Abstract
Post-kala-azar dermal leishmaniasis (PKDL) is an unusual dermatosis occurring following an attack of visceral leishmaniasis (VL). There are only few reports of PKDL after successful treatment with miltefosine. We report two cases of PKDL that developed after successful treatment of VL with miltefosine.
Keywords: Miltefosine, post-kala-azar dermal leishmaniasis, visceral leishmaniasis
Introduction
What was known?
1. Post-kala-azar dermal leishmaniasis is an unusual dermatosis occurring following an attack of visceral leishmaniasis.
2. There are only few reports of PKDL after successful treatment with miltefosine.
Post-kala-azar dermal leishmaniasis (PKDL) is an unusual dermatosis occurring between 6 months and 5 years following an attack of visceral leishmaniasis (VL).[1,2] It is characterized by hypopigmented macules, erythema, and nodules. Earlier, PKDL was considered to occur in VL cases receiving no treatment or irregular or incomplete therapy with sodium stibogluconate or pentamidine. Miltefosine is the current drug of choice for VL and is being used in its elimination program. PKDL has developed rarely in persons who received miltefosine for VL. After thorough search of literature, we could find only two such reports and both of them were published in 2009.[3,4] We report two cases of PKDL that developed after successful treatment with miltefosine.
Case Report
A 23-year-old lady was brought to our dermatology outdoor patient department by a health worker with hypopigmented macules on face, upper arms, and abdomen [Figures 1 and 2]. The lesions were nontender and sensation was intact. The peripheral nerves were not tender or thickened. On systemic examination, all findings were normal except mild anemia (9 mg/dl) and high erythrocyte sedimentation rate (ESR; 96 mm in the first hour). The recombinant K39 (rK39)-based immunochromatographic strip test was positive. Biopsy sample from hypopigmented macules did not reveal any acid fast bacilli but showed epidermal atrophy, follicular plugging with keratin, and presence of dense lymphocytic infiltrate in the dermis suggestive of PKDL. There was no demonstrable Leishman-donovan (LD) body [Figure 3]. There was no hepatosplenomegaly or lymphadenopathy. Enzyme-linked immunosorbent assay (ELISA) for HIV-1 and 2 was negative. She was treated for VL with miltefosine, 100 mg/day for 28 days about 6 years back under a phase IV WHO/TDR/ICMR-sponsored clinical trial. After treatment, she became clinically and parasitologically cured and had no complains in follow-up of 6 months. After 4 years of treatment with miltefosine, she developed skin lesions on the face, which spread to upper part of the body. As her daily activities were not hampered, she was reluctant to visit doctor for more than 2 years. A clinical diagnosis of PKDL was made and the patient was put on miltefosine, 50 mg twice daily with complete subsidence of the lesions in 3 weeks. She was advised to continue treatment for 3 months.
Figure 1.
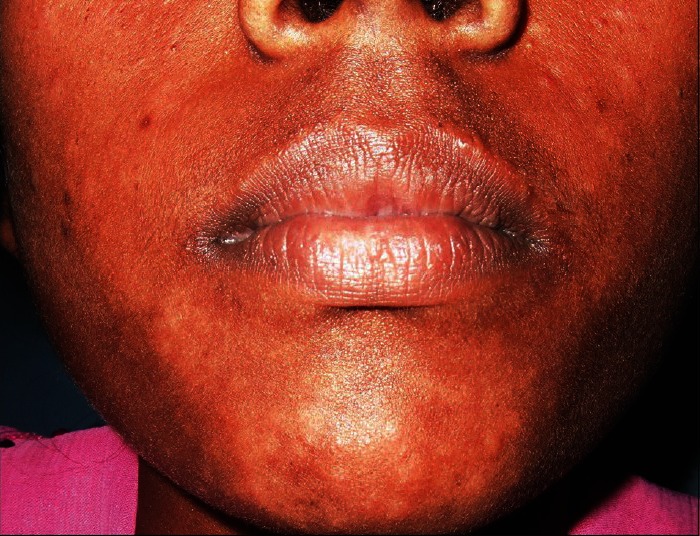
Case 1: Hypopigmented macules on face
Figure 2.
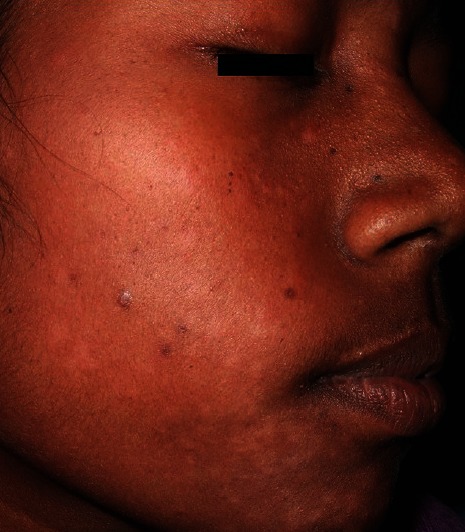
Case 1: Hypopigmented macules on face
Figure 3.
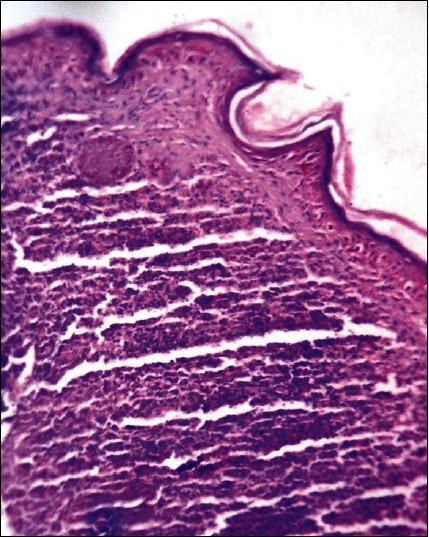
Epidermal atrophy, follicular plugging with keratin and presence of dense lymphocytic infiltrate in the dermis (H and E, ×10)
A 27-year-old patient, farmer by occupation, presented with depigmented macules widely spread all over the body since last 4 years, which were more marked on the front and back of trunk. The lesions on the face were coalescent and they appeared more hypopigmented than depigmented [Figures 4 and 5]. On touch, sensation was normal and no peripheral nerve was thickened or tender. On systemic examination, all findings were normal except mild raised eosinophils, 85 (normal range, 1-6%) and high ESR (96 mm in the first hour). The rK39-based immunochromatographic strip test was positive. Biopsy tissue sample from hypopigmented lesion from upper back showed no acid fast bacilli, but epidermal atrophy, a minimal mixed chronic infiltrate consisting of plasma cells, lymphocytes, and histiocytes in periappendageal and perivascular areas of upper dermis, suggestive of PKDL. Pityriasis lichenoides chronica was ruled out as there was no basal cell vacuolation. Ultrasonography of the abdomen suggested no pathology. The history of the patient revealed that he was treated for VL with miltefosine, 100 mg/day for 28 days about 7 years back from a tertiary hospital. Then, he had fever and enlargement of liver and spleen (symptoms for VL) and after treatment he was cured. He had appearance of hypopigmented lesions since last 3 years which gradually deepened with time. As the lesions were not disturbing, he was reluctant to visit clinic. He was started with miltefosine, 100 mg/day with complete disappearance of the lesions in 5 weeks. He was advised to continue miltefosine for 3 months.
Figure 4.
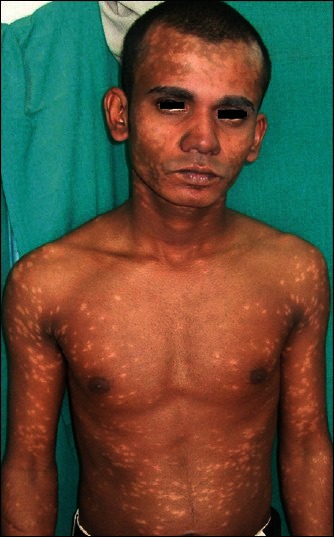
Case 2: Depigmented macules on body
Figure 5.
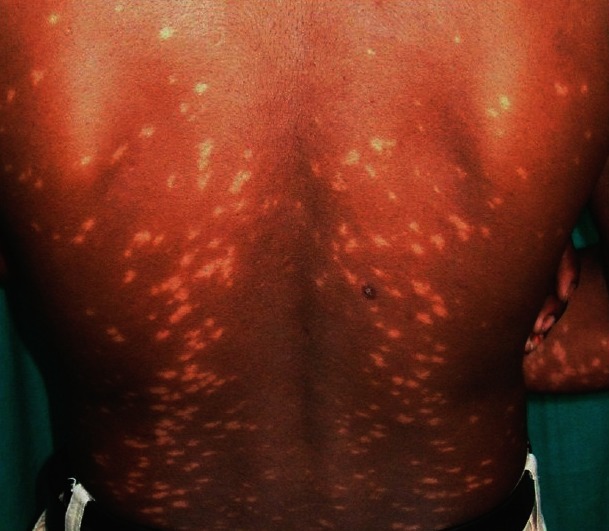
Case 2: Depigmented macules on body
Discussion
Both VL and PKDL are caused by same organism, Leishmania donovani. The exact mechanism and etiopathogenesis of PKDL are not definitely known.[1] More than 90% of the world's VL cases are found in India, Bangladesh, Brazil, Nepal, and Sudan. VL is endemic in the eastern states such as Bihar, eastern Uttar Pradesh, Jharkhand, and West Bengal.
The differential diagnoses of this symmetric hypopigmentation of body were pityriasis alba (in case 1), pityriasis versicolor, pityriasis lichenoides chronica, progressive macular hypomelanosis of trunk, and hypopigmented mycosis fungoides. Histopathology was very important in ruling out the differentials. The parasite demonstration in dermal lesions is the gold standard diagnostic criteria for PKDL.[5] The skin smears from depigmented macules did not show any amastigote. This is in compliance with other studies where no LD bodies could be demonstrated in macular lesions.[6]
rK39 is a rapid dipstick test, based on the rK39 protein, available for rapid diagnosis of VL and PKDL. Circulating anti-K39 IgG is detectable using laboratory ELISA tests. However, it cannot differentiate between recent and past infections. Previous cohort studies of past VL showed that rK39 test stayed positive upto 24 months in Sudan[7] and 4 years in India.[8] In a recent study from India, 68.2% were positive after 1-5 years, 57.7% after 5-10 years, and 42.5% after 10-15 years.[9] In our cases, rK39 tests were positive for 6-7 years after initial VL. Characteristic hypopigmented macules, history of post-kala-azar, positivity for rK39 strip test, suggestive histopathology and disappearance of lesions after starting of miltefosine helped us to diagnose the cases as PKDL. Polymerase chain reaction is the best test as it can identify the infection earliest and in any form. This facility was not available in our institute.
When the initial infection was rarely subclinical, PKDL occurred without even a preceding history of kala-azar. Parenteral amphotericin B emerged as very effective treatment for VL and incidences of PKDL decreased. Miltefosine (hexadecylphosphocholine) is comparatively a newer drug. It is cheaper and the biggest advantage is that it can be taken orally. It showed very good efficacy against VL[10] with minimal side effects. It has been found to be equally effective in PKDL but should be used for a longer duration, perhaps for 3 months.[11]
As PKDL cases are reservoirs of VL, they are of utmost epidemiological significance. Demonstration of LD bodies in slit skin smear or biopsy skin tissue is considered to be the gold standard for diagnosis of PKDL. But these methods are invasive, less sensitive, and difficult to perform at the periphery level. In hypopigmented lesions of PKDL, LD bodies are not expected to be found in biopsy. So, it is very important to diagnose all cases of PKDL clinically and start treatment.
The current VL elimination program focuses on treatment of VL with miltefosine. The initial case reports of PKDL, after treatment of VL with miltefosine, were made from India in 2009.[3,4] We report two such cases. Occurrences of more and more PKDL after treatment of VL with miltefosine may require reassessment of overall feasibility of the elimination program.
What is new?
Two cases of PKDL are reported here that developed after successful treatment of visceral leishmaniasis with miltefosine.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Das Gupta BM. A note on the parasite of dermal leishmanoid. Indian Med Gaz. 1927;62:11–2. [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmachari UN. A new form of cutaneous leishmaniasis-dermal leishmanoid. Indian Med Gaz. 1923;57:1125–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Das VN, Pandey K, Verma N, Lal CS, Bimal S, Topno RK, et al. Short report: Development of post-kala-azar dermal leishmaniasis (PKDL) in miltefosine-treated visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:336–8. [PubMed] [Google Scholar]
- 4.Kumar D, Ramesh V, Verma S, Ramam M, Salotra P. Post-kala-azar dermal leishmaniasis (PKDL) developing after treatment of visceral leishmaniasis with amphotericin B and miltefosine. Ann Trop Med Parasitol. 2009;103:727–30. doi: 10.1179/000349809X12554106963438. [DOI] [PubMed] [Google Scholar]
- 5.Sharma MC, Gupta AK, Verma N, Das VN, Saran R, Kar SK. Demonstration of Leishmania parasites in skin lesions of Indian post kala-azar dermal leishmaniasis (PKDL) cases. J Commun Dis. 2000;32:67–8. [PubMed] [Google Scholar]
- 6.Rathi SK, Pandhi RK, Chopra P, Khanna N. Post-kala-azar dermal leishmaniasis: A histopathological study. Indian J Dermatol Venereol Leprol. 2005;71:250–3. doi: 10.4103/0378-6323.16616. [DOI] [PubMed] [Google Scholar]
- 7.Zijlstra EE, Daifalla NS, Kager PA, Khalil EA, El-Hassan AM, Reed SG, et al. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998;5:717–20. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Kumari V, Singh N. Predicting kala-azar disease manifestations in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant K39 antigen. Clin Diagn Lab Immunol. 2002;9:568–72. doi: 10.1128/CDLI.9.3.568-572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidwani K, Picado A, Ostyn B, Singh SP, Kumar R, Khanal B, et al. Persistence of Leishmania donovani antibodies in past visceral leishmaniasis cases in India. Clin Vaccine Immunol. 2011;18:346–8. doi: 10.1128/CVI.00473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–46. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh V, Katara GK, Verma S, Salotra P. Miltefosine as an effective choice in the treatment of post-kala-azar dermal leishmaniasis. Br J Dermatol. 2011;165:411–4. doi: 10.1111/j.1365-2133.2011.10402.x. [DOI] [PubMed] [Google Scholar]


