Abstract
Background:
The described phenotype of the polycystic ovary syndrome (PCOS) has been primarily based on findings in a referred (self or otherwise) population. It is possible that the phenotype of PCOS would be different if the disorder were to be detected and studied in its natural (unbiased) state.
Objective:
Our objective was to compare the phenotype of PCOS detected in an unselected population with that identified in a referral population.
Participants:
Participants included 292 PCOS patients identified at a tertiary care outpatient facility (referral PCOS) and 64 PCOS women (unselected PCOS) identified through the screening of a population of 668 seeking a pre-employment physical. Among the women undergoing a pre-employment physical, 563 did not demonstrate features of the disorder (unselected controls). All PCOS subjects met the National Institutes of Health 1990 criteria for the disorder.
Main Outcome Measures:
We estimated prevalence of obesity and severity of disease burden.
Results:
Referral PCOS subjects had greater mean body mass index and hirsutism score and higher degrees of hyperandrogenemia, were more likely to be non-Hispanic White (83.90%), and demonstrated a more severe PCOS subphenotype than unselected PCOS or unselected controls. The prevalence of obesity and severe obesity in referral PCOS was 2.3 and 2.5 times greater than estimates of the same in unselected PCOS and 2.2 and 3.8 times greater than estimates in unselected controls, respectively. Alternatively, unselected PCOS subjects had a prevalence of obesity and severe obesity and a mean body mass index similar to those of the general population from which they were derived.
Conclusion:
The phenotype of PCOS, including the racial/ethnic mix, severity of presentation, and rate of obesity, is affected significantly by whether the PCOS subject arises from a referral population or through unselected screening, likely reflecting the degree of patient concern and awareness and access to healthcare.
The polycystic ovary syndrome (PCOS) is one of the most common endocrine-metabolic disorders, estimated to affect at least 7% to 10% of reproductive-aged women if the most conservative definition of PCOS is used (ie, the National Institutes of Health [NIH] 1990 definition) (1, 2). PCOS is characterized by hyperandrogenism, chronic oligo-ovulation, and polycystic ovaries and is frequently associated with a higher prevalence of insulin resistance and obesity, which increase these patients' risks for metabolic syndrome, type 2 diabetes, cardiovascular disease, and endometrial cancer (2). The phenotype of PCOS greatly influences the risk of metabolic dysfunction, with the more hyperandrogenic phenotypes and subphenotypes generally associated with a greater risk (2, 3). Consequently, PCOS has enormous economic and healthcare implications (4).
Beginning with the original description of what is now known as PCOS by Stein and Leventhal (5), much of what we know today regarding the phenotype of PCOS, including the prevalence of obesity in the disorder, stems mostly from the study of populations referred (self or otherwise) for care (5–7). Referral to a medical practice, particularly tertiary care centers, can be highly influenced by the degree of patient concern for symptoms, awareness of the disorder, socioeconomic status, and access to medical care. Thus, it is possible that the phenotype of PCOS, as we know it today, may be more a reflection of referral bias than of the disorder itself.
Using data collected prospectively during the first large-scale effort to ascertain the unbiased prevalence of PCOS among women (1, 8) and data collected prospectively during the same time period from a large referral population at a tertiary care center (7), we tested the hypothesis that referral (self or otherwise) significantly (clinically and otherwise) influences the phenotype of PCOS observed. Our data suggest that the phenotype of PCOS, including the racial/ethnic mix, the severity of the presentation, and the prevalence of obesity, depends to a significant degree on whether the patients are identified in a referred clinical population or whether they are identified in an unbiased manner in unselected populations. These results also suggest that a more exact understanding of the PCOS phenotype could arise from the study of subjects identified in relatively unselected or medically unbiased populations.
Subjects and Methods
Study participants
Unselected PCOS and controls
In brief, all prospective employees of the University of Alabama at Birmingham (UAB), from resident staff to environmental workers, undergo an entrance medical evaluation that includes a brief history and physical and blood sampling. It should be noted that at the time of the study, UAB was the single largest employer in the city of Birmingham in Jefferson County and the third largest single-site employer in the state of Alabama, and its employees represent a cross-section of the surrounding population. Consecutive premenopausal females aged 18–45 years who were being evaluated for employment at UAB were asked to participate. Exclusion criteria included endocrine disorders, use of hormonal medication within the previous 3 months, inability to establish menstruation or ovulation (eg, hysterectomy, vaginal agenesis, or postmenopausal or premenarcheal state), and missing menses data or if the patient was seen before the start of the study or if dates of the first visit were incorrect. Obesity was not used as an exclusion criterion in either the referral or unselected population.
Of 960 nonpregnant women undergoing a pre-employment physical during the study periods of 1995 through 1996 and 1998 through 1999 (1, 8) who were eligible for the study, 668 women (69.58%) agreed to be examined. The combined data of these women was assessed for this study, as previously described (8). Women diagnosed with PCOS according to the NIH 1990 diagnostic criteria (9) formed the unselected PCOS study cohort, whereas those without the evidence of the disorder served as unselected controls. We did not include any subjects in our unselected population that were already being seen in our practice. The two populations represent separate and distinct cohorts.
The diagnosis of unselected PCOS or controls was based on an evaluation that included a brief medical history and physical examination, focusing on features of menstrual and ovulatory history, and clinical and biochemical hyperandrogenism, including a modified Ferriman-Gallwey (mFG) hirsutism score (10, 11). To minimize the possibility of interobserver variability in the assessment of the mFG score, in the referral group of subjects, all were evaluated by 1 physician (R.A.). In the unselected group of subjects, the initial physical examination was performed by a research nurse, and if the mFG score was reported as ≥3, then the mFG was repeated by R.A. or by Dr Eric Knochenhauer, who had been trained by R.A. and demonstrated a high degree of concordance in mFG scoring with R.A. Weight and height were measured in all participants using standardized methods and equipment.
In subjects who had not received hormonal therapy for 3 months before their examination, serum levels of total T, free T, dehydroepiandrosterone sulfate (DHEAS), and 17-hydroxyprogesterone (17-OHP) were obtained. Subjects with clinical evidence of hirsutism but apparent eumenorrhea underwent measurement of day 22 to 24 progesterone (P4) level to determine ovulatory status. We screened all subjects with possible PCOS (including those with menstrual dysfunction) with TSH, prolactin, and 17-OHP level to exclude thyroid dysfunction, hyperprolactinemia, and 21-hydroxylase–deficient nonclassic adrenal hyperplasia, respectively. If thyroid dysfunction or hyperprolactinemia was detected, the diagnosis of PCOS was still able to be made if features persisted after correction of thyroid or prolactin abnormalities. Full details of the study protocol are as previously described (1, 8).
Because establishing the diagnosis of PCOS in an unselected population requires concerted effort, the identification of these subjects in the population may be artifactually decreased. We assigned weights to the diagnosis of PCOS, depending on whether the subject completed her evaluation (weighted diagnostic score [WDR] = 1) and on the number of subjects in the phenotype category that did complete their evaluation and were found to have PCOS (ie, number of confirmed PCOS in a category/number of patients with complete evaluation). The details of this methodology have been described previously (1, 11). For the present study, we included only unselected PCOS women with high probability (WDR ≥0.85) of having PCOS and unselected control women who appeared to not have PCOS (WDR ∼0).
Referral PCOS
All nonpregnant patients presenting for the evaluation of symptoms potentially related to androgen excess to the reproductive endocrinology clinic (to R.A.) at UAB between 1995 and 1999 were included. Patients with suspected androgen excess were evaluated as described previously (7). All patients completed a uniform history form and underwent a complete physical examination, including assessment of their mFG score and serum testing similar to that of unselected PCOS subjects above. During this time period, all consecutive women with newly diagnosed PCOS constituted the referral PCOS population.
The above subjects were all from the same population in the state of Alabama in the United States, and the referred patients come from only 1 unit. The evaluations in both groups were similar in all respects, including in hormonal testing. Data for all cohorts were recorded and maintained prospectively in a computerized database. The studies were approved by the institutional review boards of University of Alabama at Birmingham and Cedars Sinai Medical Center, Los Angeles, and all study participants provided written informed consent.
Hormonal and biochemical analyses
Serum samples were analyzed for total T, SHBG, DHEAS, prolactin (PRL), TSH, 17-OHP, and P4, per the protocol. Total T was measured by an in-house RIA method after serum extraction, as previously described (12). SHBG binding activity was measured by diffusion equilibrium dialysis, using Sephadex G-25 and [3H]T as the ligand, and the free T was calculated as previously described (12, 13). DHEAS, P4, androstenedione (A4), PRL, and 17-OHP were measured by direct RIA, using commercially available kits (DHEAS and P4 from Diagnostic Products Corp., Los Angeles, CA; A4 from Diagnostics Systems Laboratories, Webster, TX; and PRL from Nichols Institute Diagnostics, San Juan Capistrano, CA) as previously described (14). Samples were batched at regular intervals for analysis to minimize the impact of interassay variability and provide study subjects with timely information. The intra- and interassay variations for total T, SHBG, DHEAS, A4, PRL, TSH, 17-OHP, and P4 have been previously reported (8).
Statistical calculations and analysis
Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters, and subjects were categorized in obesity classes according to the classification of the World Health Organization (15) as follows: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (preobese) (BMI 25–29.9 kg/m2), and obese (BMI ≥30 kg/m2). This latter category is subgrouped into class 1 (mild) obesity (30.0–34.9 kg/m2), class 2 (moderate) obesity (35.0–39.9 kg/m2), and class 3 (severe) obesity (≥40.0 kg/m2).
We compared differences between referral PCOS, unselected PCOS, and unselected controls in the prevalence (with 95% confidence interval) of obesity and disease burden (determined by the PCOS subphenotype and degrees of hirsutism and hyperandrogenemia) and racial/ethnic make-up. Additionally, the prevalence of obesity in unselected PCOS was compared with the estimates in unselected controls and the entire unselected population. In addition, the prevalence of obesity in the unselected population was compared with estimates based on data for the state of Alabama (16, 17). Values for comparing proportions were computed with the χ2 test with Yates correction or Fisher's exact test as appropriate.
Continuous variables satisfying a normal distribution assumption were expressed as the mean ± SE and compared using ANOVA and Tukey for post hoc ANOVA test for multiple comparisons. Because the data distribution for the original scale SHGB, DHEAS, total T, and free T values was not symmetric, analyses were carried out after log-transforming the values. The geometric mean (ie, the antilog of the log scale mean) and the original scale range (ie, antilog of the log scale range) are depicted.
The significance level was set at 0.05 for all analyses, and all hypothesis tests were 2-sided. All statistical analyses were conducted using the Stats Direct statistics software package, version 2.7.8 2010 (Cheshire, United Kingdom).
Results
Of 960 women undergoing a pre-employment physical at UAB during the study periods of 1995 through 1996 and 1998 through 1999, 688 were eligible and agreed to participate (ie, considered the entire unselected population). Of these, 64 women (9.5%) had a high probability of having PCOS (WDR ≥0.85), and comprised the unselected PCOS cohort. In addition, the unselected population contained 41 subjects who had low probability (WDR of 0.05–0.35) of having PCOS and who were not studied further and 563 women who had no evidence of PCOS (WDR ∼0) and who comprised the unselected control cohort.
During this same time period, 292 consecutive patients were diagnosed as having PCOS in the clinical reproductive endocrinology practice, constituting the referral PCOS population. Table 1 displays the distribution of select demographics and characteristics of each of the 3 groups studied.
Table 1.
Comparison of Study Populations
| Referral PCOS | Unselected PCOS | Unselected Controls | |
|---|---|---|---|
| No. of total group | 292 | 64 | 563 |
| Age, y (mean ± SE) | 27.44 ± 0.44 | 27.43 ± 0.73 | 29.25 ± 0.30 |
| No. of non-HW (%) | 245.0 (83.9)a | 29.0 (45.3) | 254.0 (45.0) |
| No. of AA (%) | 44.0 (22.9)a | 34.0 (53.1) | 299.0 (52.9) |
| BMI, kg/m2 (mean ± SE) | 34.50 ± 0.55a | 27.58 ± 1.01 | 27.27 ± 0.31 |
| Prevalence of obesity, n (%) | 186.0 (63.7)a | 18.0 (28.1) | 160.0 (28.4) |
| Hirsutism (mFG) score (mean ± SE) | 8.01 ± 0.26a | 5.73 ± 0.52c | 1.09 ± 0.07 |
| Total T, ng/dLe | 77.9 (14.0–586.0)a | 59.5 (11.0–160.0)b | 44.5 (4.0–123.0) |
| Free T, ng/dLe | 0.83 (0.10–4.09)a | 0.46 (0.05–1.91)b | 0.29 (0.02–1.15) |
| DHEAS, ng/mLe | 1948 (92–6692)a | 1726 (231–5473)b | 897 (70–9460) |
| SHBG, nmol/Le | 164 (100–670)a | 233 (70–820)c | 291 (100–5800) |
| Hirsutism (mFG ≥6), % | 72.3d | 65.6b | 2.0 |
| Total T, % | 39.0a | 18.0b | 2.5 |
| Free T, % | 64.0a | 24.1b | 5.1 |
| DHEAS, % | 35.7d | 34.4b | 2.6 |
| PCOS subphenotypes, n (%) | |||
| Hirsutism+Oligo only | 58.0 (19.9)a | 29.0 (45.3) | NA |
| HA+Oligo only | 80.0 (27.4) | 22.0 (34.4) | NA |
| Hirsutism+HA+Oligo only | 154.0 (52.7)a | 13.0 (20.3) | NA |
Abbreviation: NA, not available.
P < .0001 (referral different from all others).
P < .0001 (unselected PCOS different from unselected controls).
P < .001 (unselected PCOS different from unselected controls).
P < .0001 (referral PCOS different from unselected controls only).
Geometric mean, the antilog of the log scale mean, is reported for log-transformed data.
Differences in demographics
Mean age was similar between the referral and unselected PCOS cohorts or unselected controls. However, the mean BMI was significantly higher in referral PCOS subjects compared with the 2 unselected cohorts. Most the patients among the referred PCOS (83.90%) were non-Hispanic White (non-HW), whereas slightly more than half of unselected PCOS (53.1%) or unselected controls (52.9%) were African-American (AA), a significant difference (P < .0001).
Differences in the degree of hyperandrogenism
Referral PCOS subjects had a greater mean mFG score and total T, free T, and DHEAS serum levels than unselected PCOS women or unselected controls (Table 1). The percentage of women with hirsutism (ie, an mFG score ≥6) was similar between the referral PCOS (73.2%) and unselected PCOS (65.5%) subjects and higher than in unselected controls (2%). Alternatively, the proportion of subjects demonstrating elevated total and free T was highest in referral PCOS, followed by unselected PCOS, with both higher than unselected controls. The proportion of women with elevated DHEAS was similar in the referral and unselected PCOS cohorts (∼35%) and significantly higher than in unselected controls (Table 1).
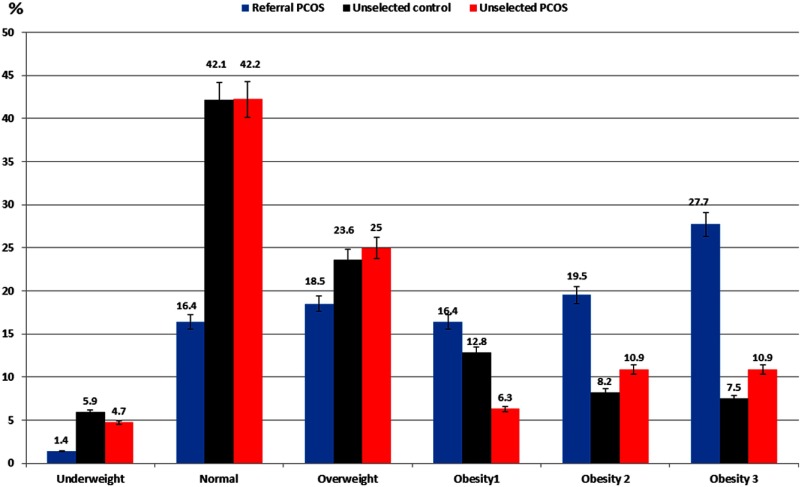
Differences in the prevalence of obesity
The distribution of study population according to BMI categories is depicted in Table 2 and Figure 1. The proportion of underweight was lower in referral PCOS than in unselected controls, and normal-weight subjects were more common in both unselected PCOS and unselected controls than in referral PCOS, whereas the prevalence of overweight subjects was similar among the 3 study groups. However, the prevalence of obesity overall (and the related odds ratio relative to controls) was higher among referral PCOS than unselected PCOS or controls.
Table 2.
Observed Distribution (Percent) of Referral PCOS, Unselected PCOS, and Unselected Controls by BMI Categories
| BMI Categories | Referral PCOS (n = 292), n (%) | Unselected PCOS (n = 64), n (%) | Unselected Controls (n = 563), n (%) | ORs (95% CI) Comparing Referral PCOS With Unselected PCOS | ORs (95% CI) Comparing Referral PCOS With Unselected Controls | ORs (95% CI) Comparing Unselected PCOS With Unselected Controls |
|---|---|---|---|---|---|---|
| Underweight (≤18.9 kg/m2) | 4.0 (1.4)a | 3.0 (4.7) | 33.0 (5.9) | 0.28 (0.05–1.99) | 0.22a (0.06–0.64) | 0.79 (0.15–2.64) |
| Normal (19.0–24.9 kg/m2) | 48.0 (16.4)b | 27.0 (42.2) | 237.0 (42.1) | 0.27b (0.15–0.51) | 0.27b (0.19–0.39) | 1 (0.57–1.75) |
| Overweight (25.0–29.9 kg/m2) | 54.0 (18.5) | 16 (25) | 133.0 (23.6) | 0.68 (0.35–1.39) | 0.74 (0.51–1.04) | 0.68 (0.35–1.35) |
| Obese (≥30.0 kg/m2) | 186.0 (63.7)b | 18.0 (28.1) | 160.0 (28.4) | 2.95b (1.02–11.68) | 4.41b (3.24–6.04) | 0.99 (0.52–1.80) |
| Obesity by class | ||||||
| Class I obesity (30.0–34.9 kg/m2) | 48.0 (16.4) | 4.0 (6.3)c | 72.0 (12.8) | 1.97c (0.84–5.40) | 1.34 (0.88–2.03) | 1.05 (0.52–1.98) |
| Class II obesity (35.0–39.9 kg/m2) | 57.0 (19.5)b | 7.0 (10.9) | 46.0 (8.2) | 3.12 (1.34–8.44) | 2.72b (1.76–4.24) | 1.38 (0.50–3.27) |
| Class III obesity (≥40.0 kg/m2) | 81.0 (27.7)b,d | 7.0 (10.9) | 42.0 (7.5) | 4.47d (2.40–8.63) | 4.75b (3.12–7.32) | 1.52 (0.55–3.64) |
Abbreviations: CI, confidence interval; OR, odds ratio.
P < .001 (referral PCOS vs unselected control [underweight BMI]).
P < .0001 (referral PCOS vs unselected PCOS or unselected control).
P < .05 (referral PCOS vs unselected PCOS [mild obesity]).
P < .01 (referral PCOS vs unselected PCOS [severe obesity]).
Figure 1.
Distribution of referral PCOS, unselected PCOS, and unselected controls by BMI categories. A, Differences in percentage of women who were underweight, of normal BMI, and obese by obesity class and trends in obesity bias and prevalence of obesity. B, Prevalence of underweight in unselected controls and normal weight in unselected PCOS and controls was higher than the referral PCOS. C, More referral PCOS were obese by BMI categories than the 2 unselected groups. Only referral PCOS showed a trend in obesity prevalence and obesity bias. The I bars represent 95% confidence intervals or SD as appropriate. *, Significant difference.
When considering the subclasses of obesity, we observed that the prevalence of mild obesity (class I) was lowest in unselected PCOS subjects, whereas the prevalence of moderate (class II) obesity was highest in referral PCOS relative to unselected controls, and the prevalence of severe (class III) obesity was higher in referral PCOS than unselected PCOS or controls (Table 1 and Figure 1).
Among referral PCOS women, the prevalence of obesity class by severity increased progressively from 16.4% to 19.5% to 27.7%, for mild, moderate, and severe obesity respectively (χ2 = 11.15; P = .0008); alternatively, there was little change in overall prevalence with increasing obesity class in unselected PCOS or unselected controls.
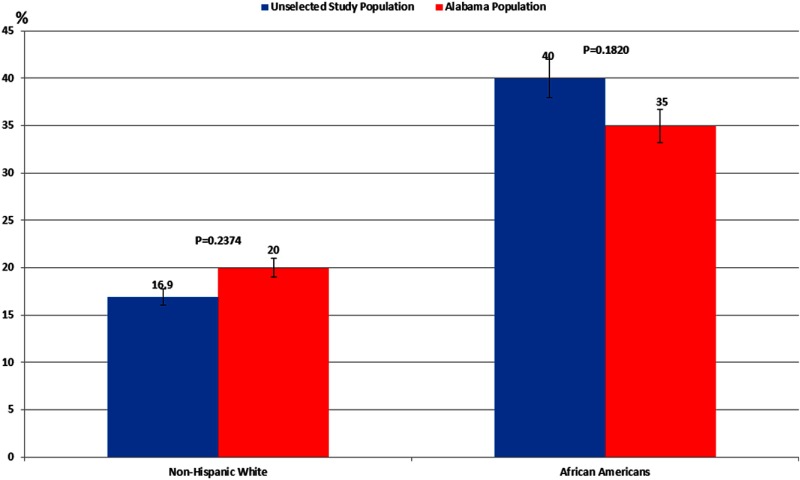
Overall, we found that the race-specific prevalence of obesity in our entire unselected population (n = 668) was similar to the estimates in the State of Alabama general female population (16.9% vs 20.0% for non-HW and 40.2% vs 35.0% AA; Figure 2), based on Alabama's Behavioral Risk Factor Surveillance System (16, 17). The prevalence of obesity in referral PCOS women was 2.2-fold greater (P < .0001) than estimates for the same in the overall unselected population and greater (3.7-fold for non-HW and 1.8-fold for AA) than the estimates for the State of Alabama general female population (Figure 2 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Alternatively, the prevalence of obesity in unselected PCOS women and unselected controls was similar to the prevalence in the overall unselected population and for the general adult female population in the State of Alabama, regardless of race (Supplemental Table 1).
Figure 2.
Race-specific prevalence of obesity (percent) in the female general population. Data for the State of Alabama was based on Alabama's Behavioral Risk Factor Surveillance System, a survey of adults ≥18 years old (16, 17). Unselected study population refers to overall unselected population from which unselected PCOS and unselected control cohorts were derived. Race-specific prevalence of obesity in unselected population is similar to the estimates in the State of Alabama general female population aged ≥18 years.
Differences in the prevalence and features of the PCOS subphenotypes
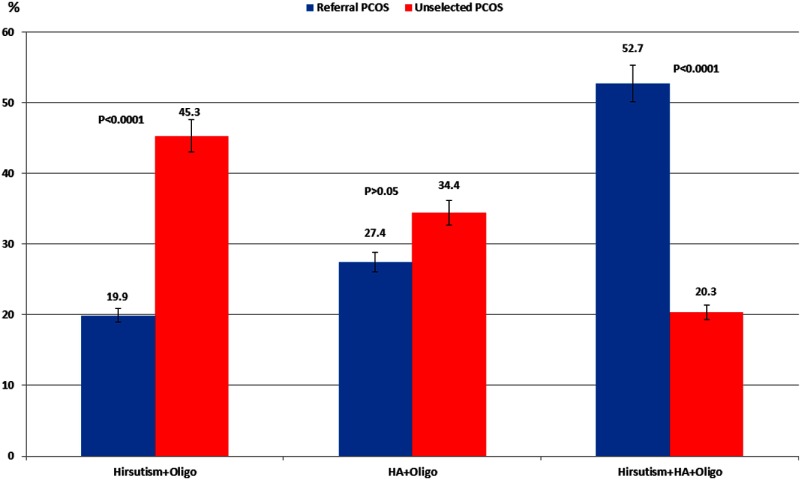
The characteristics of the 3 PCOS subphenotypes based on the NIH 1990 definition of PCOS (9, 18), ie, oligo-ovulation and hirsutism only (Oligo+Hirsutism), oligo-ovulation and hyperandrogenemia without hirsutism (Oligo+HA), and oligo-ovulation, hyperandrogenemia, and hirsutism (Oligo+Hirsutism+HA), in our referral and unselected PCOS subjects are depicted in Figure 3 and Table 3.
Figure 3.
The distribution of referral PCOS and unselected PCOS according to severity of PCOS subphenotypes by 1990 NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development criteria. A higher proportion of referral PCOS women presented with the Oligo+Hirsutism+HA subphenotype, whereas a higher percentage of unselected PCOS demonstrated the Oligo+Hirsutism subphenotype.
Table 3.
Characteristics of Phenotypes of PCOS Based on NIH Criteria in Referral and Unselected PCOSa
| Referral PCOS (n = 292) |
Unselected PCOS (n = 64) |
|||||
|---|---|---|---|---|---|---|
| Hirsutism+Oligo | HA+Oligo | Hirsutism+HA+Oligo | Hirsutism+Oligo | HA+Oligo | Hirsutism+HA+Oligo | |
| No. of total group (%) | 58.0 (19.9) | 80.0 (27.4) | 154.0 (52.7) | 29.0 (45.3) | 22 (34.4) | 13 (20.3) |
| Age, y (mean ± SE) | 30.1 ± 1.0 | 27.9 ± 0.8 | 26.2 ± 1.0 | 29.3 ± 1.0 | 26.2 ± 1.1 | 25.5 ± 1.6 |
| No. of non-NHW (%) | 49.0 (84.5) | 71.0 (88.8) | 125.0 (81.2) | 13.0 (44.8) | 13 (59.1) | 3 (23.1) |
| No. of AA (%) | 8 (13.8) | 9.0 (11.3) | 27.0 (17.5) | 16.0 (55.2) | 9 (40.9) | 9 (69.2) |
| BMI, kg/m2 (mean ± SE) | 34.8 ± 1.3e | 33.5 ± 1.1d | 34.9 ± 0.7 | 26.9 ± 1.5 | 26.9 ± 1.8 | 30.2 ± 2.3 |
| No. with obesity (%) | 36.0 (62.1)d | 48.0 (60.0)c | 102.0 (66.2)e | 7.0 (24.1) | 6 (27.3) | 5 (38.5) |
| mFG score (mean ± SE) | 9.5 ± 0.4c | 3.1 ± 0.2d | 10.0 ± 0.3e | 7.9 ± 0.4 | 1.0 ± 0.3 | 8.9 ± 1.0 |
| Total T, ng/dLf | 53.6 (16.0–83.0) | 85.6 (27.0–219.0) | 85.4 (14.0–586.0)b | 46.8 (11.0–74.0) | 75.7 (38.0–160.0) | 64.1 (29.0–160) |
| Free T, ng/dLf | 0.53 (0.10–0.75)e | 0.89 (0.30–1.75) | 0.95 (0.24–4.09)d | 0.28 (0.05–0.74) | 0.70 (0.16–1.80) | 0.63 (0.25–1.91) |
| DHEAS, ng/mLf | 1400 (234–2382) | 2051 (474–5189) | 2149c (92–6692) | 1222 (231–2366) | 1864 (786–3560 | 3019 (1145–5473) |
| SHBG, nmol/Lf | 174e (100–670) | 166 (100–310) | 159 (100–430) | 308 (100–820) | 188 (70–540) | 183 (120–300) |
| Total T, n (%) | 0.0 | 34.0 (42.5) | 60.0 (38.7) | 0.0 | 7/22 (31.8) | 4/13 (30.8) |
| Free T, n (%) | 0.0 | 70.0 (87.5)d | 132.0 (85.2)e | 0.0 | 13/22 (59.1) | 4/13 (30.8) |
| DHEAS, n (%) | 0.0 | 23.0 (28.8) | 60.0 (38.7) | 0.0 | 5/22 (22.7) | 8/13 (61.5) |
P values reflect the comparison of referral PCOS to unselected PCOS across analogous phenotypes.
P < .05.
P < .01.
P < .001.
P < .0001.
Geometric mean, the antilog of the log scale mean, is reported for log-transformed data.
PCOS women presenting with each of these 3 subphenotypes did not differ in mean BMI, prevalence of obesity, and racial composition in either the referral or unselected PCOS cohorts. There were also no differences in mean age, with the exception that in the referral PCOS cohort, women with the Hirsutism+Oligo phenotype were older at the time of their initial presentation than those with Hirsutism+HA+Oligo (30.1 vs 26.1 years, respectively, P = .0016), although there were no significant differences in age between analogous subphenotypes in referral PCOS vs unselected PCOS cohorts. Alternatively, PCOS women with the Oligo+Hirsutism+HA subphenotype had greater mean mFG scores and higher circulating androgens levels than the other two subphenotypes. Not unexpectedly, in both PCOS cohorts, women with Oligo+Hirsutism had a greater mean mFG score than those with Oligo+HA, who in turn demonstrated greater circulating androgen levels than those with Oligo+Hirsutism.
Differences in the distribution of subjects by subphenotypes between referral and unselected PCOS are depicted in Figure 3. Most referral PCOS women presented with the Oligo+Hirsutism+HA subphenotype (52.7%), whereas the majority of unselected PCOS had the Oligo+Hirsutism subphenotype (45.3%). A higher proportion of the referral PCOS cohort had the more severe subphenotype Oligo+Hirsutism+HA compared with unselected PCOS (52.7% vs 20.3%, P < .0001, respectively), whereas a higher percentage of unselected PCOS had Oligo+Hirsutism (45.3% vs 19.9%, P < .0001, respectively). The prevalence of obesity and mean BMI were greater in referral than unselected PCOS across analogous subphenotypes (except for Oligo+Hirsutism+HA where the difference in BMI did not reach significance, P < .0684). Referral PCOS women also had greater mean circulating androgens and mFG scores than unselected PCOS subjects among all 3 subphenotypes, including those manifesting Oligo+HA (only mFG score was higher), Oligo+Hirsutism (both mFG score and free T levels were higher), and Oligo+HA+Hirsutism (where mFG score, total T, free T, and DHEAS were higher) than the estimates for analogous subphenotypes in unselected PCOS. The prevalence of hyperandrogenemia was greater in referral PCOS than unselected PCOS in those presenting with the HA+Oligo (free T, P = .005) and Hirsutism+HA+Oligo (free T and DHEAS, P < .0007 and P < .0135, respectively) subphenotypes.
Effects of race on the phenotype and features of PCOS cohorts and controls
Supplemental Table 1 depicts demographic, hyperandrogenism, and prevalence of obesity profiles by race (AA and non-HW subjects). Mean age was similar between AA and non-HW subjects, both across and within diagnostic categories. Mean circulating androgen levels, mFG scores, and prevalence of obesity and hyperandrogenemia were greater, whereas SHBG levels were lower in referral PCOS than unselected PCOS or unselected controls, independent of race.
Alternatively, the prevalence of obesity, mean BMI, androgen levels (free T, total T or DHEAS), and SHBG levels were similar in PCOS identified in AA and non-HW women, whether referral or unselected PCOS. Alternatively, the mean SHBG was lower, whereas the mean BMI, circulating free T levels, and prevalence of obesity were greater in AA than in non-HW in unselected controls. No racial/ethnic differences in mean circulating total T and DHEAS levels were observed in unselected controls.
Mean mFG score was greater in AA than non-HW women in referral PCOS (P = .015), unselected PCOS (P = .0276), and unselected controls (P = .024), although the proportion of women with hirsutism and hyperandrogenemia did not differ between AA and non-HW, within each diagnostic category. See[b] Supplemental Results for additional discussion of differences in obesity by race between referral and unselected PCOS, unselected controls, the total unselected population, and the general population in the State of Alabama at the time.
Discussion
Our data indicate that PCOS patients who are seen in the clinical setting have a greater mean BMI, hirsutism score, and circulating androgen levels, are more likely to demonstrate obesity and be non-Hispanic White, and demonstrate a more severe PCOS phenotype than PCOS women detected in the general unbiased population. Our data also suggest that the prevalence of obesity in general and of severe obesity in particular, in PCOS women detected in the medically unbiased population of women seeking a pre-employment physical, is similar to that observed in unselected controls, in the overall unselected cohort, and in the population from which they were derived. Alternatively, PCOS women in a clinical (referred) population had a prevalence of obesity and severe obesity ranging from 2.2-. to 3.8-fold that of unselected PCOS or unselected controls.
PCOS is a heterogeneous disease characterized by different clinical subphenotypes. Evidence suggests that subjects manifesting the Oligo+Hirsutism+HA subphenotype demonstrate greatest degrees of insulin resistance, hyperinsulinemia, chronic inflammation, dyslipidemia, and hyperandrogenism, whereas those manifesting only Oligo+Hirsutism (without hyperandrogenemia) demonstrate lesser morbidity (18–20). In our study, a majority of PCOS subjects diagnosed in the clinical setting manifested the more complete, and morbid, subphenotype (Oligo+Hirsutism+HA), whereas most PCOS from the unselected cohort demonstrated the Oligo+Hirsutism subphenotype. Because in this study we did not routinely perform transvaginal ultrasonography, we are unable to comment on the prevalence of those subphenotypes determined solely by the presence of polycystic ovarian morphology. However, the results of other studies determining the prevalence of the PCOS subphenotypes and including transvaginal ultrasonography indicate that 25% to 70% of PCOS patients diagnosed in the clinical setting (ie, referred) present with the complete presentation (Oligo+HA/Hirsutism+polycystic ovarian morphology) (3, 21–33), whereas PCOS women detected in unbiased populations presented with this subphenotype only 10% to 25% of the time (34–36).
Hyperandrogenism has been identified as an important risk factor for dyslipidemia, metabolic syndrome, and cardiovascular disease in both pre- and postmenopausal women (19, 20, 37). Because our referral PCOS women had greater degrees of hyperandrogenism than unselected PCOS subjects, it would be logical to assume they carry a higher disease risk. Although mean mFG scores were higher in referral PCOS subjects, because the presence of hirsutism is defined by a single cutoff (mFG ≥6), the difference in the prevalence of hirsutism between the two groups is less pronounced (72% vs 66% in referral vs unselected PCOS women, respectively, a difference not reaching statistical significance).
The differences in the phenotype of women with PCOS depending on whether they were diagnosed in the clinical setting or detected in an unselected population may be due to several reasons. First, as various quality-of-life studies have demonstrated, obesity and the severity of hirsutism are the complaints most likely to detrimentally affect quality of life (38) and potentially are the symptoms most likely to drive women to seek medical care. Furthermore, obesity may exacerbate the other clinical features of PCOS, including menstrual irregularities (39), which makes them more likely to seek medical care. Second, because access to medical care in the United States is greater for non-HW, who also are more likely to see themselves as overweight or obese than minority women (40, 41), it is not surprising that the former race/ethnicity predominates in the population of PCOS women diagnosed in the clinical setting in contrast to those women detected in an unbiased population.
The present study has several potential limitations. First, the use of an unselected population based on those seeking pre-employment physical examination might not have captured some PCOS subjects who were unable to seek employment due to depression or other issues associated with the disorder. We should also note that all currently used criteria for PCOS (Rotterdam 2003 and Androgen Excess & PCOS Society 2006) are simply extensions of the NIH 1990 criteria. In addition, the NIH 1990 criteria are considered to be the strictest and less ambiguous criteria for PCOS and represent the criteria denoting the group of PCOS subjects at highest risk for metabolic dysfunction.
In conclusion, our data provide strong evidence of referral bias in the PCOS phenotype, primarily driven by obesity and the severity of disease burden. Furthermore, as for other disorders, there appears to be significant racial/ethnic disparity in accessing or seeking medical care among women with PCOS. Women with PCOS seeking clinical care (self or otherwise referred for evaluation) are generally more severely affected, more obese, and more likely to be non-HW. Because obesity and the degree of hyperandrogenism are important risk factors for metabolic and cardiovascular dysfunction in pre- and postmenopausal women (19, 20, 37), it is also likely that PCOS women diagnosed in the clinical setting have a higher risk for these morbidities than patients detected in the general population. More striking, the overall prevalence and degree of obesity among women with PCOS who were detected through the study of an unbiased population was not different from that of the general population in which the subjects are found, suggesting that the relationship between PCOS and obesity has been exaggerated by the study of populations of PCOS subjects primarily recruited from the clinical setting. The results of our study have important public health implications and suggest that a more accurate picture of the PCOS phenotype may arise from the study of PCOS women detected through the screening of unselected or minimally biased populations.
Supplementary Material
Acknowledgments
This work was supported by Grants 1-K24-HD01346, R01-DK073632, and R01-HD29364 from the National Institutes of Health and an endowment from the Helping Hand of Los Angeles, Inc.
Results from this work were presented in part at the 92nd Annual Meeting of The Endocrine Society, San Diego, California, 2010.
Disclosure Summary: U.E., B.O.Y., and R.A. have nothing to declare.
Footnotes
- A4
- androstenedione
- AA
- African-American
- BMI
- body mass index
- DHEAS
- dehydroepiandrosterone sulfate
- HA
- hyperandrogenemia
- mFG
- modified Ferriman-Gallwey
- non-HW
- non-Hispanic White
- 17-OHP
- 17-hydroxyprogesterone
- P4
- progesterone
- PCOS
- polycystic ovary syndrome
- PRL
- prolactin
- WDR
- weighted diagnostic score.
References
- 1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 2. Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488 [DOI] [PubMed] [Google Scholar]
- 3. Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf). 2007;67:735–742 [DOI] [PubMed] [Google Scholar]
- 4. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–4658 [DOI] [PubMed] [Google Scholar]
- 5. Stein I, Leventhal M. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181–185 [Google Scholar]
- 6. Glueck CJ, Dharashivkar S, Wang P, et al. Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol. 2005;122:206–212 [DOI] [PubMed] [Google Scholar]
- 7. Azziz R, Sanchez LA, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462 [DOI] [PubMed] [Google Scholar]
- 8. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082 [DOI] [PubMed] [Google Scholar]
- 9. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome. In: Dunaif A, Givens J, Haseltine F, Merriam GR, eds. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific Publications; 1992:377–384 [Google Scholar]
- 10. Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boots LR, Potter S, Potter D, Azziz R. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril. 1998;69:286–292 [DOI] [PubMed] [Google Scholar]
- 13. Azziz R, Bradley EL, Jr, Potter HD, Parker CR, Jr, Boots LR. Chronic hyperinsulinemia and the adrenal androgen response to acute corticotropin-(1–24) stimulation in hyperandrogenic women. Am J Obstet Gynecol. 1995;172:1251–1256 [DOI] [PubMed] [Google Scholar]
- 14. Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: a prospective study. Fertil Steril. 1999;72:915–925 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization 1997 Obesity: preventing and managing the global epidemic. Report of a WHO Consultation presented at the World Health Organization; June 3–5 Geneva, Switzerland: World Health Organization; 1997 [PubMed] [Google Scholar]
- 16. Alabama Obesity Task Force Strategic Plan for the Prevention and Control of Overweight and Obesity in Alabama. Montgomery, AL: Alabama Department of Public Health; 2005. Available at: http://adph.org/obesity/assets/ObesityPlan.pdf [Google Scholar]
- 17. Hataway H, Rees S, Chapman K. Obesity and Overweight in Alabama. Montgomery, AL: Alabama Center for Health Statistics; 2003. Available at: http://adph.org/administration/obesityfactsheet.pdf [Google Scholar]
- 18. Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–1723 [DOI] [PubMed] [Google Scholar]
- 19. Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril. 2009;92:626–634 [DOI] [PubMed] [Google Scholar]
- 20. Alemzadeh R, Kichler J, Calhoun M. Spectrum of metabolic dysfunction in relationship with hyperandrogenemia in obese adolescent girls with polycystic ovary syndrome. Eur J Endocrinol. 2010;162:1093–1099 [DOI] [PubMed] [Google Scholar]
- 21. Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927 [DOI] [PubMed] [Google Scholar]
- 22. Welt CK, Gudmundsson JA, Arason G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848 [DOI] [PubMed] [Google Scholar]
- 23. Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88:1389–1395 [DOI] [PubMed] [Google Scholar]
- 24. Hsu MI, Liou TH, Chou SY, Chang CY, Hsu CS. Diagnostic criteria for polycystic ovary syndrome in Taiwanese Chinese women: comparison between Rotterdam 2003 and NIH 1990. Fertil Steril. 2007;88:727–729 [DOI] [PubMed] [Google Scholar]
- 25. Chae SJ, Kim JJ, Choi YM, et al. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod. 2008;23:1924–1931 [DOI] [PubMed] [Google Scholar]
- 26. Zhang HY, Zhu FF, Xiong J, Shi XB, Fu SX. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large-scale Chinese population. BJOG. 2009;116:1633–1639 [DOI] [PubMed] [Google Scholar]
- 27. Guastella E, Longo RA, Carmina E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94:2197–2201 [DOI] [PubMed] [Google Scholar]
- 28. Guo M, Chen ZJ, Macklon NS, et al. Cardiovascular and metabolic characteristics of infertile Chinese women with PCOS diagnosed according to the Rotterdam consensus criteria. Reprod Biomed Online. 2010;21:572–580 [DOI] [PubMed] [Google Scholar]
- 29. Kavardzhikova S, Pechlivanov B. [Clinical, hormonal and metabolic characteristics of different phenotypes of polycystic ovary syndrome, in Bulgarian population. Akush Ginekol (Sofiia). 2010;49:32–37 [PubMed] [Google Scholar]
- 30. Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females]. J Res Med Sci. 2011;16:763–769 (Bulgarian) [PMC free article] [PubMed] [Google Scholar]
- 31. Melo AS, Vieira CS, Romano LG, Ferriani RA, Navarro PA. The frequency of metabolic syndrome is higher among PCOS Brazilian women with menstrual irregularity plus hyperandrogenism. Reprod Sci. 2011;18:1230–1236 [DOI] [PubMed] [Google Scholar]
- 32. Cupisti S, Haeberle L, Schell C, et al. The different phenotypes of polycystic ovary syndrome: no advantages for identifying women with aggravated insulin resistance or impaired lipids. Exp Clin Endocrinol Diabetes. 2011;119:502–508 [DOI] [PubMed] [Google Scholar]
- 33. Yilmaz M, Isaoglu U, Delibas IB, Kadanali S. Anthropometric, clinical and laboratory comparison of four phenotypes of polycystic ovary syndrome based on Rotterdam criteria. Obstet Gynaecol Res. 2011;37:1020–1026 [DOI] [PubMed] [Google Scholar]
- 34. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551 [DOI] [PubMed] [Google Scholar]
- 35. Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27:3067–3073 [DOI] [PubMed] [Google Scholar]
- 37. Shaw LJ, Bairey Merz CN, Azziz R, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health–National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2008;14:15–25 [DOI] [PubMed] [Google Scholar]
- 39. Kiddy DS, Sharp PS, White DM, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf). 1990;32:213–220 [DOI] [PubMed] [Google Scholar]
- 40. Health Care for Minority Women: Recent Findings. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.ahrq.gov/qual/nhqrdr11/nhqrdrminority11.pdf [Google Scholar]
- 41. Paeratakul S, White MA, Williamson DA, Ryan DH, Bray GA. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obes Res. 2002;10:345–350 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.