Abstract
Objective:
Resistance to thyroid hormone is a syndrome characterized by high serum free T4 levels and unsuppressed serum TSH concentration. Thyroxine-binding globulin complete deficiency manifests with low serum total T4 and T3 levels and normal serum TSH concentration. Our objective is to describe a family with the coexistence of resistance to thyroid hormone and thyroxine-binding globulin complete deficiency.
Methods:
We conducted clinical studies and genetic analyses.
Results:
The proband presented with mental retardation, hearing loss, and recurrent upper respiratory tract infections accompanied by high serum levels of TSH, T3, T4, and high thyroglobulin antibody titers. His elder sister presented with normal TSH and T3 and high serum T4 levels. Both patients were found to be heterozygous for the mutation P453A in the thyroid hormone receptor beta (THRB) gene. One of the proband's brothers had low serum total T3 and T4 and normal TSH concentrations, without any clinical manifestations. He was hemizygous for the mutation P50fs51X in the TBG gene. The proband's mother showed slightly elevated TSH, normal total T3 and T4, and elevated titers of thyroperoxidase antibodies and thyroglobulin antibodies. She was heterozygous for both THRB and TBG genes mutations.
Conclusions:
To our knowledge, this is the first report of the coexistence of THRB and TBG gene mutations in the same individual (mother of the proband), whereas other affected family members had only 1 of the 2 genes mutated. The case illustrates the difficulty that might be encountered in the interpretation of thyroid function tests when different genetic defects affecting thyroid function coexist.
Resistance to thyroid hormone (RTH) is an autosomal-dominant disorder characterized by high serum levels of thyroid hormones (TH) in the presence of unsuppressed serum TSH concentration. Clinically, RTH is highly variable with manifestations suggestive of TH deficiency and excess. At the molecular level, in the majority of cases, RTH has been associated with mutations in the thyroid hormone receptor β (THRB, alias TRβ) gene (1).
Inherited thyroxine-binding globulin (TBG) deficiency is caused by mutations in the TBG gene, which results in defective synthesis or changes in the physical properties or biological function of the protein. The defect shows an X-linked transmission, according to the location of the gene on the X-chromosome. TBG deficiency has 2 forms: complete deficiency (TBG-CD) and partial deficiency (TBG-PD), denoting complete absence and reduction of TBG in affected males, respectively. As TBG deficiency does not affect the serum concentration of free TH, it does not produce any clinical manifestations (2).
Herein, we describe, for the first time, the coexistence of RTH and TBG-CD and its consequences on the thyroid phenotype.
Materials and Methods
Patient
The proband (II-2; Figure 1), a 27-year-old man, born to nonconsanguineous Turkish parents, presented with mental retardation, hearing loss, and recurrent upper respiratory tract infections. Since the age of 9 months, he showed delayed neuropsychomotor development, ascribed to infantile febrile convulsions.
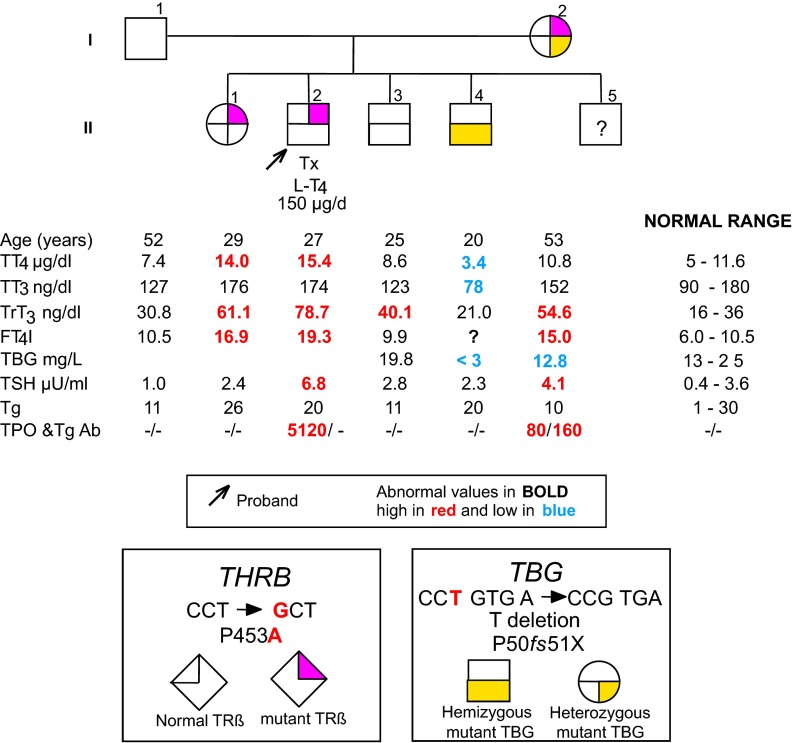
Figure 1.
Family pedigree showing the genotype and TFT results. Results are aligned with each symbol. Ages represent those at the time of blood sampling. Tx, thyroidectomy. Note that FT4I cannot be obtained in the complete absence of TBG. TT4, total T4; TT3, total T3; FT4I, free T4 index; Tg, thyroglobulin; TPO, thyroperoxidase; THRB and TRβ, thyroid hormone receptor beta.
At the age of 21 years, serum concentration of free T3 was 4.89 pg/mL (normal 2.57–4.43); free T4 was 2.04 ng/dL (normal 0.9–1.8), and TSH was 2.38 μIU/mL (normal 0.23–4.2). These results and the presence of a goiter led to the diagnosis of Graves disease and he was given 10 mCi of 131I. His TSH increased to >100 μIU/mL and 150 μg of l-thyroxine (L-T4) was started.
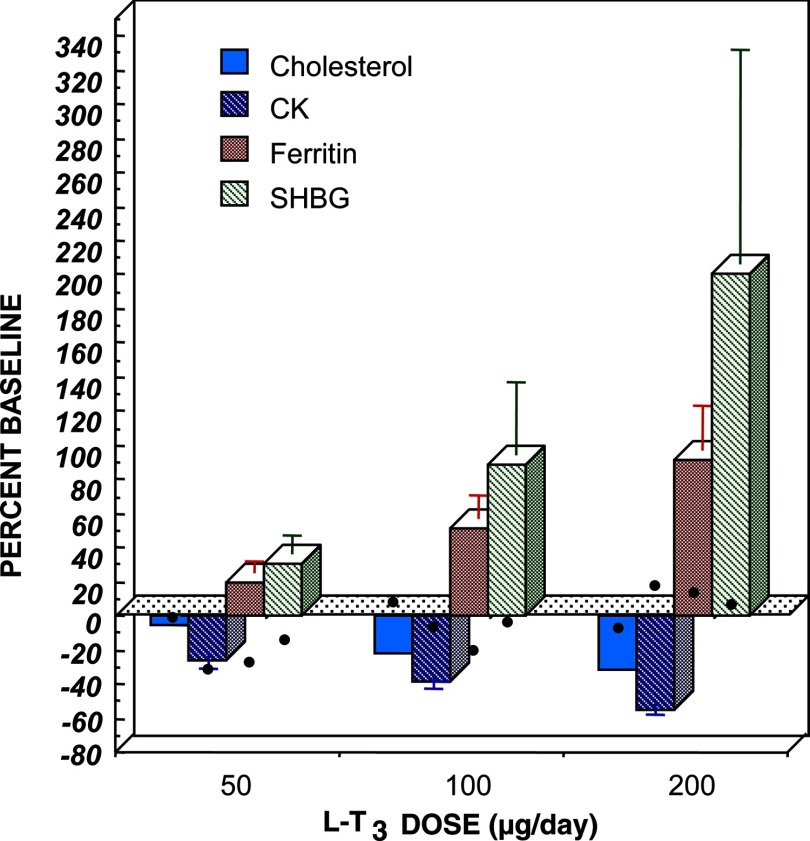
On a recent admission to the Konya University Hospital, the proband presented severe mental retardation (intelligence quotient 35–49), dysphasia, difficulty in walking, and dystonic movements of the neck, chin, and wrists. He had a moderate to severe hearing loss. Results of pure tone audiometry were as follows: air 72 dB and bone 55 dB. His height was 180 cm, weight 57 kg, and heart rate 72 beats per minute. On thyroid ultrasonography, the right lobe was 0.95 × 0.64 × 3.6 cm and the left lobe was 0.74 × 1.09 × 2.08 cm. On L-T4 his thyroid function test (TFT) results were: free T3 4.66 pg/mL (normal 2.5–3.9), free T4 2.51 ng/dL (normal 0.61–1.12), and TSH 1.52 μIU/mL (normal 0.35–5.5). RTH was considered because of the unsuppressed TSH in face of elevated free T3 and free T4 concentrations. A T3-suppression test was performed off L-T4 treatment for 4 days. Incremental doses of liothyronine (L-T3) (50–100-200 μg) were given once rather than twice daily, as suggested in a standardized protocol (3). As the results were suggestive of RTH (Figure 2), blood samples of the proband and family members were sent to the University of Chicago for genetic studies. Contextually, TFT were tested and results are shown in Figure 1. At the time of blood sampling, the proband was on 150 μg L-T4.
Figure 2.
L-T3 suppression test results for the proband (black dots) compared to normal subjects (columns). Changes are expressed as percentage baseline ± SE. CK, creatinine kinase.
Family members
The proband's mother (I-2, Figure 1), 52 years old, had free T4 of 1.88 ng/dL (normal 0.74–1.79) and TSH 12.3 μIU/mL (normal 0.35–5.5); antibodies to thyroperoxidase were negative and antibodies to thyroglobulin were 95 IU/mL (normal 0–40). Accordingly, she was given L-T4 50 μg per day. As on repeated testing free T4 declined to 1.64 ng/dL (normal 0.61–1.12) and TSH to 3.44 μIU/mL (normal 0.35–5.5), L-T4 treatment was stopped. At 53 years of age, her TFTs are indicated in Figure 1. She was reported to have tachycardia that required recurrent hospitalizations but no hearing loss. A sister (II-1; Figure 1) had elevated serum total T4 and normal TSH concentrations, but no tachycardia or hearing loss. A younger brother (II-4; Figure 1) had low serum total T4 and T3 and normal TSH levels. His father (I-1; Figure 1) and another brother (II-3; Figure 1) had normal test results. One more brother (II-5; Figure 1) declined investigation.
Clinical data
Studies were approved by the review board of the University of Chicago and written consents were obtained from all subjects studied.
Thyroid function tests
Blood was collected locally and shipped to Chicago for analysis. Total T4, T3, rT3, TSH, free T4 index (FT4I), thyroglobulin antibodies, and thyroperoxidase antibodies were measured as previously described (4).
DNA mutation analysis
DNA was isolated from peripheral blood leukocytes using QIAamp DNA Mini Kit (Qiagen, Valencia, California) and was amplified by PCR and all coding exons of THRB and TBG genes along with intron/exon boundaries were sequenced (3730XL; Applied Biosystems, Carlsbad, California). Sequences were compared with those published for THRB (NM 001252634; exons 7 through 10) and TBG (NG 021252) genes (http://www.ncbi.nlm.nih.gov/). Sequences of primers are available on request.
Results
Thyroid function tests
The proband (II-2), his mother (I-2), and his elder sister (II-1) had TFTs consistent with RTH, high serum free T4, and normal or slightly elevated TSH concentration (Figure 1). The probands's brother (II-4) had TFT suggestive of TBG deficiency, low total T4 and T3, but normal TSH. This was confirmed by serum TBG determination (Figure 1). The proband and his mother had autoimmune thyroid disease based on positive thyroglobulin antibodies (I-2) and thyroperoxidase antibodies (I-2 and II-2).
Mutation identification and genotyping of family members
The proband (II-2) was found to be heterozygous for a single nucleotide substitution in the THRB gene. A C to G transversion resulted in the substitution of the normal amino acid proline 453 (CCT) with an alanine (GCT) (P453A). The same mutation was found in the elder sister (II-1). In addition, one of the brothers (II-4) had a deletion of the T 214 in the first coding exon of the TBG gene. The mutation resulted in a frameshift at codon 50 with a premature stop (TGA) at position 51, causing an early truncation of the TBG protein (P50fs51X). The proband's mother (I-2) was the only family member to have both THRB and TBG gene mutations.
Discussion
This study describes the first family with coexistence of RTH and TBG-CD, due to mutations in the THRB and TBG genes, respectively. One family with a THRB P453S, reported in 1994 from our laboratory, had also slight reduction in serum TBG (5). However, subsequent sequencing of the TBG gene failed to show a mutation (determined by A.M.F. and S.R.).
The mutation P453A in the THRB gene, found in the proband, his mother, and his elder sister, was previously reported (6–11). The affinity of the mutant protein for T3 is 17% the wild-type (WT) receptor (7). This mutation was identified in 10 other families, excluding the one reported herein, and 6 of these have been published. Among THRB codons involved in RTH, 453 is the most common to have undergone mutations, resulting in 7 different amino acid substitutions. These are R543T (22 families, 16 published), P453S (13 families, 8 published), P453N (1 unpublished family), P453Y (1 published family), P453H (5 families, 3 published), P453L (1 published family), and P453A reported herein. In terms of impairment of T3-binding, P453H is the most severe, with affinity 7% that of the WT TRβ and P453S the least severe with 36% that of the WT TRβ (12). In fact, TRβ P453H requires the highest amount of T3 to reach the in vitro level of transactivation of the WT TRβ using both positively and negatively regulated genes. The preservation of intact dimerization domain maintains heterodimerization with the retinoid-X-receptor and the manifestation of dominant negative effect (12).
The mutation P50fs51X in the TBG gene, found in the proband's mother and younger brother (I-2 and II-4, Figure 2), was previously identified in another family (13). This mutation, producing an early termination of translation, failed to generate a functional TBG molecule as demonstrated by the inability to detect TBG using isoelectric focusing, a method capable of measuring as little as 0.3% of the average normal TBG concentration in serum (13).
The first thing to consider is whether TRβ P453A had a role in producing the severe clinical phenotype in the proband. Mental retardation, hearing loss, and recurrent upper respiratory tract infections are uncommon findings in patients with RTH (1). Most likely, the severe neurological impairment in the proband was caused by brain damage, during the febrile convulsions that occurred in his infancy. This argument is supported by the following observations: 1) the concomitant presence of dysphasia, difficulty in walking, and dystonic movements suggests a broader cerebral damage rather than the effect of the mutant TRβ, and 2) the less severe clinical presentation of RTH in the proband's mother (I-2) and elder sister (II-1), both harboring the same THRB gene mutation, suggests that this mutation, in the heterozygous state, does not cause a severe clinical phenotype. This is further supported by the mild phenotype of other patients with TRβ P453A (6–11). However, it cannot be excluded that the neurological phenotype of the proband might be worsened by the actual hypothyroidism as indicated by the elevated levels of serum TSH (Figure 1). The insufficient TH dose given to the proband underlines the difficulty in replacing TH in patients with RTH who, due to misdiagnosis, have received ablative therapy (14).
As expected for the TBG deficiency, the thyroid function tests were consistent with the X-linked transmission of the defect. In particular, the affected male member II-4, expressing only the mutant P50fs51X TBG gene, showed undetectable serum TBG with normal TSH values and levels of total T4 and T3 below the lower limit of normal (Figure 1). On the contrary, the mother (I-2), who is heterozygous also for the P50fs51X mutation of the TBG gene, had values of total T4 and T3 within the normal range (Figure 1) and not mildly reduced as expected in females with TBG-CD, harboring heterozygous mutation in the TBG gene (2). This discrepancy might be the consequence of the coexistent THRB gene mutation that, conceivably, mitigates the decreasing effect of TBG-CD on TH. It could have been possible to clarify this point if a male subject of the family had had both mutations in the THRB and TBG genes as in the case of the mother. However, the normal values of total T4 and T3 in the proband's mother could have been also due to the effect of the underlying autoimmune thyroid disease, which can overcome the RTH-dependent elevation of T3 and T4, because of thyroid gland insufficiency. In this situation, the elevated serum TSH indicates reduced thyroidal reserve and hypothyroid state at the tissue level.
In conclusion, we report an interesting association of THRB and TBG gene mutations in a Turkish family. Both mutations were present in 1 of the 5 blood relatives (the mother of the proband), whereas other affected family members had only 1 of the 2 genes mutated. Taking into consideration the reported prevalence for RTH of 1:40 000 (15) and that of TBG-CD of 1:15 000 (16), the estimated incidence of coexistence in the same individual would be 1:600 000 000. This family illustrates how challenging could be the interpretation of thyroid function tests in the presence of coexistent inherited thyroid defects.
Acknowledgments
This work was supported in part by Grants R37DK15070 and 5M01RR04999 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: A.M.F., M.C., and P.H.H. have nothing to declare. S.R. is academic associate for Quest Diagnostic Laboratories and, therefore, is considered as a consultant.
Footnotes
- L-T3
- liothyronine
- L-T4
- l-thyroxine
- RTH
- resistance to thyroid hormone
- TBG-CD
- thyroxine-binding globulin-complete deficiency
- TBG-PD
- thyroxine-binding globulin-partial deficiency
- TFT
- thyroid function test
- TH
- thyroid hormones
- TRβ
- thyroid hormone receptor β
- WT
- wild type.
References
- 1. Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone [published online August 15, 2012]. Biochim Biophys Acta. doi:10.1016/j.bbagen.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Refetoff S. Inherited thyroxine-binding globulin abnormalities in man. Endocr Rev. 1989;10:275–293 [DOI] [PubMed] [Google Scholar]
- 3. Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399 [DOI] [PubMed] [Google Scholar]
- 4. Ferrara AM, Onigata K, Ercan O, Woodhead H, Weiss RE, Refetoff S. Homozygous thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J Clin Endocrinol Metab. 2012;97:1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Refetoff S, Weiss RE, Wing JR, Sarne D, Chyna B, Hayashi Y. Resistance to thyroid hormone in subjects from two unrelated families is associated with a point mutation in the thyroid hormone receptor beta gene resulting in the replacement of the normal proline 453 with serine. Thyroid. 1994;4:249–254 [DOI] [PubMed] [Google Scholar]
- 6. Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor beta gene. J Clin Invest. 1994;94:506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Margotat A, Sarkissian G, Malezet-Desmoulins C, et al. [Identification of eight new mutations in the c-erbAB gene of patients with resistance to thyroid hormone]. Ann Endocrinol (Paris). 2001;62:220–225 [PubMed] [Google Scholar]
- 8. Aksoy DY, Gurlek A, Ringkananont U, Weiss RE, Refetoff S. Resistance to thyroid hormone associated with autoimmune thyroid disease in a Turkish family. J Endocrinol Invest. 2005;28:379–383 [DOI] [PubMed] [Google Scholar]
- 9. Florkowski CM, Brownlie BE, Croxson MS, et al. Thyroid hormone resistance: the role of mutational analysis. Intern Med J. 2006;36:738–741 [DOI] [PubMed] [Google Scholar]
- 10. Liu JF, Shi BY. [Study on TR beta gene mutation in a thyroid hormone resistance syndrome family]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:423–426 [PubMed] [Google Scholar]
- 11. Bayraktaroglu T, Noel J, Alagol F, Colak N, Mukaddes NM, Refetoff S. Thyroid hormone receptor beta gene mutation (P453A) in a family producing resistance to thyroid hormone. Exp Clin Endocrinol Diabetes. 2009;117:34–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collingwood TN, Adams M, Tone Y, Chatterjee VK. Spectrum of transcriptional, dimerization, and dominant negative properties of twenty different mutant thyroid hormone beta-receptors in thyroid hormone resistance syndrome. Mol Endocrinol. 1994;8:1262–1277 [DOI] [PubMed] [Google Scholar]
- 13. Mannavola D, Vannucchi G, Fugazzola L, et al. TBG deficiency: description of two novel mutations associated with complete TBG deficiency and review of the literature. J Mol Med (Berl). 2006;84:864–871 [DOI] [PubMed] [Google Scholar]
- 14. Weiss RE, Refetoff S. Treatment of resistance to thyroid hormone—primum non nocere. J Clin Endocrinol Metab. 1999;84:401–404 [DOI] [PubMed] [Google Scholar]
- 15. Lafranchi SH, Snyder DB, Sesser DE, et al. Follow-up of newborns with elevated screening T4 concentrations. J Pediatr. 2003;143:296–301 [DOI] [PubMed] [Google Scholar]
- 16. Refetoff S. Abnormal thyroid hormone transport. In: Thyroid Disease Manager. South Dartmouth, Massachusetts: Endocrine Education; 2012. http://www.thyroidmanager.org/chapter/abnormal-thyroid-hormone-transport/ [Google Scholar]