans. Endocrinol Metab Clin North Am. 2007;36:37–55 [DOI] [PubMed] [Google Scholar]
- 15. Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6:515–525 [DOI] [PubMed] [Google Scholar]
- 16. Sotiropoulos A, Ohanna M, Kedzia C, et al. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci USA. 2006;103:7315–7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255 [DOI] [PubMed] [Google Scholar]
- 18. Lipworth WL, Ho K, Kerridge IH, Day RO. Drug policy at the margins: the case of growth hormone replacement for adults with severe growth hormone deficiency. Med J Aust. 2012;197:204–205 [DOI] [PubMed] [Google Scholar]
- 19. Olshansky SJ, Perls TT. New developments in the illegal provision of growth hormone for “anti-aging” and bodybuilding. JAMA. 2008;299:2792–2794 [DOI] [PubMed] [Google Scholar]
- 20. Birzniece V, Nelson AE, Ho KK. Growth hormone and physical performance. Trends Endocrinol Metab. 2011;22:171–178 [DOI] [PubMed] [Google Scholar]
- 21. Melmed S. Supplemental growth hormone in healthy adults: the endocrinologist's responsibility. Nat Clin Pract Endocrinol Metab. 2006;2:119. [DOI] [PubMed] [Google Scholar]
- 22. Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57:555–559 [DOI] [PubMed] [Google Scholar]
- 23. Shalet SM. Partial growth hormone deficiency in adults; should we be looking for it? Clin Endocrinol (Oxf). 2010;73:432–435 [DOI] [PubMed] [Google Scholar]
- 24. Gleeson HK, Gattamaneni HR, Smethurst L, Brennan BM, Shalet SM. Reassessment of growth hormone status is required at final height in children treated with growth hormone replacement after radiation therapy. J Clin Endocrinol Metab. 2004;89:662–666 [DOI] [PubMed] [Google Scholar]
- 25. Aimaretti G, Baffoni C, Bellone S, et al. Retesting young adults with childhood-onset growth hormone (GH) deficiency with GH-releasing-hormone-plus-arginine test. J Clin Endocrinol Metab. 2000;85:3693–3699 [DOI] [PubMed] [Google Scholar]
- 26. Schneider HJ, Rovere S, Corneli G, et al. Endocrine dysfunction in patients operated on for non-pituitary intracranial tumors. Eur J Endocrinol. 2006;155:559–566 [DOI] [PubMed] [Google Scholar]
- 27. Snyder PJ, Fowble BF, Schatz NJ, Savino PJ, Gennarelli TA. Hypopituitarism following radiation therapy of pituitary adenomas. Am J Med. 1986;81:457–462 [DOI] [PubMed] [Google Scholar]
- 28. Benvenga S, Campenni A, Ruggeri RM, Trimarchi F. Clinical review 113: hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353–1361 [DOI] [PubMed] [Google Scholar]
- 29. Gasco V, Corneli G, Rovere S, et al. Diagnosis of adult GH deficiency. Pituitary. 2008;11:121–128 [DOI] [PubMed] [Google Scholar]
- 30. Chanson P, Cailleux-Bounacer A, Kuhn JM, et al. Comparative validation of the growth hormone-releasing hormone and arginine test for the diagnosis of adult growth hormone deficiency using a growth hormone assay conforming to recent international recommendations. J Clin Endocrinol Metab. 2010;95:3684–3692 [DOI] [PubMed] [Google Scholar]
- 31. Yuen KC, Biller BM, Molitch ME, Cook DM. Clinical review: is lack of recombinant growth hormone (GH)-releasing hormone in the United States a setback or time to consider glucagon testing for adult GH deficiency? J Clin Endocrinol Metab. 2009;94:2702–2707 [DOI] [PubMed] [Google Scholar]
- 32. Biller BM, Samuels MH, Zagar A, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079 [DOI] [PubMed] [Google Scholar]
- 33. Popovic V, Leal A, Micic D, et al. GH-releasing hormone and GH-releasing peptide-6 for diagnostic testing in GH-deficient adults. Lancet. 2000;356:1137–1142 [DOI] [PubMed] [Google Scholar]
- 34. Colao A, Di Somma C, Savastano S, et al. A reappraisal of diagnosing GH deficiency in adults: role of gender, age, waist circumference, and body mass index. J Clin Endocrinol Metab. 2009;94:4414–4422 [DOI] [PubMed] [Google Scholar]
- 35. Bonert VS, Elashoff JD, Barnett P, Melmed S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab. 2004;89:3397–3401 [DOI] [PubMed] [Google Scholar]
- 36. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veldhuis JD, Roelfsema F, Keenan DM, Pincus S. Gender, age, body mass index, and IGF-I individually and jointly determine distinct GH dynamics: analyses in one hundred healthy adults. J Clin Endocrinol Metab. 2011;96:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aimaretti G, Fanciulli G, Bellone S, et al. Enhancement of the peripheral sensitivity to growth hormone in adults with GH deficiency. Eur J Endocrinol. 2001;145:267–272 [DOI] [PubMed] [Google Scholar]
- 39. Clasey JL, Weltman A, Patrie J, et al. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab. 2001;86:3845–3852 [DOI] [PubMed] [Google Scholar]
- 40. Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64:1039–1044 [DOI] [PubMed] [Google Scholar]
- 41. Luque RM, Lin Q, Cordoba-Chacon J, et al. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One. 2011;6:e15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 2002;87:477–485 [DOI] [PubMed] [Google Scholar]
- 43. Holmes SJ, Shalet SM. Characteristics of adults who wish to enter a trial of GH repacement. 1995;42:613–618 [DOI] [PubMed] [Google Scholar]
- 44. Cuneo RC, Salomon F, McGauley GA, Sonksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol (Oxf). 1992;37:387–397 [DOI] [PubMed] [Google Scholar]
- 45. Brabant G, Poll EM, Jonsson P, Polydorou D, Kreitschmann-Andermahr I. Etiology, baseline characteristics, and biochemical diagnosis of GH deficiency in the adult: are there regional variations? Eur J Endocrinol. 2009;161(suppl 1):S25–S31 [DOI] [PubMed] [Google Scholar]
- 46. van Nieuwpoort IC, van Bunderen CC, Arwert LI, et al. Dutch National Registry of GH Treatment in Adults: patient characteristics and diagnostic test procedures. Eur J Endocrinol. 2011;164:491–497 [DOI] [PubMed] [Google Scholar]
- 47. Nystrom HF, Saveanu A, Barbosa EJ, et al. Detection of genetic hypopituitarism in an adult population of idiopathic pituitary insufficiency patients with growth hormone deficiency. Pituitary. 2011;14:208–216 [DOI] [PubMed] [Google Scholar]
- 48. Bates AS, Van't Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996;81:1169–1172 [DOI] [PubMed] [Google Scholar]
- 49. Bulow B, Hagmar L, Eskilsson J, Erfurth EM. High incidence of cardiovascular disease and increased prevalence of risk factors in females with hypopituitarism. In: Program of the 81st Annual Meeting of The Endocrine Society; June 12–15, 1999; San Diego, CA; Abstract OR14-13 [Google Scholar]
- 50. Tomlinson JW, Holden N, Hills RK, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431 [DOI] [PubMed] [Google Scholar]
- 51. Attanasio AF, Lamberts SW, Matranga AM, et al. Adult growth hormone (GH)-deficient patients demonstrate heterogeneity between childhood onset and adult onset before and during human GH treatment. J Clin Endocrinol Metab. 1997;82:82–88 [DOI] [PubMed] [Google Scholar]
- 52. Klose M, Jonsson B, Abs R, et al. From isolated GH deficiency to multiple pituitary hormone deficiency: an evolving continuum—a KIMS analysis. Eur J Endocrinol. 2009;161(Suppl 1):S75–S83 [DOI] [PubMed] [Google Scholar]
- 53. Murray RD, Bidlingmaier M, Strasburger CJ, Shalet SM. The diagnosis of partial growth hormone deficiency in adults with a putative insult to the hypothalamo-pituitary axis. J Clin Endocrinol Metab. 2007;92:1705–1709 [DOI] [PubMed] [Google Scholar]
- 54. Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab. 2001;86:2752–2756 [DOI] [PubMed] [Google Scholar]
- 55. Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369:1461–1470 [DOI] [PubMed] [Google Scholar]
- 56. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429–1438 [DOI] [PubMed] [Google Scholar]
- 57. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827–831 [DOI] [PubMed] [Google Scholar]
- 58. Gasperi M, Cecconi E, Grasso L, et al. GH secretion is impaired in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2002;87:1961–1964 [DOI] [PubMed] [Google Scholar]
- 59. De Bellis A, Pane E, Bellastella G, et al. ; Italian Autoimmune Hypophysitis Network Study Detection of antipituitary and antihypothalamus antibodies to investigate the role of pituitary or hypothalamic autoimmunity in patients with selective idiopathic hypopituitarism. Clin Endocrinol (Oxf). 2011;75:361–366 [DOI] [PubMed] [Google Scholar]
- 60. Bellastella G, Rotondi M, Pane E, et al. ; Italian Autoimmune Hypophysitis Network Study Predictive role of the immunostaining pattern of immunofluorescence and the titers of antipituitary antibodies at presentation for the occurrence of autoimmune hypopituitarism in patients with autoimmune polyendocrine syndromes over a five-year follow-up. J Clin Endocrinol Metab. 2010;95:3750–3757 [DOI] [PubMed] [Google Scholar]
- 61. Yamamoto M, Iguchi G, Takeno R, et al. Adult combined GH, prolactin, and TSH deficiency associated with circulating PIT-1 antibody in humans. J Clin Invest. 2011;121:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gasperi M, Aimaretti G, Cecconi E, et al. Impairment of GH secretion in adults with primary empty sella. J Endocrinol Invest. 2002;25:329–333 [DOI] [PubMed] [Google Scholar]
- 63. Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab. 2000;85:1789–1793 [DOI] [PubMed] [Google Scholar]
- 64. Alatzoglou KS, Dattani MT. Genetic causes and treatment of isolated growth hormone deficiency—an update. Nat Rev Endocrinol. 2010;6:562–576 [DOI] [PubMed] [Google Scholar]
- 65. Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gotherstrom G, Elbornsson M, Stibrant-Sunnerhagen K, Bengtsson BA, Johannsson G, Svensson J. Ten years of growth hormone (GH) replacement normalizes muscle strength in GH-deficient adults. J Clin Endocrinol Metab. 2009;94:809–816 [DOI] [PubMed] [Google Scholar]
- 67. Rosilio M, Blum WF, Edwards DJ, et al. Long-term improvement of quality of life during growth hormone (GH) replacement therapy in adults with GH deficiency, as measured by questions on life satisfaction-hypopituitarism (QLS-H). J Clin Endocrinol Metab. 2004;89:1684–1693 [DOI] [PubMed] [Google Scholar]
- 68. Holt RI, Erotokritou-Mulligan I, Sonksen PH. The history of doping and growth hormone abuse in sport. Growth Horm IGF Res. 2009;19:320–326 [DOI] [PubMed] [Google Scholar]
- 69. Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484 [DOI] [PubMed] [Google Scholar]
- 70. Graham MR, Baker JS, Evans P, et al. Physical effects of short-term recombinant human growth hormone administration in abstinent steroid dependency. Horm Res. 2008;69:343–354 [DOI] [PubMed] [Google Scholar]
- 71. Meinhardt U, Nelson AE, Hansen JL, et al. The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Intern Med. 2010;152:568–577 [DOI] [PubMed] [Google Scholar]
- 72. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karges B, Pfaffle R, Boehm BO, Karges W. Acromegaly induced by growth hormone replacement therapy. Horm Res. 2004;61:165–169 [DOI] [PubMed] [Google Scholar]
- 74. Liu H, Bravata DM, Olkin I, et al. Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med. 2008;148:747–758 [DOI] [PubMed] [Google Scholar]
- 75. Chipman JJ, Attanasio AF, Birkett MA, Bates PC, Webb S, Lamberts SW. The safety profile of GH replacement therapy in adults. Clin Endocrinol (Oxf). 1997;46:473–481 [DOI] [PubMed] [Google Scholar]
- 76. Hartman ML, Xu R, Crowe BJ, et al. ; International HypoCCS Advisory Board 2013 Prospective safety surveillance of GH-deficient adults: comparison of GH-treated vs untreated patients. J Clin Endocrinol Metab. 2013;98:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Attanasio AF, Jung H, Mo D, et al. ; HypoCCS International Advisory Board Prevalence and incidence of diabetes mellitus in adult patients on growth hormone replacement for growth hormone deficiency: a surveillance database analysis. J Clin Endocrinol Metab. 2011;96:2255–2261 [DOI] [PubMed] [Google Scholar]
- 78. Monson JP. Long-term experience with GH replacement therapy: efficacy and safety. Eur J Endocrinol. 2003;148(suppl 2):S9–S14 [DOI] [PubMed] [Google Scholar]
- 79. Young J, Anwar A. Strong diabetes. Br J Sports Med 2007;41:335–336; discussion 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cittadini A, Berggren A, Longobardi S, et al. Supraphysiological doses of GH induce rapid changes in cardiac morphology and function. J Clin Endocrinol Metab. 2002;87:1654–1659 [DOI] [PubMed] [Google Scholar]
- 81. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355:2558–2573 [DOI] [PubMed] [Google Scholar]
- 82. Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160 [PubMed] [Google Scholar]
- 83. Melmed S, Ho K. Pituitary physiology and diagnostic evaluation. In: Melmed S, Polonsky KS, Reed Larsen P, Kronenberg HM, eds. Williams textbook of endocrinology, 12th ed Philadelphia, PA: Elsevier; 2010 [Google Scholar]