Abstract
Background and Aims:
The cellular mechanisms responsible for initiating or limiting the tumors including skin types are of great importance. The p53 is a tumor-inhibiting gene which is believed to be defective in many malignant situations. Ki67 is a non-histonic protein which is mainly interfere with the proliferation and has many controlling effects during the cell cycle. Because of their importance in skin tumor cell growth, this study aimed at evaluating the p53 and Ki67 expression in skin epithelial tumors by immunohistochemical method.
Materials and Methods:
In a descriptive setting, 50 biopsy samples (30 basal cell carcinomas (BCCs), 10 squamous cell carcinomas (SCCs), 8 keratoacanthomas (KAs), and 2 trichoepitheliomas (TEs)) were immunohistochemically evaluated for p53 and Ki67 expression during a 14-month period. The incidence and expression rate of these two variables were separately reported in each group of samples.
Results:
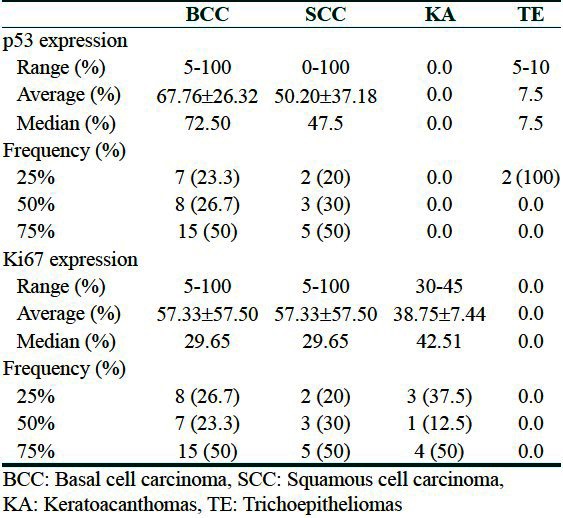
The expression rate of p53 was 67.77% for the BCCs, 50.20% for the SCCs, and null for the KAs. For both TEs, it was 50%. The expression rate of Ki67 was 57.33% for the BCCs, 47.70% for the SCCs, 37.5% for the KAs, and 0.0% for TEs. The incidence of P53+ cells was 100% and 90% in the BCC and SCC samples, respectively. The both TEs were positive in this regard. The incidence of Ki67+ cells was 100% for the BCC, SCC, and KA samples. The both TEs were negative in this regard.
Conclusion:
This study showed that the incidence rate of p53- and Ki67-positive cells is very high in skin malignant epithelial tumors. The expression rate of these two variables is comparable with reports in the literature. Further studies with large sample size are recommended to be carried out for KA and TE samples.
Keywords: Epithelial tumors, tumor marker, p53, Ki67
Introduction
What was known?
1. The p53 is a tumor-suppressor gene and its mutation has been implicated in the genesis of skin cancers.
2. The expression of proliferation marker, Ki67, is also increased in sundamaged skin.
Basal cell carcinoma (BCC) is the most common malignancy in humans. Although rarely metastatic, it is capable of significant local destruction and disfigurement.[1–5] Incidence rates have been reported to be between 20 and 300/1,000,000 in Europe and Northern America.[6]
Squamous cell carcinoma (SCC) is the second most frequent skin cancer and occurs most frequently in the sun-exposed regions of the skin and in immunocompromised patients. Approximately, 250,000 patients per year develop skin SCC in the USA.[7] SCC harbors significant risk of metastasis that can eventually lead to death.[8,9]
Keratoacanthoma (KA) is a common cutaneous neoplasm.[10] KA and well-differentiated SCC are two cutaneous neoplasms that most often occur in sun-exposed sites of light-skinned persons. It is often difficult to distinguish these two from each other either clinically or histologically. Earlier, complete excision is the treatment of choice for all skin neoplasms thought to be KA.[11]
Accurate histopathological distinction between trichoepithelioma (TE) and BCC may be challenging. A constellation of histopathological criteria may help to discriminate problematic examples of TE from BCC.[12]
Some factors have related with biological behavior of these tumors, including histopathological subtype, degree of differentiation, depth of invasion, invasion and some biological markers that their prognostic importance has been recently described.[6,13–15] Some of the biological markers that we investigated in this study were p53 and Ki67.
The p53 is a tumor-suppressor gene and its mutation has been implicated in the genesis of malignant neoplasms, including skin cancers. The importance of p53 mutation in the development of ultraviolet (UV)-induced skin tumors such as BCC is well known. The p53 overexpression has been reported in 42-92% of BCC cases in various studies. Although a positive correlation between clinicopathological aggressiveness and p53 immunoreactivity has usually been suggested in the literature, some authors have reported contradictory results.[6]
Inactivation of the p53 pathway that controls cell cycle progression, apoptosis, and senescence, has been proposed to occur in virtually all human tumors and p53 is the protein most frequently mutated in human cancer.[16] The p53 is a transcription factor that can bind to promoter regions of hundreds of genes where it either activates or suppresses gene expression.[17] Thereby, p53 serves as a tumor suppressor by inducing cell cycle arrest, apoptosis, and DNA repair.[18] During DNA damages and other stresses, the amount of p53 is increased due to disruption of its degradation.[16,19,20] Notably, inactivation of p53 is one of the characteristics of cancer. Indeed, p53 is found mutated in approximately half of all human tumors [Figure 1].[16]
Figure 1.
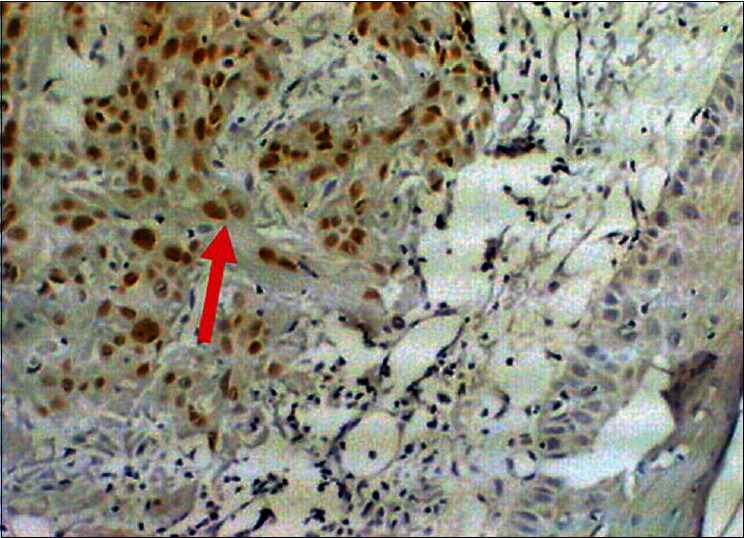
Squamous cell carcinoma of skin, Immunostained for p53, nuclear positivity (arrow), 10×40
Ki67 antigen is a high molecular weight non-histone protein and the most reliable marker of proliferating cells. Ki67 expression is seen in G1, S, G2, and M phases of the cell cycle except for G0 phase. The proliferation index determined by Ki67 immunostaining has a prognostic value in certain types of cancers.[6] The expression of proliferation marker, Ki67, is also increased in sun-damaged skin [Figure 2].[21]
Figure 2.
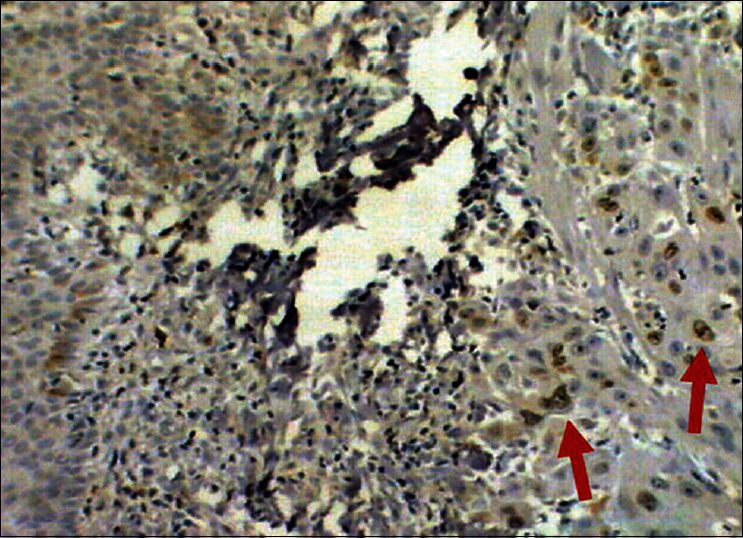
Squamous cell carcinoma of skin, Immunostained for Ki67, nuclear positivity (arrows), 10×40
Distinction between benign and malignant melanocytic lesions may be difficult by today's methods, even for highly skilled dermatopathologists, emphasizing the need for improved diagnostic tools.[22] Early diagnosis is essential to improve the management of patients with aggressive types of skin cancers. There is no single specific marker available for distinguishing aggressive skin cancers from nonaggressive ones.[4]
To gain insight into the molecular pathogenesis of epithelial skin cancer, we examined the immunohistochemical expression of p53 and Ki67 proteins in BCCs, SCCs, KAs, and TEs in Iranian patients.
Materials and Methods
This descriptive study was performed on skin biopsies of patients with skin epithelial tumors, presenting to Dermatology clinic of Tabriz Sina Hospital from 2008 to 2010, where their biopsy samples were referred to pathology laboratory of Tabriz Imam Reza Hospital.
The skin lesion biopsy was performed by one expert dermatologist and the samples were sent to pathology laboratory for evaluation about Ki67 and p53 expression. In total, 30 BCC, 10 SCC, 8 KA, and 2 TE samples were studied.
Tumor sections were mounted on glass slides and stained by avidin–biotin complex system for Ki67 and p53, and then the study was classified as staining method. The main antibodies against these markers had been used only on frozen skin samples. However, monoclonal antibodies for formalin-resistant epitopes, (MIB-1 and MIB-3 monoclonal antibodies), have application on paraffin blocks, and the aim of this study was these types.
After scoring of paraffinized sections using avidin–biotin method specific for SCC, BCC, and KA, and staining for Ki67 and p53 (MIB-1), the samples were classified according to the staining marks as follows:
Score 1: Weak staining (if any),
Score 2: <5%,
Score 3: 5-50%, and
Score 4: >50%.
All tissue samples were paraffin-embedded and formalin-fixed for immunohistochemistry (IHC) evaluation for p53 and Ki67. Then tissue cuts were prepared in 4 μm thick and placed on glassy slides. The used glasses were special silanized slides. Then the samples were deparaffinized and put into buffer solution for 20 min at 95-99°C and then were cooled in room temperature for 20 min. The method used was Dako Cytomation EnVision + System - horseradish peroxidase (HRP) - which is a two-level IHC staining method.
Then ready-to-use Anti-p53 and Anti-Ki67 solutions were applied for staining the prepared tissue samples by special IHC methods. The endogenous peroxidase activity was inhibited by incubation of slices inside peroxidase inhibitor for 10 min. The slices were incubated inside primary diluted antibody for 30 min and then on labeled polymers for another 30 min. After staining, the slices were put in 3,3-diaminobenzidine (DAB) + substrate chromogen which lead to detection of antigen with brown color. Then, the stained samples were reviewed under light microscope and labeled cells were counted in both pathological and normal tissues for 100 cells in microscopic field. The rate of positivity of tissues according to p53 and Ki67 was registered as the percent of labeled cells. Then, the scoring was performed.
IHC staining (by Envision method) in each of these sections was performed in following steps:
Deparaffinization: Putting the sample in the Four (dry heat sterilizer) at 60°C for 1 h.
Transparent rehydration: Using xylol, 100% and 96% of ethanol, and distilled water.
Inactivation of endogenous peroxidase: Using 3% hydrogen peroxide and ethanol.
Rinse with Tris-buffered saline (TBS).
Incubation of slides with primary antibodies against p53 and Ki67 (DAKO, Denmark) at room temperature for 1 h.
Washing with TBS for 5 min.
Adding envision solution for 30 min.
Washing with TBS for 5 min.
Adding substrate DAB (secondary antibody).
Washing with TBS for 5 min.
Counterstaining with hematoxylin (Gill's hematoxylin).
After all these steps, slides were examined by light microscopy. In a field with high magnification (×400), all cells were counted. The number of stained cells was counted and the percentage was calculated.
The types of applied antibodies were as follows:
p53: Monoclonal mouse Anti-human P53 protein; Clone DO-7; N1581; 10041283; 11 ml.
Ki67: FLEX Monoclonal Mouse Anti-Human; Clone MIB-1; Autostainer plus; IS626; 00074527; 6 ml.
These ready-to-use antibodies did not need dilution. Written informed consent was obtained from all patients prior to any of these measures and the study was performed in adherence to the Declaration of Helsinki Principles. The study protocol was accepted by the Ethical Committee of Tabriz University of Medical Sciences. The obtained results were analyzed and then compared with standard values in references.
Statistical analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS) Software, (SPSS Version 16, Chicago, USA). Comparison of the mean numbers of p53 and Ki67 was performed using Student's t-test. P values less than 0.05 were considered as statistically significant.
Results
The study group included 50 patients with epithelial skin tumors including 30 BCCs (60%), 10 SCCs (20%), 8 KAs (16%), and 2 TEs (4%). Table 1 shows the findings of immunohistochemical staining of BCC, SCC, KA, and TE samples for p53 protein and Ki67 antigen.
Table 1.
Immunohistochemical staining of basal cell carcinomas, squamous cell carcinoma, keratoacanthomas, and trichoepitheliomas samples for p53 protein and Ki67 antigen
The occurrence of p53 protein in BCC samples were as follows: 5% (one case), 25% (two cases), 30% (two cases), 45% (one case), 46% (one case), 55% (two cases), 60% (one case), 65% (two cases), 67% (one case), 70% (two cases), 75% (two cases), 80% (four cases), 85% (one case), 90% (two cases), 95% (one case), and 100% (five cases) [Diagram 1].
Diagram 1.
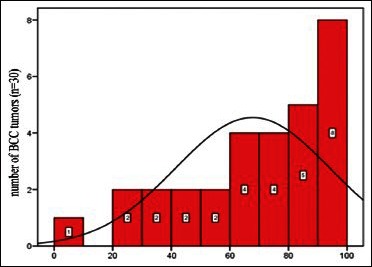
Abundance distribution of p53 protein in basal cell carcinomas
The occurrence of Ki67 antigen in BCC samples were as follows: 5% (one case), 10% (two cases), 15% (one case), 20% (one case), 25% (one case), 30% (two cases), 40% (one case), 45% (one case), 65% (one case), 70% (three cases), 75% (two cases), 80% (three cases), 85% (one case), 90% (one case), and 100% (four cases) [Diagram 2].
Diagram 2.
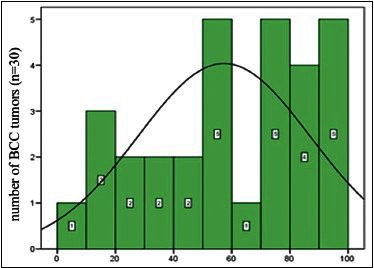
Abundance distribution of Ki67 antigen in basal cell carcinomas
The occurrence of p53 protein in SCC samples were as follows: 0% (one case), 25% (two cases), 37% (two cases), 45% (one case), 50% (one case), 55% (two cases), 90% (one case), and 100% (two cases) [Diagram 3].
Diagram 3.
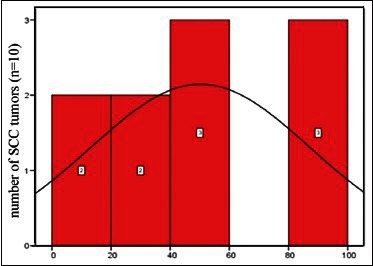
Abundance distribution of p53 protein in squamous cell carcinomas
The occurrence of Ki67 antigen in SCC samples were as follows: 15% (one case), 20% (one case), 25% (one case), 37% (one case), 39% (one case), 30% (one case), 48% (one case), 66% (one case), 70% (one case), 73% (one case), and 84% (one case) [Diagram 4].
Diagram 4.
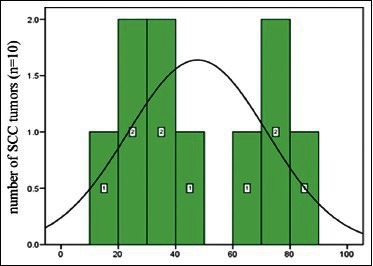
Abundance distribution of Ki67 antigen in squamous cell carcinomas
The occurrence of p53 protein in KA samples was 0.0%. The occurrence of Ki67 antigen in KA samples were as follows: 30% (three cases), 40% (one case), and 45% (four cases).
The occurrence of p53 protein in TE samples were as follows: 5% (one case) and 10% (one case). The occurrence of Ki67 antigen in TE samples was 0.0%.
The p53 immunoreactivity was observed in a total of 44 cases (88%); 30 BCCs (100%), 10 SCCs (100%); 0 KAs (0.0%) and two TEs (100%).
Ki67 immunoreactivity was observed in a total of 49 cases (98%); 30 BCCs (100%), 10 SCCs (100%); 8 KAs (100%) and 0 TEs (0.0%).
Discussion
It is believed that molecular events regulating cell survival, apoptosis, growth arrest, and differentiation play an important role in the development, progression, and regression of benign and malignant cell growth.[23–26]
Neoplastic disorders are characterized by uncontrolled cell growth. Activation of proto-oncogenes and inactivation of tumor-suppressor genes are critical molecular events that lead to neoplastic transformation. The p53 tumor-suppressor gene is the classic example of these genes, as it is found mutated in 50-90% of human malignant tumors including skin cancers.[4,23,27] The tumor-suppressor gene p53, located on the short arm of chromosome 17 (17p13.1), encodes for a nuclear protein which regulates cell proliferation by inhibiting cells entering S-phase. The p53 mutations are alleged to be the commonest genetic abnormality in human cancer.[28]
Expression of the p53 protein has also been detected in normal sun-exposed epidermis and in the normal epidermis adjacent to BCCs. These observations provide strong evidence that UV exposure is the major cause of p53 mutations.[4,29] Increased expression of p53 has been found in the majority of BCCs; however, UV light-induced signature mutations are present in only about 50% of cases.[30]
Proliferative activity in tumor cells is a common prognostic factor and correlates with metastatic potential in several malignancies.[29] Immunohistochemical analysis of p53 protein expression has long been regarded as a useful marker of p53 mutations. In cutaneous malignancies, p53 overexpression is detected frequently and has been considered as an early event in skin carcinogenesis.[27,31]
Protein p53 is a well-described tumor suppressor that has a central role in the initiation of apoptosis and in cell cycle control.[23,32] Its critical role in maintaining integrity of the human genome is evident because p53 is the most commonly altered gene in human cancer.[23,25]
Our study shows the highest prevalence of p53 protein immunoreactivity in 100% of SCCs and BCCs. Stratigos et al. showed a high prevalence of p53 protein immunoreactivity in 64.3% of SCCs and 84.2% of BCCs.[27] Their findings are comparable with those in previous studies, in which p53 protein overexpression in SCC has been reported to be approximately 50% with a range of 15-60%. [33–35]
The p53 expression is found to be related to the aggressive histopathological feature, which may be of predictive value for the behavior of BCC. However, this result does not support the relation between sun exposure inducing BCC and p53 protein expression.[36]
The p53 is a tumor-suppressor gene, whose mutation has been involved in the genesis of malignant neoplasms, including skin cancers. The p53 mutation appears to be an early target in UV-induced skin tumors and plays an important role in the development of BCC. Various studies have reported that overexpression of the p53 protein was detected in 42-92% of BCCs. This immunoreactivity was first believed to be related to sun exposure and an age-dependent process.[36,37]
Extensive study of the p53 gene has established its role as a tumor-suppressor gene and the involvement of mutant p53 in a wide spectrum of human malignancy.[38] In a study by Gibson et al. on kidney transplant recipients, immunoreactivity of p53 was observed in 59% of BCCs and more than 60% of SCCs, and dysplastic lesions.[38]
In Shea et al's. study, overexpression of p53 protein was detected immunohistochemically in 83% of specimens of BCC of the head and neck. Keratinocytes of chronically sun-exposed epidermis adjacent to BCCs also focally overexpressed p53 protein in the majority of cases, whereas those of sun-protected buttock skin did not. Mutation of p53 may form an important part of the pathogenetic sequence in a majority of cases of BCC.[39]
The Ki67 antigen, a high molecular weight non-histone protein, is generally accepted as the most reliable marker of proliferating cells.[27,40] It is expressed in all phases of the cell cycle, except G0 and is a marker of proliferation expressed in many cancers.[29,40,41] Positive Ki67 immunostaining has been used prognostically in patients with certain types of cancers.[27] The Ki67 level has been shown to correlate with tumor progression, metastatic potential, and decreasing overall survival.[23,29] The number of cells with Ki67 is used to mark cell proliferation. Ki67 expression is higher in aggressive and recurring BCCs.[3]
The highest Ki67 expression found in SCC, particularly in poorly differentiated SCC, confirms that its aggressive behavior is at least partly due to enhanced cell proliferation.[23,42]
Expression of p53 might be used as a marker to predict the aggressiveness of BCCs.[4] On the other hand, Healy et al. failed to confirm any association between p53 expression and tumor aggressiveness in BCCs; however, they demonstrated higher expression of another proliferation marker Ki67 in aggressive BCCs. Also, Bolshakov et al. found a higher rate of p53 expression in aggressive BCCs than nonaggressive ones, but the difference was not significant.[43] In another study, Ki67 expression did not correlate with the degree of proliferation and malignancy.[40] However, as a study limitations, we did not evaluate the correlation of tumor markers and the aggressiveness of the skin tumors.
Ki67 antigen expression differs between BCCs which later recur and BCCs that do not recur. The p53 protein expression, as assessed by IHC, was similar in each group.[44,45] Hernberg et al. suggest that Ki67 may be useful for predicting the prognosis of melanoma patients, because patients with a low Ki67 index in their first metastasis had a better prognosis when compared with patients with high indexes.[46]
In this study, p53 immunoreactivity was observed in 88% of cases; 30 BCCs (100%), 10 SCCs (100%), 0 KAs (0.0%), and 2 TEs (100%). In comparison, in Batinac et al. study, p53 immunostaining of normal skin, KA, BCC, and SCC was detected in 39.0%, 66.7%, 80%, and 86.7% of cases, respectively. They found a significant correlation between p53 and Ki67 expression. Their findings suggest that p53 overexpression occurs mainly in neoplastic skin lesions and reflects the degree of malignancy in the examined cutaneous neoplasms.[31]
In Perez et al's. study, immunohistochemical staining of 16 KA specimens detected p53 protein in 15 cases (94%), distributed as strong in four (25%), moderate in two (12%) mild in nine (56%), and negative in one case (6%).[47]
Ki67 was expressed in 0.8%, 23.7%, and 19.3% of the cells in the normal skin, actinic keratosis (AK), and SCC groups, respectively. No significant difference was seen between the three pathological conditions regarding the expression rate of Ki67. The p53 expression was detected in 26.6% and 54.6% of the assessed cells in the AK and SCC groups, respectively. There was no expression of p53 in the normal skin specimens.[48]
The p53 expression has been reported as 75% (on 18 BCCs),[1] 39% (on 30 BCCs),[3] and 0-92% in another studies (in BCC cases),[30,36,49,39] in comparison with 67.76% in this study.
The p53 expression has been reported as 40.3% (3.7-94% on 16 SCCs),[27] 28.75-43.65% (on 30 SCCs),[23] and 15-60% in another studies (in SCC cases),[28,38] in comparison with 50.20% in this study.
Ki67 expression has been reported as 40.6%,[27] 45%,[50] 51%,[1] 80%,[40] 39%[3] 26%,[51] and 34.6%,[27] in comparison with 57.33% in this study.
Ki67 expression has been reported as 54% (on 30 SCCs)[40] and 36.5-46.2% in another study on SCC cases,[23] in comparison with 47.70% in this study.
In this study, we had eight cases of KA which were positive for Ki67 and negative for p53, with average expression rate of 38.75% for Ki67. Batinac et al. studied 50 cases of KA and found the expression rate of 66.7% and appearance rate of 9.2% (0-24%) for p53.[31] Also, in another study by Batinac et al., on 30 cases of KA, immunoreactivity was observed in 8.05-17.65% for p53 and 10-25.75% for Ki67.[23] The technical differences in evaluation systems for expression of p53 and Ki67 could be responsible for discrepancies in results of various studies.[47] Also, Baum et al. suggested that expression of p53 and Ki67 varies according to microarchitecture (nodular, superficial, or fibrosing) classification.[52]
In this study, we had two cases of TE which were negative for Ki67 and positive for p53, with average expression rate of 50% for p53. Another study suggested that TE tumors are rarely positive for Ki67.[53]
The quantitative and qualitative evaluations of the expression of Ki67 and p53 may be helpful in differentiating malignant and premalignant epidermal lesions.[48] Significantly, higher p53 and Ki67 expressions were observed in all tumor lesions examined as compared with normal skin.[23] Staining of both Ki67 and p53 appears useful in resolving challenging differential diagnoses and thereby helps in directing appropriate treatment strategies.[54]
Conclusion
In conclusion, the high prevalence of p53 protein and Ki67 antigen immunoreactivity in malignant skin lesions further supports their pathogenetic role in cutaneous carcinogenesis. This high expression is a useful indicator of proliferative activity in cutaneous carcinomas and appears to correspond to a nonfunctional protein that is unable to exert its control on cell cycle and apoptosis.[11]
Limitations
Our study has several limitations. First, directly assessing mutations in p53 through genetic methods would be more accurate than immunohistochemical staining. In addition, we did not use clinical criteria of aggressiveness; neither did we follow the clinical course of the tumors to detect cases with recurrence or distant metastasis. Also, paucity of KA and TE cases was another limitation. Yet, our findings support the value of p53 overexpression to predict aggressive behavior in BCCs and SCCs. This may serve as a useful tool for prediction of tumor progression and help to design a more rational treatment protocol.
What is new?
The high prevalence of p53 protein and Ki67 antigen malignant skin lesions is a useful indicator of proliferative carcinomas and appears to correspond to a nonfunctional to exert its control on cell cycle and apoptosis.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Dyer RK, Weinstock MA, Cohen TS, Rizzo AE, Bingham SF. Predictors of basal cell carcinoma in high-risk patients in the VATTC (VA topical tretinoin chemoprevention) Trial. J Invest Dermatol. 2012;132:2544–51. doi: 10.1038/jid.2012.227. [DOI] [PubMed] [Google Scholar]
- 2.Amirnia M, Babaie-Ghazani A, Fakhrjou A, Khodaeiani E, Alikhah H, Naghavi-Behzad M, et al. Immunohistochemical study of cyclooxygenase-2 in skin tumors. J Dermatolog Treat. 2012. Jun 5, http://www.ncbi.nlm.nih.gov/pubmed/22667343. [Epub ahead of print] [DOI] [PubMed]
- 3.Vidal D, Matías-Guiu X, Alomar A. Efficacy of imiquimod for the expression of Bcl-2, Ki67, p53 and basal cell carcinoma apoptosis. Br J Dermatol. 2004;151:656–62. doi: 10.1111/j.1365-2133.2004.06094.x. [DOI] [PubMed] [Google Scholar]
- 4.Ansarin H, Daliri M, Soltani-Arabshahi R. Expression of p53 in aggressive and non-aggressive histologic variants of basal cell carcinoma. Eur J Dermatol. 2006;16:543–7. [PubMed] [Google Scholar]
- 5.Khodaeiani E, Amirnia M, Nazhad SB, Esmaeili H, Karimi ER, Alikhah H. Clinicopathologic study of basal cell carcinoma. Res J Biol Sci. 2010;5:293–6. [Google Scholar]
- 6.Koseoglu RD, Sezer E, Eyibilen A, Aladag I, Etikan I. Expressions of p53, cyclinD1 and histopathological features in basal cell carcinomas. J Cutan Pathol. 2009;36:958–65. doi: 10.1111/j.1600-0560.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 7.Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA. 2011;108:7431–6. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108:7425–30. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 10.Canas GC, Robson KJ, Arpey CJ. Persistent keratoacanthoma: Challenges in management. Dermatol Surg. 1998;24:1364–9. doi: 10.1111/j.1524-4725.1998.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 11.Manstein CH, Frauenhoffer CJ, Besden JE. Keratoacanthoma: Is it a real entity? Ann Plast Surg. 1998;40:469–72. doi: 10.1097/00000637-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bettencourt MS, Prieto VG, Shea CR. Trichoepithelioma: A 19-year clinicopathologic re-evaluation. J Cutan Pathol. 1999;26:398–404. doi: 10.1111/j.1600-0560.1999.tb01864.x. [DOI] [PubMed] [Google Scholar]
- 13.Cernea CR, Ferraz AR, De Castro IV, Sotto MN, Logullo AF, Bacchi CE, et al. p53 and skin carcinomas with skull base invasion: A case-control study. Otolaryngol Head Neck Surg. 2006;134:471–5. doi: 10.1016/j.otohns.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Stratigos AJ, Kapranos N, Petrakou E, Anastasiadou A, Pagouni A, Christofidou E, et al. Immunophenotypic analysis of the p53 gene in non-melanoma skin cancer and correlation with apoptosis and cell proliferation. J Eur Acad Dermatol Venereol. 2005;19:180–6. doi: 10.1111/j.1468-3083.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 15.Ansarin H, Daliri M, Soltani-Arabshahi R. Expression of p53 in aggressive and non-aggressive histologic variants of basal cell carcinoma. Eur J Dermatol. 2006;16:543–7. [PubMed] [Google Scholar]
- 16.Houben R, Hesbacher S, Schmid CP, Kauczok CS, Flohr U, Haferkamp S, et al. High-level expression of wild-type p53 in melanoma cells is frequently associated with inactivity in p53 reporter gene assays. PLoS One. 2011;6:e22096. doi: 10.1371/journal.pone.0022096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Xu Y. Gain of function of p53 cancer mutants in disrupting critical DNA damage response pathways. Cell Cycle. 2007;6:1570–3. doi: 10.4161/cc.6.13.4456. [DOI] [PubMed] [Google Scholar]
- 18.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006;126:30–2. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–50. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. Divorcing ARF and p53: An unsettled case. Nat Rev Cancer. 2006;6:663–73. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva TA, Coelho G, Lorenzetti Bocca A, Figueiredo Cavalcante Neto F. Expression of apoptotic, cell proliferation regulatory, and structural proteins in actinic keratosis and their association with dermal elastosis. J Cutan Pathol. 2007;34:315–23. doi: 10.1111/j.1600-0560.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen PS, Riber-Hansen R, Steiniche T. Immunohistochemical double stains against Ki67/MART1 and HMB45/MITF: Promising diagnostic tools in melanocytic lesions. Am J Dermatopathol. 2011;33:361–70. doi: 10.1097/DAD.0b013e3182120173. [DOI] [PubMed] [Google Scholar]
- 23.Batinac T, Zamolo G, Coklo M, Hadzisejdic I, Stemberger C, Zauhar G. Expression of cell cycle and apoptosis regulatory proteins in keratoacanthoma and squamous cell carcinoma. Pathol Res Pract. 2006;202:599–607. doi: 10.1016/j.prp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser HE, Bodey B Jr, Siegel SE, Gröger AM, Bodey B. Spontaneous neoplastic regression: The significance of apoptosis. In vivo. 2000;14:773–88. [PubMed] [Google Scholar]
- 25.Batinac T, Zamolo G, Jonjić N, Gruber F, Petrovecki M. P53 protein expression and cell proliferation in non-neoplastic and neoplastic proliferative skin diseases. Tumori. 2004;90:120–7. doi: 10.1177/030089160409000124. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402–12. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 27.Stratigos AJ, Kapranos N, Petrakou E, Anastasiadou A, Pagouni A, Christofidou E, et al. Immunophenotypic analysis of the p53 gene in non-melanoma skin cancer and correlation with apoptosis and cell proliferation. J Eur Acad Dermatol Venereol. 2005;19:180–6. doi: 10.1111/j.1468-3083.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson TJ, Royds J, Silcocks PB, Bleehen SS. Mutant p53 oncogene expression in keratoacanthoma and squamous cell carcinoma. Br J Dermatol. 1992;127:566–70. doi: 10.1111/j.1365-2133.1992.tb14866.x. [DOI] [PubMed] [Google Scholar]
- 29.Väisänen A, Kuvaja P, Kallioinen M, Turpeenniemi-Hujanen T. A prognostic index in skin melanoma through the combination of matrix metalloproteinase-2, Ki67, and p53. Hum Pathol. 2011;42:1103–11. doi: 10.1016/j.humpath.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Barrett TL, Smith KJ, Hodge JJ, Butler R, Hall FW, Skelton HG. Immunohistochemical nuclear staining for p53, PCNA, and Ki-67 in different histologic variants of basal cell carcinoma. J Am Acad Dermatol. 1997;37:430–7. doi: 10.1016/s0190-9622(97)70145-2. [DOI] [PubMed] [Google Scholar]
- 31.Batinac T, Zamolo G, Jonjić N, Gruber F, Petrovecki M. P53 protein expression and cell proliferation in non-neoplastic and neoplastic proliferative skin diseases. Tumori. 2004;90:120–7. doi: 10.1177/030089160409000124. [DOI] [PubMed] [Google Scholar]
- 32.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077–85. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 33.Stephenson TJ, Royds J, Silcocks PB, Bleehen SS. Mutant p53 oncogene expression in keratoacanthoma and squamous cell carcinoma. Br J Dermatol. 1992;127:566–70. doi: 10.1111/j.1365-2133.1992.tb14866.x. [DOI] [PubMed] [Google Scholar]
- 34.McGregor JM, Yu CC, Dublin EA, Levison DA, MacDonald DM. Aberrant expression of p53 tumour-suppressor protein in non-melanoma skin cancer. Br J Dermatol. 1992;127:463–9. doi: 10.1111/j.1365-2133.1992.tb14841.x. [DOI] [PubMed] [Google Scholar]
- 35.Gibson GE, O'Grady A, Kay EW, Leader M, Murphy GM. P53 tumor suppressor gene protein expression in premalignant and malignant skin lesions of kidney transplant recipients. J Am Acad Dermatol. 1997;36:924–31. doi: 10.1016/s0190-9622(97)80274-5. [DOI] [PubMed] [Google Scholar]
- 36.Auepemkiate S, Boonyaphiphat P, Thongsuksai P. P53 expression related to the aggressive infiltrative histopathological feature of basal cell carcinoma. Histopathology. 2002;40:568–73. doi: 10.1046/j.1365-2559.2002.01393.x. [DOI] [PubMed] [Google Scholar]
- 37.Boonchai W, Walsh M, Cummings M, Chenevix-Trench G. Expression of p53 in arsenic-related and sporadic basal cell carcinoma. Arch Dermatol. 2000;136:195–8. doi: 10.1001/archderm.136.2.195. [DOI] [PubMed] [Google Scholar]
- 38.Gibson GE, O'Grady A, Kay EW, Leader M, Murphy GM. P53 tumor suppressor gene protein expression in premalignant and malignant skin lesions of kidney transplant recipients. J Am Acad Dermatol. 1997;36:924–31. doi: 10.1016/s0190-9622(97)80274-5. [DOI] [PubMed] [Google Scholar]
- 39.Shea CR, McNutt NS, Volkenandt M, Lugo J, Prioleau PG, Albino AP. Overexpression of p53 protein in basal cell carcinomas of human skin. Am J Pathol. 1992;141:25–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Conscience I, Jovenin N, Coissard C, Lorenzato M, Durlach A, Grange F, et al. P16 is overexpressed in cutaneous carcinomas located on sun-exposed areas. Eur J Dermatol. 2006;16:518–22. [PubMed] [Google Scholar]
- 41.van Diest PJ, Brugal G, Baak JP. Proliferation markers in tumours: Interpretation and clinical value. J Clin Pathol. 1998;51:716–24. doi: 10.1136/jcp.51.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stratigos AJ, Kapranos N, Petrakou E, Anastasiadou A, Pagouni A, Christofidou E, et al. Immunophenotypic analysis of the p53 gene in non-melanoma skin cancer and correlation with apoptosis and cell proliferation. J Eur Acad Dermatol Venereol. 2005;19:180–6. doi: 10.1111/j.1468-3083.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 43.Bolshakov S, Walker CM, Strom SS, Selvan MS, Clayman GL, El-Naggar A, et al. P53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res. 2003;9:228–34. [PubMed] [Google Scholar]
- 44.Healy E, Angus B, Lawrence CM, Rees JL. Prognostic value of Ki67 antigen expression in basal cell carcinomas. Br J Dermatol. 1995;133:737–41. doi: 10.1111/j.1365-2133.1995.tb02748.x. [DOI] [PubMed] [Google Scholar]
- 45.Yerebakan O, Ciftçioglu MA, Akkaya BK, Yilmaz E. Prognostic value of Ki-67, CD31 and epidermal growth factor receptor expression in basal cell carcinoma. J Dermatol. 2003;30:33–41. [PubMed] [Google Scholar]
- 46.Hernberg M, Turunen JP, Von Boguslawsky K, Muhonen T, Pyrhönen S. Prognostic value of biomarkers in malignant melanoma. Melanoma Res. 1998;8:283–91. doi: 10.1097/00008390-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Perez MI, Robins P, Biria S, Roco J, Siegel E, Pellicer A. P53 oncoprotein expression and gene mutations in some keratoacanthomas. Arch Dermatol. 1997;133:189–93. [PubMed] [Google Scholar]
- 48.Talghini S, Halimi M, Baybordi H. Expression of P27, Ki67 and P53 in squamous cell carcinoma, actinic keratosis and Bowen disease. Pak J Biol Sci. 2009;12:929–33. doi: 10.3923/pjbs.2009.929.933. [DOI] [PubMed] [Google Scholar]
- 49.Campbell C, Quinn AG, Angus B, Rees JL. The relation between p53 mutation and p53 immunostaining in non-melanoma skin cancer. Br J Dermatol. 1993;129:235–41. doi: 10.1111/j.1365-2133.1993.tb11840.x. [DOI] [PubMed] [Google Scholar]
- 50.Tilli CM, Ramaekers FC, Broers JL, Hutchison CJ, Neumann HA. Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma. Br J Dermatol. 2003;148:102–9. doi: 10.1046/j.1365-2133.2003.05026.x. [DOI] [PubMed] [Google Scholar]
- 51.Al-Sader MH, Doyle E, Kay EW, Bennett M, Walsh CB, Curran B, et al. Proliferation indexes–a comparison between cutaneous basal and squamous cell carcinomas. J Clin Pathol. 1996;49:549–51. doi: 10.1136/jcp.49.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum HP, Meurer I, Unteregger G. Ki-67 antigen expression and growth pattern of basal cell carcinomas. Arch Dermatol Res. 1993;285:291–5. doi: 10.1007/BF00371599. [DOI] [PubMed] [Google Scholar]
- 53.Smith KJ, Williams J, Corbett D, Skelton H. Microcystic adnexal carcinoma: An immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464–71. doi: 10.1097/00000478-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Lum CA, Binder SW. Proliferative characterization of basal-cell carcinoma and trichoepithelioma in small biopsy specimens. J Cutan Pathol. 2004;31:550–4. doi: 10.1111/j.0303-6987.2004.00230.x. [DOI] [PubMed] [Google Scholar]


