Abstract
Introduction:
One of the most important complications after burning is hypo/depigmentation. This study was designed to compare two methods of cell spray and intradermal injection of epidermal cell suspension for treatment of burn induced hypopigmentation.
Material and Methods:
In this study, 28 patients with post burn hypo/depigmentation were selected and divided in 2 groups. A small skin biopsy was taken from normal skin of patients in operation room and epidermal cell suspension was prepared using NaBr 4N and trypsin. In the first group, the epidermal cell suspension was sprayed on the wound surface and then the area was dressed with amniotic membrane and gauze. In the second group, the cell suspension was injected in intradermal manner in the hypopigmented area. The patients were followed up and to evaluate the effect of the cells, photos were taken from the area before operation and also at follow-up. Clinical evaluation was done by the surgeon and a clinical score between “0” to “4” was used to demonstrate the clinical status from poor to excellent pigmentation. Skin biopsies were taken from depigmented area before and after interventions. Melanocytes were stained using anti S100 antibody and were counted in ×400 magnification fields.
Results:
Eighteen patients were in cell spray and 10 were in cell injection groups. Mean change of pigmentation in two group showed that there was no statistical significant differences in pigmentation between two groups, (P value = 0.52) although a limited improvement in pigmentation status was observed in both groups. Regarding melanocyte numbers per field, there was not a significant difference between two groups and also before and after interventions, but melanocyte number increased after treatment in both groups.
Conclusion:
We did not find noticeable differences between cell spray and intradermal injection methods. Although both methods showed a limited effect on pigmentation of depigmented skin, the clinical results were not satisfactorily for both patients and clinicians.
Keywords: Cell spray, hypopigmentation, intradermal injection, melanocytes
Introduction
What was known?
Administration of epidermal cell suspension had been shown to be effective in patients with vitiligo. Here, we used it for burn induced hypo-pigmentation. Previously, we showed that NaBr 4N in combination with trypsin is more effective in preparation of epidermal cell suspension than trypsin alone. Therefore, we used this method in this experiment.
Some changes are observed in pigmentation state of skin after burns, mostly in the form of hypo/depigmentation especially after deep burns. This post burn hypo/depigmentation is also called leukoderma.[1] In normal skin, melanin is produced in melanocytes within basal layer of epidermis, deposited in melanosomes and transferred to keratinocytes via melanocyte processes. Therefore, communication of melanocytes and keratinocytes is responsible for maintaining the skin colour.[2] One possible explanation for post burn hypopigmentation may be the physical barriers that are produced due to secondary healing which prevent melanocyte migration and melanin transfer while the majority of melanocytes are destroyed due to thermal injury.[1] Just like vitiligo, post burn hypopigmentation may cause phsychological problems for the patient due to effects on body image.[3]
Post burn leukoderma is resistant to UV based therapies like UV-B and psoralen plus UV-A.[4] Although fractional ablative lasers are able to improve the skin texture to some extent, they are unable to influence the pigmentation status.[5] Autologous skin grafts are the main surgical interventions for leukoderma nowadays, whilst donor site area limitations are still a challenge.[6] In addition, hyperpigmentation of grafted skin is difficult to manage.[7]
Reconstructing the removed hypopigmented skin area with co-cultured keratinocyte-melanocyte sheets is another treatment for leukoderma. Although large areas of hypopigmentation can be treated with this method, preparation of sheets needs a long time and trained personnel which in turn increases the cost of treatment.[8,9] Also, some results were not satisfactory for vitiligo.[10]
Gauthier et al. introduced autologous grafting of non-cultured keratinocyte-melanocytes for treatment of depigmented lesions for the first time.[11] Since that time, a couple of articles have been published using non cultured keratinocyte melanocyte suspension for treatment of skin depigmentation in diseases such as vitiligo and post burn leukoderma.[3,4,8,12–19] Some articles are in favour of good results with this method[14,17,19] ; some of them claim that good results are just observed in a portion of patients,[8,12,13] while others have disappointing outcomes.[3]
There are two methods for applying autologous non cultured keratinocyte-melanocyte suspension for treatment of hypopigmented areas. Most investigators spread the suspension on the dermabraded skin via a syringe or cell spray method, but others injected the cell suspension in the dermis of depigmented area.[12,13] As there is a controversy about the efficacy of autologous non cultured keratinocyte-melanocyte suspension for treatment of hypopigmention and the best method of applying the suspension is not known, we applied the epidermal cell suspension in post burn leukoderma patients and compared intradermal injection with spraying the cells on dermoabraded skin.
Materials and Methods
Preparing epidermal cell suspension
Patients who had desire to participate in this study were chosen based on their follow-up possibility and a written consent form was taken to enter the study. Patients in both groups underwent general anesthesia in order to skin graft surgery for hypopigmentation. While they were under general anesthesia for the major surgery, we prepared the cell suspension and applied it on or within the part of their hypopigmented skin that will explain as the treatment. Under general anesthesia, a small biopsy (between 1-2 cm2) was taken from normal skin area of the patients with burn induced hypopigmentation in operation room who were under operation for removal of depigmented skin. Then, preparation of epidermal cell suspension was done in operation room under sterile condition. The skin was washed with PBS for two times in order to remove extra hair and blood. As we showed previously[20] NaBr 4N is able to separate dermis and epidermis in a short time. Therefore, 5cc NaBr 4N was added to a small Petri dish in which the skin biopsy was floating. The dish was incubated in an incubator at 37 degree centigrade for 20 minutes. Afterwards NaBr 4N was removed by washing the dish with PBS. Epidermis was scratched with a scalpel blade and the remaining dermis was removed and disposed. Trypsin (2cc) was added to the plate to detach inter cellular connections. After 15 minutes incubation, 2cc αMEM (Invitrogen) media plus 10% fetal bovine serum (FBS; Gibco-RBL), 1% L-glutamin (Gibco/Invitrogen, Carlsbad, CA, United States), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco/Invitrogen, Carlsbad, CA, United States) was added to inactivate the trypsin. Centrifugation was done and the cells within the pellet were resuspended in 2cc αMEM media plus 10% FBS.
Applying epidermal cell suspension
Twenty eight patients were divided into two study groups. In the first group, the epidermal cell suspension from normal skin was sprayed on the hypopigmented skin area which was dermabraded by a dermatome. In the second group, cell injection group, the cell suspension was injected in the site of burn without removal of hypopigmented skin. In both groups’ of patients, the area was dressed with the amniotic membrane and gauze to prevent spreading of the cells and also contamination. The patients were followed between 9 months to 15 months. Besides taking the photograph, the patients’ wound was evaluated by the surgeon and a clinical score (0 to 4) was given based on the pigmentation state of the wound. In our scoring system, score 0 was considered as complete depigmentation, 1 as poor pigmentation, 2 as mild pigmentation, 3 as near normal pigmentation and 4 as normal pigmentation.
Immunohistochemical staining of melanocytes and counting of these cells within skin biopsies
We took skin biopsies from depigmented area before and after interventions. Melanocytes were stained immunohistochemically using anti S100 antibody and were counted in ×400 magnification fields.
Statistical analysis
Continuous variables were compared using independent samples t-test for normally distributed data, and Mann-Whitney U test for non-normally distributed data. Comparison of dichotomous values was done using Chi-square test. Normal distribution was checked by Q-Q-plots and tested using the Kolmagorov-Smirnov test. A two-tailed P < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Comparison between cell spray and intra dermal injection of epidermal cell suspension
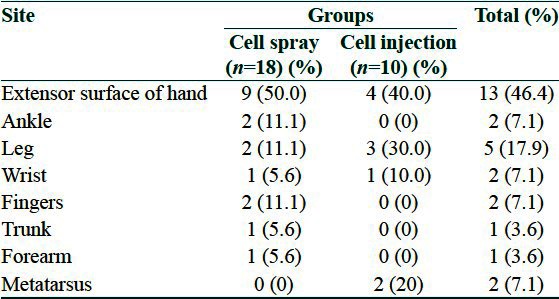
From 28 participants in the study, 18 patients were in the cell spray and 10 patients were in cell injection group. Eight patients (44%) in cell spray group, and five patients (50%) in cell injection group were male (P value = 1). Mean age of patients in cell spray group was 28.5 ± 9.9 and in cell injection group was 29.2 ± 4 years. Distribution of site of burns is shown in Table 1. The mean time between onset of the burn and intervention in cell spray and cell injection group were 1.77 ± 0.57 and 1.9 ± 0.56 years, respectively (P value = 0.59).
Table 1.
Distribution of sites of burns in two groups
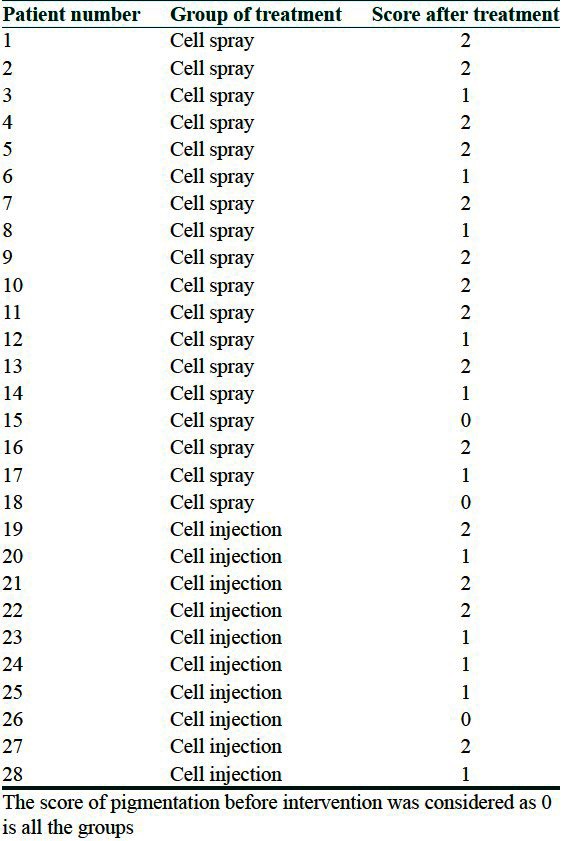
Mean change of pigmentation (based on clinical score) in two group showed no statistical significant difference between two groups (P value = 0.52) [Table 2] although a limited improvement in pigmentation status was observed in both [Figures 1 and 2]. In Table 3 the score of pigmentation related to each patient is shown considering that the score for all the patients before surgery is given 0 [Table 3].
Table 2.
Comparison of pigmentation between two groups
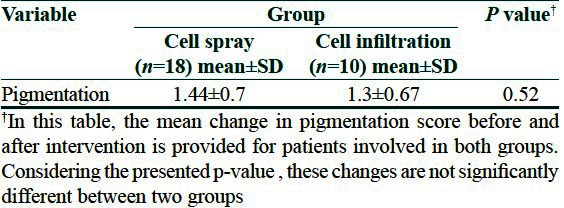
Figure 1.
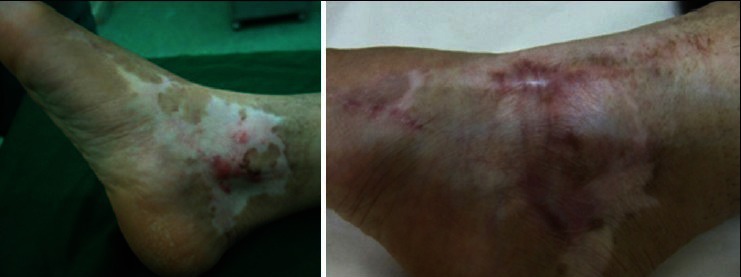
A 27 years woman with depigmented skin after a third degree burning of right foot. Left Figure is before cell spray and right one is 10 months after cell spray. The patient takes score 0 before and score 2 after treatments
Figure 2.
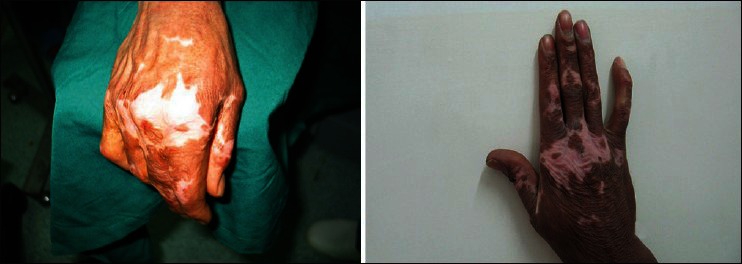
A 31 years man with a second degree burn induced depigmented skin of the right hand. Left Figure is before cell injection and right one is 3 months after injection. The patient takes score 0 before and score 1 after treatment
Table 3.
Score of pigmentation in cell spray and cell injection groups
Melanocyte count results
Regarding melanocyte counts per field, there was no significant difference between two groups and also before and after interventions, although the number was increased after treatment in both groups [Figure 3].
Figure 3.
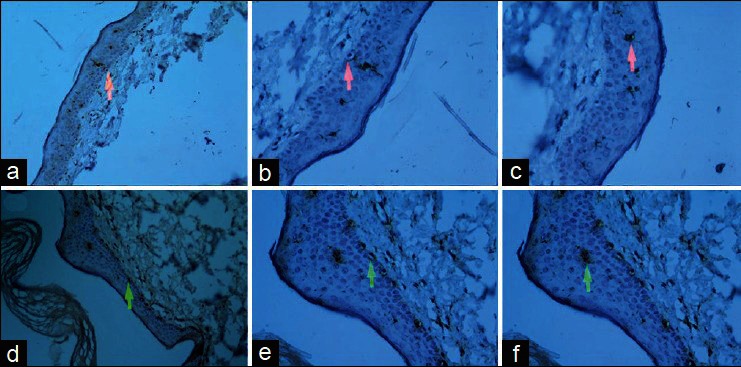
(a) ×200 magnification of immunohistochemically stained skin biopsy of hypopigmented skin before cell spay (red arrow shows a melanocyte), (b) ×400 magnification of the same sample (red arrow shows a melanocyte), (c) ×400 magnification of the same sample (red arrow shows a dendritic cell), (d) ×200 magnification of skin biopsy of hypopigmented skin after cell spay treatment (green arrow shows a melanocyte), (e) ×400 magnification of the same sample after treatment (green arrow shows a melanocyte), (f) ×400 magnification of the same sample after treatment (green arrow shows a dendritic cell)
Discussion
Post burn hypopigmentation is a complication of burning which is important regarding the cosmetic respect and its psychological effects on the patient.[3] There are a few treatment options for this condition. The most applicable one is the surgical option which uses skin grafts to recover the hypopigmented skin areas.[1] This is not applicable for large hypopigmented lesions due to limitations of donor area.[7]
Autologous cultured keratinocyte-melanocyte transplantation has been used in some studies for treatment of hypopigmented lesions. In the study of Lontz et al., both cultured melanocytes and cultured epidermal sheets were able to induce repigmentation in most of their vitiligo patients albeit to some extent.[21] Stoner et al. used cultured epithelial autograft for a post burn hypopigmented lesion in a 5 year old patient and reported complete repigmentation two years post-surgery.[22] In addition to high expenses for preparation, mutagenesis and risks of cancer formation are possible disadvantages of this technique.[7] However, Olsson et al. observed better results from epidermal sheets than basal layer suspension or melanocyte culture for treatment of hypopigmentation in a follow up of 132 patients.[9]
The other approach which has been used for leukoderma is autologous non cultured keratinocyte melanocyte transplantation in the form of a cell suspension. There is a controversy in the results. Henderson et al. used this method in two sites of a patient with post burn leukoderma and reported good results.[4] Mulekar team showed repigmentation ranging from 90% to 100% with good color matching in 7 patients with post burn leukoderma. [19] All the vitiligo patients in the study of Van Geel et al. gained 85-100% repigmentation.[16] In another study, 79% of vitiligo patients gained >90% repigmentation at the end of 1 year post transplantation.[17] On the other hand some groups reported fair results. Back et al. described their results as disappointing ones.[3] In a study in United States, just 17% of vitiligo patients depicted excellent repigmentation.[15] Some other studies had moderate results. In the study of El-Zawahry et al. 23% of vitiligo patients showed excellent results.[12] Khodadadi et al. observed a marked repigmentation in 40% of their vitiligo patients.[13] In another study of Mulekar team on 142 vitiligo patients, 56% of patients showed excellent repigmentation.[8]
We applied autologous epidermal cell suspension for treatment of post burn hypopigmentation in two different ways: Intradermal injection and cell suspension spray on the dermabraded skin. Although both methods showed a limited effect on pigmentation status of depigmented skin, the clinical results were not satisfactorily for both patients and clinicians. In addition there was not a significant change in melanocyte counts before and after interventions.
There may be some reasons for a variety of results obtained in different studies. In some studies, investigators used UV therapy for the patients after the procedure to promote melanocyte replication.[12,16,18] Another possible defect in this procedure may be due to difficult attachment of suspension cells on the skin surface. To overcome this defect, some groups mixed the cell suspension with hyaluronic acid to increase the cell attachment possibility.[12,18] Different studies have used different volume of cell suspension in addition to different media to resuspend the cells. For example, a group used phosphate buffer saline[17] while another used melanocyte media for making cell suspension.[18] The other difference is the route of transplantation. While most groups applied the cell suspension on the dermabraded skin surface, El-Zawahry et al. injected the cell suspension in the blisters that they formed on the skin.[12] Also Khodadadi et al. injected the cells within the epidermis.[13]
In conclusion, we did not get good results from this method. Probably some alterations in our method like adding hyaloronic acid and changing the resuspension media to a melanocyte culture media may improve the results. Also, UV therapy after the procedure should be tested to evaluate the repigmentation.
What is new?
Neither intradermal injection of autologous epidermal cell suspension nor spraying of these cells on dermabraded surface of skin in patients with burn-induced hypo-pigmentation is so effective to significantly improve the skin color. It may be related to pathologic nature of skin in these areas due to presence of fibrotic tissue that does not provide optimal condition for existence and migration of melanocytes.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Grover R, Morgan BD. Management of hypopigmentation following burn injury. Burns. 1996;22:627–30. doi: 10.1016/s0305-4179(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 2.Morgan JE, Gilchrest B, Goldwyn RM. Skin pigmentation: Current concepts and relevance to plastic surgery. Plast Reconstr Surg. 1975;56:617–28. doi: 10.1097/00006534-197512000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Back C, Dearman B, Li A, Neild T, Greenwood JE. Noncultured keratinocyte/melanocyte cosuspension: Effect on reepithelialization and repigmentation-a randomized, placebo-controlled study. J Burn Care Res. 2009;30:408–16. doi: 10.1097/BCR.0b013e3181a28c4d. [DOI] [PubMed] [Google Scholar]
- 4.Henderson MD, Huggins RH, Mulekar SV, Ozog D, Lim HW, Hamzavi IH. Autologous noncultured melanocyte-keratinocyte transplantation procedure in an African American man with postburn leukoderma. Arch Dermatol. 2011;147:1025–8. doi: 10.1001/archdermatol.2011.238. [DOI] [PubMed] [Google Scholar]
- 5.Waibel J, Beer K. Fractional laser resurfacing for thermal burns. J Drugs Dermatol. 2008;7:59–61. [PubMed] [Google Scholar]
- 6.Falabella R. Surgical approaches for stable vitiligo. Dermatol Surg. 2005;31:1277–84. doi: 10.1111/j.1524-4725.2005.31203. [DOI] [PubMed] [Google Scholar]
- 7.Mysore V, Salim T. Cellular grafts in management of leucoderma. Indian J Dermatol. 2009;54:142–9. doi: 10.4103/0019-5154.53194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulekar SV. Long-term follow-up study of 142 patients with vitiligo vulgaris treated by autologous, non-cultured melanocyte-keratinocyte cell transplantation. Int J Dermatol. 2005;44:841–5. doi: 10.1111/j.1365-4632.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 9.Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. Br J Dermatol. 2002;147:893–904. doi: 10.1046/j.1365-2133.2002.04837.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips J, Gawkrodger DJ, Caddy CM, Hedley S, Dawson RA, Smith-Thomas L, et al. Keratinocytes suppress TRP-1 expression and reduce cell number of co-cultured melanocytes-implications for grafting of patients with vitiligo. Pigment Cell Res. 2001;14:116–25. doi: 10.1034/j.1600-0749.2001.140207.x. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier Y, Surleve-Bazeille JE. Autologous grafting with noncultured melanocytes: A simplified method for treatment of depigmented lesions. J Am Acad Dermatol. 1992;26:191–4. doi: 10.1016/0190-9622(92)70024-a. [DOI] [PubMed] [Google Scholar]
- 12.El-Zawahry BM, Zaki NS, Bassiouny DA, Sobhi RM, Zaghloul A, Khorshied MM, et al. Autologous melanocyte-keratinocyte suspension in the treatment of vitiligo. J Eur Acad Dermatol Venereol. 2011;25:215–20. doi: 10.1111/j.1468-3083.2010.03759.x. [DOI] [PubMed] [Google Scholar]
- 13.Khodadadi L, Shafieyan S, Sotoudeh M, Dizaj AV, Shahverdi A, Aghdami N, et al. Intraepidermal injection of dissociated epidermal cell suspension improves vitiligo. Arch Dermatol Res. 2010;302:593–9. doi: 10.1007/s00403-010-1034-7. [DOI] [PubMed] [Google Scholar]
- 14.Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol. 2004;140:1211–5. doi: 10.1001/archderm.140.10.1211. [DOI] [PubMed] [Google Scholar]
- 15.Huggins RH, Henderson MD, Mulekar SV, Ozog DM, Kerr HA, Jabobsen G, et al. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: The experience of an academic medical center in the United States. J Am Acad Dermatol. 2012;66:785–93. doi: 10.1016/j.jaad.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.van Geel N, Ongenae K, De Mil M, Naeyaert JM. Modified technique of autologous noncultured epidermal cell transplantation for repigmenting vitiligo: A pilot study. Dermatol Surg. 2001;27:873–6. doi: 10.1046/j.1524-4725.2001.01045.x. [DOI] [PubMed] [Google Scholar]
- 17.Sahni K, Parsad D, Kanwar AJ. Noncultured epidermal suspension transplantation for the treatment of stable vitiligo in children and adolescents. Clin Exp Dermatol. 2011;36:607–12. doi: 10.1111/j.1365-2230.2011.04065.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Geel N, Ongenae K, Vander Haeghen Y, Vervaet C, Naeyaert JM. Subjective and objective evaluation of noncultured epidermal cellular grafting for repigmenting vitiligo. Dermatology. 2006;213:23–9. doi: 10.1159/000092833. [DOI] [PubMed] [Google Scholar]
- 19.Mulekar SV, Issa AA, Eisa AA. Treatment of post-burn leucoderma with non-cultured melanocyte-keratinocyte transplantation (MKTP) Burns. 2011;37:448–52. doi: 10.1016/j.burns.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Maharlooei MK, Mohammadi AA, Farsi A, Ahrari I, Attar A, Monabati A. A camparison between different existing methods used to seprate epidermal cells from cells from skin biopsies for autologus transplantation. Indian J Dermatol. 2011;56:666–9. doi: 10.4103/0019-5154.91825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Löntz W, Olsson MJ, Moellmann G, Lerner AB. Pigment cell transplantation for treatment of vitiligo: A progress report. J Am Acad Dermatol. 1994;30:591–7. doi: 10.1016/s0190-9622(94)70067-2. [DOI] [PubMed] [Google Scholar]
- 22.Stoner ML, Wood FM. The treatment of hypopigmented lesions with cultured epithelial autograft. J Burn Care Rehabil. 2000;21:50–4. doi: 10.1097/00004630-200021010-00010. [DOI] [PubMed] [Google Scholar]


