Abstract
Introduction:
Cutaneous leishmaniasis (CL) is a parasitic disease which has different clinical forms. The aim of this study is to compare the response to leishmanin skin test (LST) in three forms of CL including plaque type, lupoid type, and sporotrichoid type.
Materials and Methods:
This was a descriptive cross-sectional study. The patients enrolled in this study had three clinical forms of CL confirmed by positive smear of their lesions and then LST was performed for them. Results were categorized as negative (0-5 mm induration), positive (6-14 mm), and strongly positive (≥15 mm). The data were documented in the patients’ files and analyzed with SPSS windows software version 16 (Inc.Chicago, USA).
Results:
200 patients were enrolled in the study. In the group with plaque type, 86% had a positive LST, 13.3% were negative, and 0.7% were strongly positive. In the lupoid group, these figures were 45.8%, 8.4%, 45.8%, respectively. In the sporotrichoid group, LST was positive in 27.3%, negative in 72.7%, and none of the patients had a strongly positive reaction (P < 0.05). Discussion: The most of the positive LST were belong to plaque and lupoid groups, the most of strongly positive were belong to lupoid, and the most of negative LST were related with sporotrichoid type.
Conclusion:
It can be suggested that lupoid and sporotrichoid types of CL are parts of a continuous spectrum of the disease with an enhanced cellular immunity in lupoid form and a decreased state in sporotrichoid type.
Keywords: Cutaneous leishmaniasis, lupoid, skin test, sporotrichoid
Introduction
What was known?
Cutaneous leishmaniasis is an endemic parasitological disease with different clinical variants.
Cutaneous leishmaniasis (CL) is an endemic parasitic disease in Middle-East countries including Iran.[1] Most skin lesions evolve from papular, nodular, and plaques.[1] However, there are rare clinical forms including, disseminated CL (DCL), sporotrichoid, and lupoid which are related to host immunity.[1,2] Delayed-type hypersensitivity is an important feature of the disease pathogenesis, and it can be measured by leishmanin skin test (LST), also called Montenegro test.[3] In disseminated type, LST and other tests of specific cellular immunity are negative, and affected patients with lupoid leishmaniasis have a vigorous cellular immune response but low antibody titer.[4,5] In sporotrichoid type of CL, there is lymphatic dissemination in the form of subcutaneous nodules (SCN). However, there is no report of evaluation of host cellular immunity in these cases. The aim of this study was to determine and compare the patients’ reactivity to LST in three forms of CL, including common plaque type [Figure 1], lupoid type [Figure 2], and sporotrichoid type [Figure 3].
Figure 1.
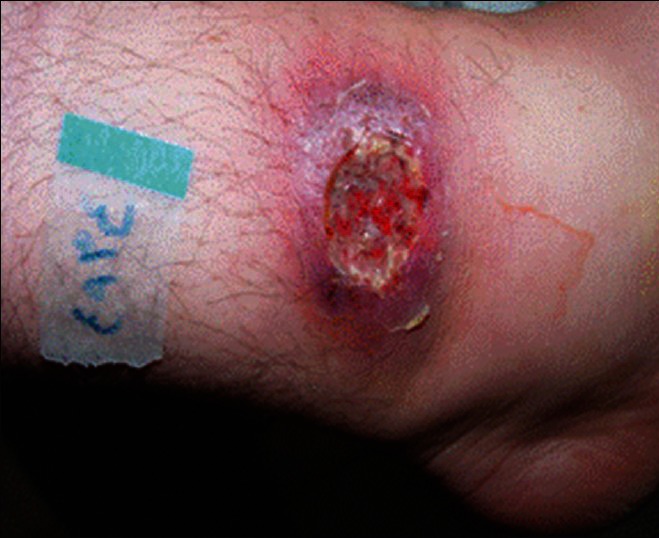
Plaque type of cutaneous leishmaniasis
Figure 2.
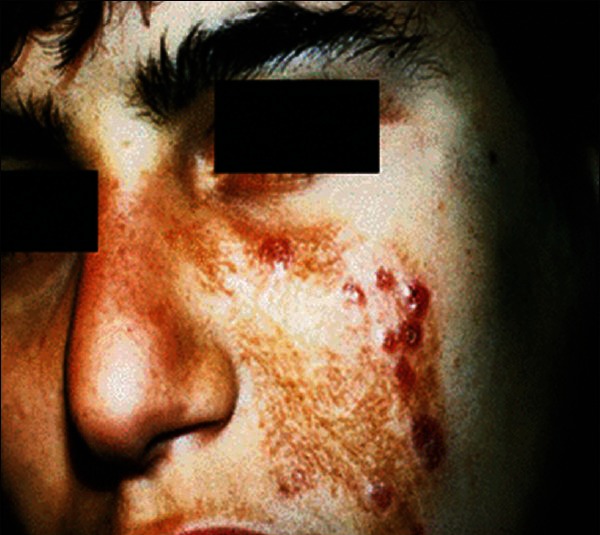
Lupoid type of cutaneous leishmaniasis
Figure 3.
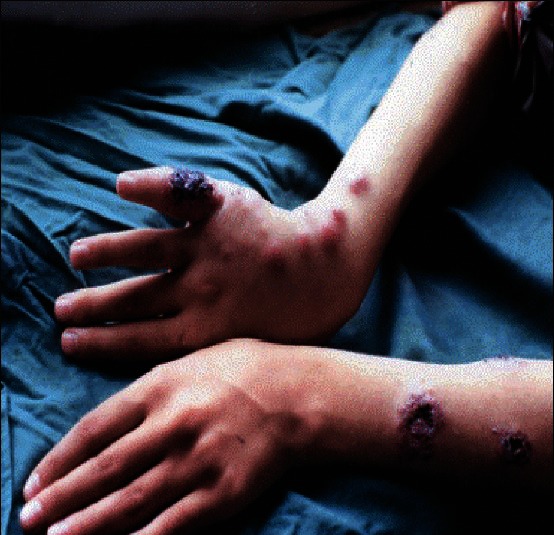
Sporotrichoid type of cutaneous leishmaniasis
Materials and Methods
This was a descriptive cross-sectional study performed in Skin Disease and Leishmaniasis Research Centre, Isfahan, Iran. The study was carried out from August 2010 to December 2011. The patients enrolled in this study had CL confirmed by positive direct smear of their lesions and had three clinical forms of common plaque type, lupoid type, and sporotrichoid type. They were of both sex and different age groups. Patients more than 60 and less than 5 years old, those with any kind of state which affects the immune system such as underlying disease, pregnancy, hypersensitivity disorders, and steroids intake and clinical course shorter than 2 months were excluded from the study. After being informed about the study, the patients or the guardians signed a consent form, and then LST was performed for them. The LST was supplied from Pastor Institution, Tehran, Iran. Each test vial contains 1 ml phosphate buffered saline, 0.1% thiomersal, and 6×10 6 killed L.Major promastigotes. LST was carried out by injecting 0.1 ml of a suspension of antigen intradermal with a 27-g needle in volar aspect of right forearm. In order to minimize the placebo effect, we also injected a control solution supplied from the same institute containing 1 ml PBS and 0.1% thiomersal in the contralateral side. The reaction was read after 48 h and the induration was measured by ballpoint technique. In the case of no reactivity, the test would be observed at 72 h of the injection.[6] Results were categorized as negative (0-5 mm induration), positive (6-14 mm), and strongly positive (≥15 mm).[7] With a special notice to the fact that in the site of injection of control solution, there should be no reaction. The data were documented in the patients’ files and analyzed with SPSS 16 Inc. (Chicago, USA) version 16 software using Kruskal–Wallis and Analysis of variance.
Results
Totally, 500 patients suspected to CL were referred to Skin diseases and leishmaniasis research center from August 2010 to December 2011, of which 200 parasitologically confirmed cases with three different types of CL were enrolled in the study and underwent LST. Fifty patients (25%) were female and 150 were male (75%). The overall mean age of the studied groups was 29.17 ± 1.2. Hundred and forty-three patients had common plaque type, 24 patients had lupoid type, and 33 were diagnosed with sporotrichoid type of CL. The mean duration of the disease was 5.99 ± 1.04 (min: 2 months and max: 96 months). In the group with plaque type, 86% had a positive LST, 13.3% were negative, and 0.7% were strongly positive. In the lupoid group, these figures were 45.8%, 8.4%, 45.8%, respectively. In the sporotrichoid group, LST was positive in 27.3%, negative in 72.7%, and none of the patients had a strongly positive reaction (P < 0.05). The minimum mean induration was in sporotrichoid group which was 3.30 ± 2.24 mm and the maximum mean induration with 11.2 ± 6.10 mm belonged to the patients with lupoid type [Table 1; Figures 4 and 5].
Table 1.
The results of leishmanin skin test in three groups of cutaneous leishmaniasis with different clinical forms
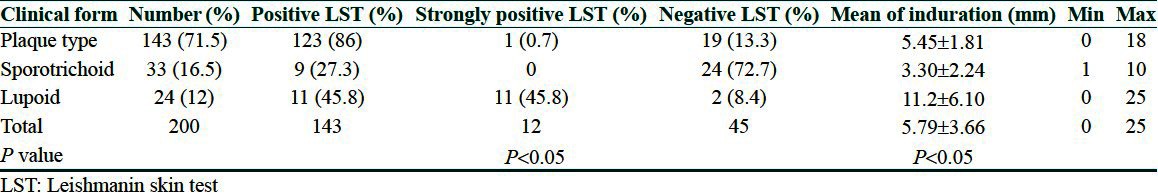
Figure 4.
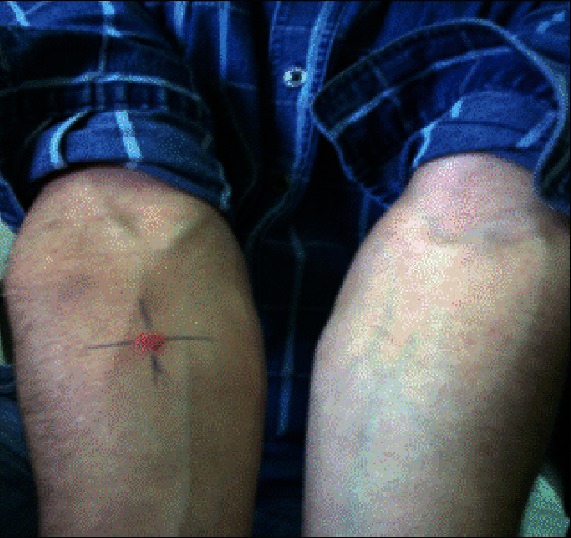
Positive leishmanin skin test
Figure 5.
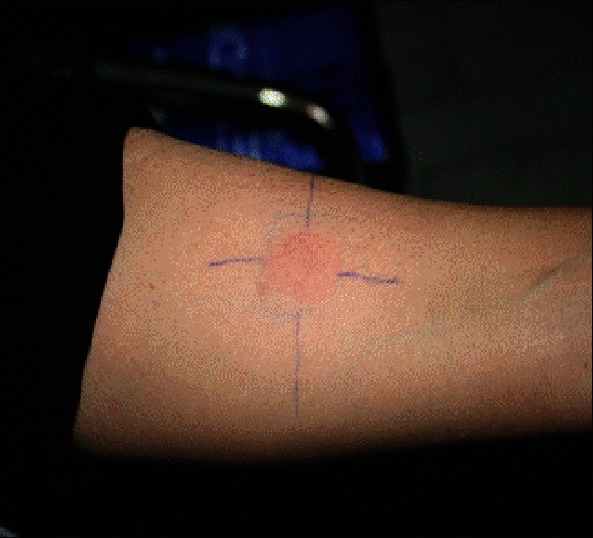
Exaggerated positive leishmanin test in lupoid type
Discussion
The LST or Montenegro test is an important tool for the diagnosis and epidemiological surveys of CL. Because of the high sensitivity of LST in CL, the test has been frequently applied for diagnosis.[8] The test becomes positive a few weeks after infection and it is a decisive method for the diagnosis of older leishmanial lesions, when the number of parasites is low and therefore, difficult to detect. The test is useful for follow-up in vaccination programs, and is also used as a parameter for evaluating the development of immune protection.[9,10] The LST consists of the intradermal injection of a suspension of antigen prepared from dead promastigotes, and relies on the cell-mediated immune response that usually occurs with CL. The reaction read at 48-72 h.[9] Understanding the timing of LST is of significant importance to avoid false-negative results in those few cases that present with early disease; therefore, we excluded the patients with a clinical course shorter than 2 months. We also excluded patients with any kind of possible impairment in the immune system in order to avoid possible negative effects on test results. Protective immunity against leishmaniasis is largely, if not wholly T-cell mediated and associated with production of interferon gamma.[11] In this study, the test was supplied from Pastor Institution, Tehran, Iran, where the safety and efficacy of it have been previously documented.[12]
LST is positive in the majority of patients with localized CL. It is negative in patients with very recent CL infection and DCL.[5] Whereas in lupoid leishmaniasis which is a rare peculiar form of chronic CL, a vigorous cellular immunity response and low number of parasites in lesions have been shown. This recurrent form of the disease refers to the development of the new lesions within the scar of the healed lesions mimicking lupus vulgaris.[5] Sporotrichoid type of CL is a clinical form of CL that represents lymphatic dissemination, a phenomenon not widely recognized in the form of SCN and is seen in 10% of CL patients.[13] The SCN were usually inconspicuous, painless, proximal to the primary skin lesions when multiplied they show sporotrichoid configuration. The biopsies of these nodules have shown that they contain active promastigotes.[14]
Conclusion
The results of our study showed that most of the positive LST results belong to plaque and lupoid groups, the majority of strongly positives belong to lupoid, and the most of negative LST results were related to sporotrichoid type, The negativity of leishmanin test in some patients with plaque and lupoid types can be explained by the sensitivity of the test used which is 88% according to our results.
In addition, the mean induration in sporotrichoid group was less than the other two groups. According to results obtained from this study, we show that variation in LST reactivity was remarkable. It can be suggested that lupoid and sporotrichoid types of CL are parts of a continuous spectrum of the disease with an enhanced cellular immunity in lupoid form and a decreased state in sporotrichoid type.
What is new?
There is decreased cellular immunity in sporotrichoid form of cutaneous leishmaniasis
Acknowledgments
Our grateful appreciation is extended to all the staff of Skin Diseases and Leishmaniosis Research Center for their help in this project.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Ayatollahi J. Sporotrichoid cutaneous leishmaniasis in central Iran. Iran J Med Sci. 2006;31:173–5. [Google Scholar]
- 2.Calvopina M, Gomez EA, Uezato H, Kato H, Nonaka S, Hashiguchi Y. Atypical clinical variants in New World cutaneous leishmaniasis: Disseminated, erysipeloid, and recidiva cutis due to Leishmania (V.) panamensis. Am J Trop Med Hyg. 2005;73:281–4. [PubMed] [Google Scholar]
- 3.Manzur A, Bari A. Sensitivity of leishmanin skin test in patients of acute cutaneous leishmaniasis. Dermatol Online J. 2006;12:2. [PubMed] [Google Scholar]
- 4.Bryceson AD. Diffuse cutaneous leishmaniasis in Ethiopia. I. The clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63:708–37. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- 5.Salman SM, Rubeiz NG, Kibbi AG. Cutaneous leishmaniasis: Clinical features and diagnosis. Clin Dermatol. 1999;17:291–6. doi: 10.1016/s0738-081x(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 6.Sokal JE. Editorial: Measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–2. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 7.Wong HT, Shahrizal TA, Prepageran N, Lim WK, Raman R. Cell-mediated immunity in nasopharyngeal carcinoma and allergic rhinitis: A controlled study. Ear Nose Throat J. 2007;86:292–4. [PubMed] [Google Scholar]
- 8.Faber WR, Oskam L, van GT, Kroon NC, Knegt-Junk KJ, Hofwegen H, et al. Value of diagnostic techniques for cutaneous leishmaniasis. J Am Acad Dermatol. 2003;49:70–4. doi: 10.1067/mjd.2003.492. [DOI] [PubMed] [Google Scholar]
- 9.da Costa CA, de Toledo VP, enaro O, Williams P, Mayrink W. Montenegro skin test–evaluation of the composition and stability of the antigen preparation. Mem Inst Oswaldo Cruz. 1996;91:193–4. doi: 10.1590/s0074-02761996000200013. [DOI] [PubMed] [Google Scholar]
- 10.Mayrink W, Coelho GL, Guimarães TM, de Andrade HM, de Castro PE, da Costa CA, et al. Immuno-biochemical evaluations of phenol and thimerosal as antigen preservatives in Montenegro skin test. Acta Trop. 2006;98:87–93. doi: 10.1016/j.actatropica.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Alexander J, Russell DG. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 12.Alimohammadian MH, Kivanjah M, Pak F, Gaznavia A, Kharazmi A. Evaluation of the efficacy of Iran leishmanin and comparison with leishmanins from Wellcome (UK) and Roma (Italy) in cured cutaneous leishmaniasis patients. Trans R Soc Trop Med Hyg. 1993;87:550–1. doi: 10.1016/0035-9203(93)90083-3. [DOI] [PubMed] [Google Scholar]
- 13.Kubba R, al-Gindan Y, el-Hassan AM, Omer AH, Kutty MK, Saeed MB. Dissemination in cutaneous leishmaniasis. II. Satellite papules and subcutaneous induration. Int J Dermatol. 1988;27:702–6. doi: 10.1111/j.1365-4362.1988.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 14.Masmoudi A, Ayadi N, Khabir A, Bouzid L, Bouassida S, Meziou TJ, et al. Sporotrichoid cutaneous leishmaniasis in Tunisia: A clinical and histological study. Ann Dermatol Venereol. 2008;135:63–7. doi: 10.1016/j.annder.2007.04.005. [DOI] [PubMed] [Google Scholar]


