Abstract
Pityriasis rosea (PR) is an acute or subacute inflammatory skin disease characterized by erythematous papulosquamous eruptions localized on the trunk and arms. The eruptions are self-limiting and usually disappear gradually in 2-10 weeks, without any treatment. Typical PR is much easier to diagnose than the rare atypical forms. There is a passing mention of PR with erythema multiforme-like lesions in the literature, but no extensive case series have been published till date. We present a series of five patients for whom we believe atypical PR is the likely diagnosis.
Keywords: Erythema multiforme, papulosquamous eruption, pityriasis rosea
Introduction
What was known?
Atypical variants of PR are rare. PR can be atypical with respect to morphology, size, distribution, number, site and course of disease.
Pityriasis rosea (PR) literally ‘rose-colored scale,’ was named as such by the French physician Camille Melchoir Gibert in 1860, but recognized by Willan as early as 1798.[1,2] The overall prevalence of PR has been estimated at 1.31% by Bjornberg and Hellgren, but taking into account the atypical forms that may go unnoticed, this figure is probably an underestimation.[3] A history of a herald patch and a few characteristic lesions in a ‘Christmas tree’ pattern aid the diagnosis of typical PR. It may present with atypical clinical features in around 20% cases. It is difficult to make a clear distinction between typical and atypical PR, and therefore it is important not to ascribe any unusual skin eruption with PR unless other dermatoses have been excluded. PR with erythema multiforme (EM)-like lesions is a very rarely reported variant. Only a handful of authors have reported this variant earlier.[4–9] Sharma et al. have mentioned one patient with target type lesions in their clinicoepidemiological study on PR,[8] more recently, Sinha et al. have reported EM-like lesions coexisting with papular PR lesions in an Indian patient.[9] In this paper, we report several patients who presented with EM-like PR [Table 1].
Table 1.
Clinical details of the patients studied
Case Reports
Case 1
A 15-year-old boy presented with a cutaneous eruption of 2 weeks duration. He gave history of appearance of a single erythematous scaly plaque on the left side of the abdomen initially. Within 7-10 days, he developed multiple erythematous slightly scaly papules and plaques over the trunk and both upper limbs, and few over both the thighs. Along with these lesions, he also noticed numerous erythematous plaques with depressed centers and peripheral elevated rings. All the lesions were arranged in a Christmas tree pattern. They were largely asymptomatic. He denied any history of painful fluid-filled lesions on the skin or mucosae, sexual contact or drug intake before the eruption. Physical examination revealed a herald patch measuring 2.5 × 1.5 cm over the abdomen and several smaller erythematous papules and plaques ranging from 0.2-1.5 cm in size, located on the trunk, both upper limbs, and proximal lower limbs. Numerous lesions were targetoid in appearance and were intermixed with typical looking PR lesions [Figure 1]. An active search was made for lesions suggestive of herpes simplex or dermatophytic infection but no such lesions were found. Examination also did not reveal any palpable lymph nodes, oral lesions or other significant pathology. A full blood count and blood chemistry profile yielded normal results. Antistreptolysin O (ASLO), C-reactive protein, venereal disease research laboratory (VDRL), Immunoglobulin M (IgM) for herpes simplex virus1 (HSV1) and HSV2 and throat swab were all normal. He was diagnosed as PR with EM-like lesions. He responded well to a 2 week course of 2.5% hydrocortisone cream and emollients.
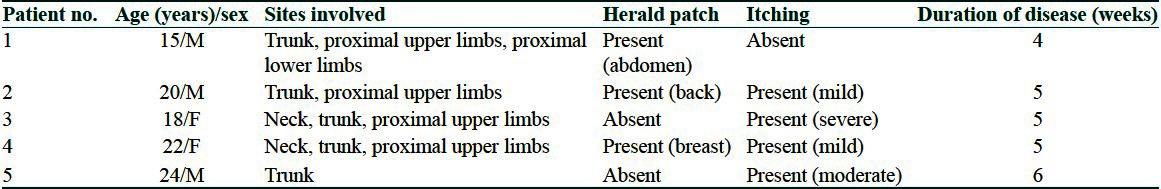
Figure 1.
Multiple scaly papules and plaques arranged in Christmas tree pattern. Herald patch on left side of abdomen can be seen. Numerous targetoid lesions are intermixed with typical looking PR lesions
Case 2
A 20-year-old man presented with a mildly pruritic eruption for 3 weeks. He gave history of a similar but larger lesion on his back 1 week prior to the eruption. Examination revealed numerous discrete patches on his trunk and upper limbs. There was a herald patch measuring 2 × 1 cm on the left side of the back. Few lesions showed collarette of scales while few showed iris like appearance [Figure 2]. Most of the lesions were oriented along the lines of skin cleavage. All blood investigations were within normal limits and VDRL and HSV serology was nonreactive. Scrapings from the lesions did not reveal any fungal hyphae or spores. He was prescribed emollients and oral fexofenadine 180 mg once a day for 2 weeks after which the lesions healed without any sequelae.
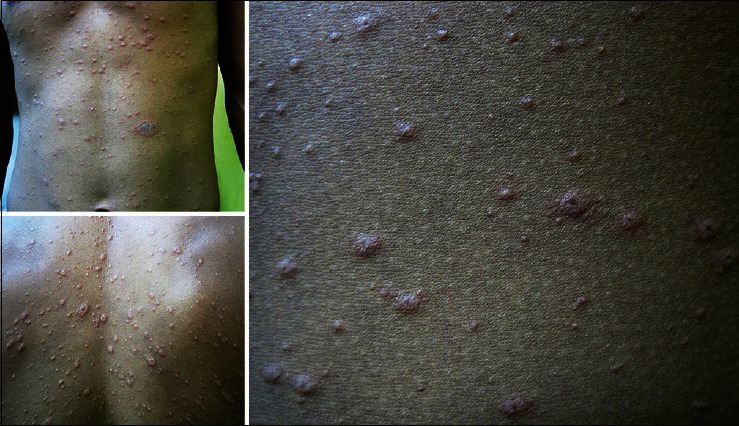
Figure 2.
Right forearm shows numerous targetoid lesions admixed with scaly papules and plaques
Case 3
An 18-year-old woman patient presented with a severely pruritic eruption for 1 week. She gave history of sore throat before the onset of the lesions. She complained of itching severe enough to disturb her sleep. Examination revealed multiple erythematous papules and plaques ranging in size from 0.2 to 1.5 cm present over the neck, trunk, and upper extremities. There was no herald patch. The lesions were of two types wherein some showed peripheral fine scaling while others showed central necrotic areas imitating EMlesions. All blood investigations were within normal limits. The patient was prescribed emollients and oral levocetrizine 5 mg daily. Complete symptomatic remission was seen after 4 weeks.
Case 4
A 22-year-old woman patient complained of an erythematous rash over her neck, upper trunk, and upper limbs for around 12 days. She also gave history of mild pruritus in the lesions. The patient had noticed a single large lesion over her left breast around a week prior to the other lesions. On examination, we noticed a herald patch over the left mammary area measuring around 1.5 × 1 cm. Multiple erythematous papules and plaques with fine scales and few with EM-like appearance, were present over the neck, upper trunk, and upper extremities [Figure 3]. We diagnosed her as PR with EM-like lesions. All blood investigations were within normal limits. Scrapings from the lesions were examined in 10% potassium hydroxide (KOH), no fungal hyphae or spores were found. She responded to a 3 week course of oral Loratadine 10 mg daily and liberal use of emollients.
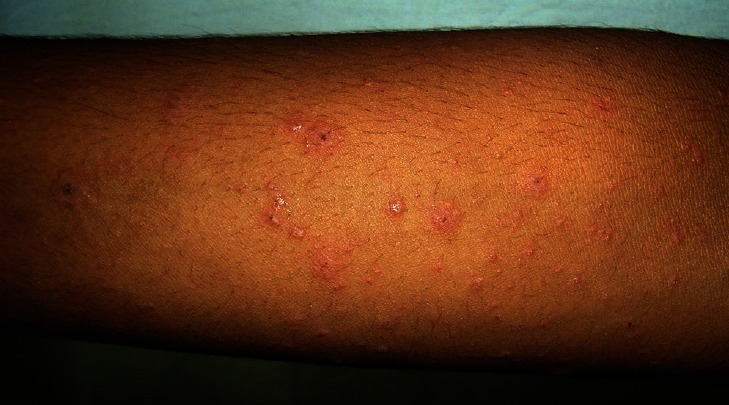
Figure 3.
Front of abdomen shows numerous targetoid lesions intermixed with PR lesions
Case 5
Our last case was a 24-year-old man who presented with a generalized eruption over his trunk for 2 weeks. He did not give history of any lesion preceding the rash. He complained of itching in the lesions that was discomforting but not severe enough to hamper routine activities and sleep. Physical examination revealed numerous erythematous plaques on his trunk and the upper limbs. Few of the lesions were typical papulosquamous lesion of PR and showed peripheral collarette of scales while few showed an iris like appearance suggestive of the EM variant of PR [Figure 4]. Most of the lesions were oriented along the lines of skin cleavage. All blood investigations were within normal limits and VDRL and HSV serology were nonreactive. He was prescribed topical betamethasone cream and oral levocetrizine 5 mg once daily for 4 weeks after which the lesions healed without any sequelae.
Figure 4.
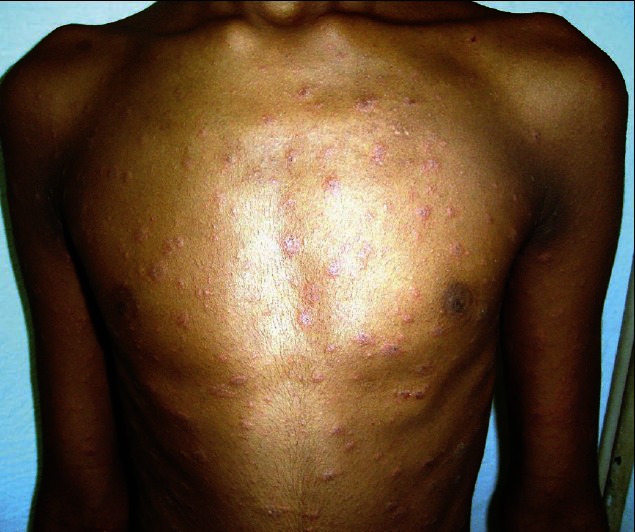
Multiple scaly papules and plaques arranged in Christmas tree pattern. Numerous targetoid lesions intermixed with typical looking PR lesions
These five patients presented to our outpatients department in the last 2 years. Age range at presentation was 15-22 years, and the male:female ratio was 3:2. Three of them had history of a herald patch preceding the eruption. Majority of the patients (4/5) reported mild to severe itching in the lesions. One patient also had multiple smaller lesions suggestive of papular PR along with the EM-like lesions. Blood tests including VDRL of all the patients were unremarkable.
In all the five patients two 4mm punch biopsies were done, one from a typical papulosquamouslesion of PR and the other from an EM-like lesion. The papulosquamous lesion revealed focal parakeratosis, prominent spongiosis, and perivascular lymphocytic infiltrate in the upper dermis. Extravasation of RBCs and exocytosis of lymphocytes into the epidermis was also seen [Figure 5]. The EM-like lesions showed focal thinning of the epidermis and significant spongiosis. The superficial dermis showed perivascular lymphocytic infiltrate. However, no characteristic features of EM such as vacuolar degeneration of the basal layer or satellite cell necrosis could be appreciated in any of the fivebiopsies [Figure 6].
Figure 5.
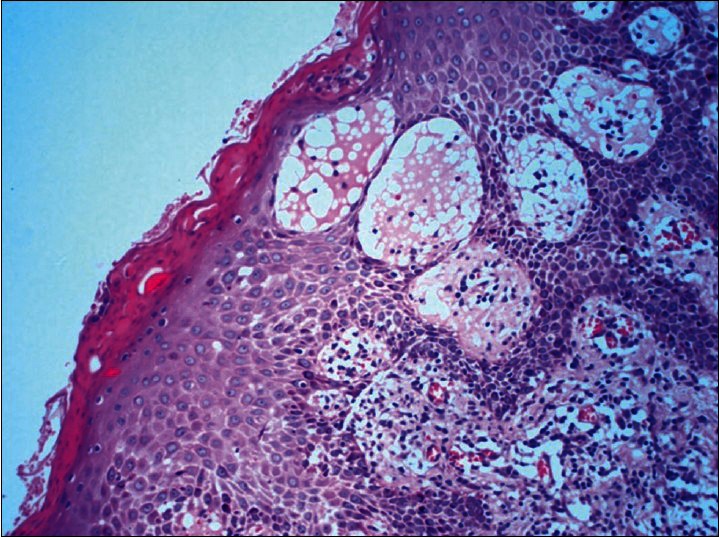
Biopsy from papulosquamous lesion reveal focal parakeratosis, prominent spongiosis and perivascular lymphocytic infiltrate in the upper dermis. Extravasation of RBCs and exocytosis of lymphocytes into the epidermis is also seen (H and E, ×400)
Figure 6.
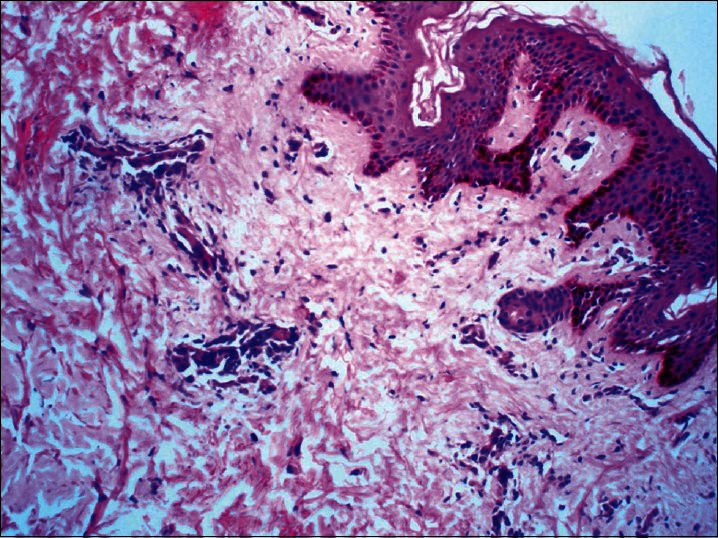
Biopsy from EM – Like lesions showed focal thinning of the epidermis and significant spongiosis. The superficial dermis showed perivascular lymphocytic infiltrate. However, no characteristic features of EM such as vacuolar degeneration of the basal layer or satellite cell necrosis could be seen (H and E, ×100)
Discussion
PR is a common disease reported in all races with an incidence of 6.8 per 1000 dermatological patients.[10] The overall male to female ratio is 1:1.5.[11] PR may occur in patients of all ages; however, approximately 75% of cases occur between the ages of 10 and 35 years.[12] The exact cause of PR is uncertain but Human Herpes Virus 6 and 7 have been suggested as the most likely cause.[13,14]
Atypical variants of PR are rare and occur in only 20% of cases. PR can be atypical with respect to morphology, size, distribution, number, site, and course of disease.[15] The various atypical morphological types include vesicular, purpuric, urticarial, generalized papular, lichenoid, erythrodermic, and EM-like PR.[15–20]
Vesicular PR presents as a generalized eruption of 2-6 mm vesicles or as a rosette of vesicles.[16] It may be severely pruritic, is most commonly seen in children and young people, and can be generalized in distribution, affecting the head, palms, and soles. It needs to be differentiated from varicella and dyshidrosis.
Purpuric (hemorrhagic) PR presents as macular purpura on skin and sometimes over the oral mucosa too. It has been described in adults as well as children.[17,18]
Urticarial PR (PR urticata) presents with lesions similar to urticarial wheals often accompanied by intense pruritus.[15]
Generalized papular PR is a rare form ofthe disorder that is more common in young children, pregnant women, and Afro-Caribbeans. It presents as multiple small 1-2 mm papules, which may occur along with classic patches and plaques.[19,20]
Lichenoid lesions can be observed in the course of atypical PR, but is more commonly caused by drugs like gold, captopril, barbiturates, D-penicillamine, and clonidine.
Recently, another atypical presentation of PR has been described by Bukhariin 2005 with plaques on the palms and soles, a very uncommonly involved site in PR.[21]
PR with EM-like lesions is an extremely uncommonly reported variant,[4–9] it is mentioned as one of the rarer atypical presentations in reviews but detailed case reports or series are unfortunately lacking in the current literature.
Histopathological findings of PRare relatively nonspecific and represent those of a subacuteor chronic dermatitis. Typically focal parakeratosis, a diminished granular layer, and spongiosis are seen. Small spongiotic vesicles are a characteristic but infrequently observed feature.[22] The papillary dermis shows some edema and mild-to-moderate lymphohistiocytic perivascular infiltrate. Exocytosis of the infiltrate is also noticed into the epidermis.
Two rather characteristic findingsof PR have been described: Extravasated erythrocytes in the dermis in 66% of patients as described by Ackermann, and dyskeratotic cells in the epidermis in 55% of cases as described by Okamoto et al.[23–25]
EM lesions show many similar features histologically. Epidermal changes include spongiosis and, characteristically, vacuolar degeneration of stratum basale.[22] Lymphocytic exocytosis into the epidermis is seen in EM as also in PR. Satellite cell necrosis is a distinguishing feature seen only in EM where lymphocytes are seen attached to scattered necrotic keratinocytes. Dermal changes are similar to those of PR including papillary dermal edema, dense mononuclear infiltrate, and extravasation of erythrocytes.[22]
We postulate that the EM-like lesions seen in our patients were morphological variants of PR itself and not a separate entity as all the patients had classic papulosquamous lesions arranged along the Langer's lines as well.
The cases could represent the occurrence of EM as a cutaneous reaction pattern that might have been manifested in response to the postulated causative viruses, HHV 6 or 7.
Conclusion
EM-like lesions may appear along with typical lesions of PRand may go un-recognized or mis-diagnosed and hence we report this case series.
What is new?
EM-like PR is a rare variant which should be considered in the differential diagnosis of targetoid lesions occurring in the setting of typical PR or even otherwise.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Gibert M. 3rd ed. Paris: Plon; 1860. Traitépratique des maladies de peauet de la syphilis; p. 402. [Google Scholar]
- 2.Percival GH. Pityriasisrosea. Br J Dermatol. 1932;44:241–53. [Google Scholar]
- 3.Bjornberg A, Hellgren L. Pityriasisrosea: A statistical, clinical, and laboratory investigation of 826 patients and matched healthy controls. Acta Derm Venereol Suppl (Stockh) 1962;42:1–68. doi: 10.2340/0001555542168. [DOI] [PubMed] [Google Scholar]
- 4.Niles HD, Klumpp MM. Pityriasisrosea: Review of literature and report of two hundred and nineteen cases, in thirty eight of which convalescent serum was used. Arch Dermatol Syph. 1940;41:265–94. [Google Scholar]
- 5.Benedek T. Statistical Research to the knowledge of Pityriasis rosea. Acta Dermatol Venereol. 1936;17:151. [Google Scholar]
- 6.Parsons JM. Pityriasisrosea update: 1986. J Am Acad Dermatol. 1986;15:159–67. doi: 10.1016/s0190-9622(86)70151-5. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SJ. Pityriasisrosea with erythema multiforme like lesions. J Am Acad Dermatol. 1987;17:135–6. doi: 10.1016/s0190-9622(87)80542-x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma L, Srivastava K. Clinicoepidemiological study of pityriasisrosea. Indian J Dermatol Venereol Leprol. 2008;74:647–9. doi: 10.4103/0378-6323.45113. [DOI] [PubMed] [Google Scholar]
- 9.Sinha S, Sardana K, Garg VK. Coexistence of two atypical variants of pityriasisrosea: A case report and review of literature. Pediatr Dermatol. 2012;29:538–40. doi: 10.1111/j.1525-1470.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- 10.Tay YK, Goh CL. One year review of pityriasisrosea at the National Skin Centre, Singapore. Ann Acad Med Singapore. 1999;28:829–31. [PubMed] [Google Scholar]
- 11.Chuang TY, Ilstrup DM, Perry HO, Kurland LT. Pityriasisrosea in Rochester, Minnesota: 1969 to 1978. J Am Acad Dermatol. 1982;7:80–9. doi: 10.1016/s0190-9622(82)80013-3. [DOI] [PubMed] [Google Scholar]
- 12.Truhan AP. Pityriasisrosea. Am Fam Physician. 1984;29:193–6. [PubMed] [Google Scholar]
- 13.Watanabe T, Kawamura T, Jacob SE, Aquilino EA, Orenstein JM, Black JB, et al. Pityriasisrosea is associated with systemic active infection with both human herpesvirus 7 and human herpesvirus 6. J Invest Dermatol. 2002;119:793–7. doi: 10.1046/j.1523-1747.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 14.Vag T, Sonkoly E, Kemery B, Ongradi J. Avidity of antibodies to HHV – 7 suggests primary infection in young adults with pityriasisrosea. J Eur Acad Dermatol Venereol. 2004;18:738–40. doi: 10.1111/j.1468-3083.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 15.Chuh A, Zawar V, Lee A. Atypical presentations of pityriasisrosea: Case presentations. J Eur Acad Dermatol Venereol. 2005;19:120–6. doi: 10.1111/j.1468-3083.2004.01105.x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia RL. Vesicular pityriasisrosea. Arch Dermatol. 1976;112:410. [PubMed] [Google Scholar]
- 17.Pierson JC, Dijikstra JW, Elston DM. Purpuricpityriasisrosea. J Am Acad Dermatol. 1993;28:1021. doi: 10.1016/s0190-9622(08)80661-5. [DOI] [PubMed] [Google Scholar]
- 18.Verbov J. Purpuricpityriasisrosea. Dermatologica. 1980;160:142–4. doi: 10.1159/000250488. [DOI] [PubMed] [Google Scholar]
- 19.Bernardin RM, Ritter SE, Murchland MR. Papularpityriasisrosea. Cutis. 2002;70:51–5. [PubMed] [Google Scholar]
- 20.Vano-Galvan S, Ma DL, Lopez-Neyra A, Perez B, Muñoz-Zato E, Jaén P. Atypical pityriasisrosea in a black child. Cases J. 2009;2:6796. doi: 10.1186/1757-1626-2-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukhari I. Pityriasisrosea with palmoplantar plaque lesions. Dermatol Online J. 2005;11:27. [PubMed] [Google Scholar]
- 22.Weedon D. New York, NY: Churchill Livingstone; 1998. Skin pathology; pp. 96–7. [Google Scholar]
- 23.Imamura S, Ozaki M, Oguchi M, Okamoto H, Horiguchi Y. Atypical pityriasisrosea. Dermatologica. 1985;171:474–7. doi: 10.1159/000249476. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman AG. Philadelphia: Lea and Febiger; 1978. Histologic diagnosis of inflammatory skin diseases; pp. 233–5. [Google Scholar]
- 25.Okamoto H, Imamura S, Aoshima T, Komura J, Ofuji S. Dyskeratotic degeneration of epidermal cells in pityriasisrosea: Light and electron microscopic studies. Br J Dermatol. 1982;107:189–94. doi: 10.1111/j.1365-2133.1982.tb00337.x. [DOI] [PubMed] [Google Scholar]


