Abstract
Background
Isoflurane can increase pro-inflammatory cytokine interleukin (IL)-6 levels. However, the up-stream mechanism remains unknown. Nuclear factor-kappa B (NF-κB) promotes the generation of pro-inflammatory cytokines. We examined the effects of isoflurane and sevoflurane on the NF-κB signalling pathway and its association with IL-6 levels in cultured cells.
Methods
H4 human neuroglioma cells (H4 cells), and mouse primary neurones and microglia were treated with 2% isoflurane or 4.1% sevoflurane for 6 h, for analysis of IL-6 and NF-κB. Pyrrolidine dithiocarbamate (an NF-κB inhibitor) or 2-deoxy-d-glucose (2-DG) (an inhibitor of glucose glycolysis) was applied 1 h before anaesthetic treatment.
Results
Isoflurane or sevoflurane treatment increased the levels of IL-6 [isoflurane: 410% (54); sevoflurane: 290% (24)], the nuclear levels of NF-κB [isoflurane: 170% (36); sevoflurane: 320% (30)], and the transcription activity of NF-κB in H4 cells. Moreover, isoflurane enhanced the transcription activity of NF-κB in mouse microglia, but not primary neurones. Finally, pyrrolidine dithiocarbamate and 2-DG attenuated isoflurane-induced increases in IL-6 and NF-κB, and the transcription activity of NF-κB.
Conclusions
These studies in H4 cells suggest that the NF-κB signalling pathway could contribute to isoflurane or sevoflurane-induced neuroinflammation. This could lead to the targeted intervention of anaesthetic-induced neuroinflammation.
Keywords: anaesthetic, interleukin-6, NF-κB
Editor's key points.
Isoflurane can produce cognitive dysfunction in rodents, possibly involving pro-inflammatory signalling pathways.
The role of nuclear factor-kappa B (NF-κB) in anaesthetic-induced increases in the inflammatory cytokine interleukin (IL)-6 was investigated in vitro.
Activation of inflammatory signalling by isoflurane and sevoflurane increased IL-6, which might contribute to neuroinflammation and cognitive dysfunction.
Postoperative cognitive dysfunction (POCD) is a common complication in senior patients,1 and is associated with increased cost, morbidity, and mortality.2–4 Age is a known risk factor for POCD, but its pathogenesis remains largely unknown. This lack of knowledge has become a barrier that impedes further studies of POCD, and thus a lack of treatment or prevention for POCD.
The commonly used inhalation anaesthetic isoflurane has been shown to induce cognitive impairment in rodents,5–7 and might also be associated with a higher incidence of POCD in humans.8 Isoflurane can increase levels of interleukin (IL)-6,9 and IL-6 has been associated with learning and memory impairment in animals,10–12 and cognitive dysfunction,13,14 mild cognitive impairment (MCI)15 and delirium in medical patients.16 It is conceivable that isoflurane induces neurobehavioural deficits through increasing brain IL-6 levels. However, the up-stream mechanism by which isoflurane increases IL-6 levels remains largely to be determined. This gap in knowledge prevents further mechanistic studies and potential interventions of isoflurane-induced neurobehavioural deficits.
Isoflurane can increase cytosolic calcium levels in H4 human neuroglioma cells (H4 cells) and other tumour cell lines.17–20 The elevation of cytosolic calcium can activate the nuclear factor-kappa B (NF-κB) signalling pathway,21–26 which is associated with increased levels of pro-inflammatory cytokines.27 Pyrrolidine dithiocarbamate (PDTC) can inhibit BNF-κB signalling pathway by attenuating the entrance of activated NF-κB into the nucleus and binding of NF-κB to the promoter region of multiple genes.28–30 2-Deoxy-d-glucose (2-DG), an inhibitor of glycolysis, can attenuate isoflurane-induced elevation of cytosolic calcium.18 We, therefore, assessed the effects of isoflurane on the levels of IL-6 and NF-κB in H4 cells and determined whether the effects of isoflurane on IL-6 and NF-κB could be attenuated by PDTC and 2-DG. We also assessed the effects of isoflurane on the transcription activity of NF-κB in mouse primary neurones and microglia.
Methods
H4 human neuroglioma cells
H4 cells have been extensively used in the Alzheimer's disease research as an in vitro cellular model,7,17,31 and these in vitro findings have been confirmed in primary neurones and brain tissue of mice.7,32 Cells were cultured in Dulbecco's Modified Eagle Medium (high glucose) containing 10% (v/v) heat-inactivated fetal calf serum, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin, and 2 mM glutamine. Oxygen (21%), 5% CO2, and 2% isoflurane or 4.1% sevoflurane were delivered from an anaesthesia machine to a sealed plastic box containing the cells in an incubator at 37°C. A Datex infrared gas analyser (Puritan-Bennett, Tewksbury, MA, USA) was used to continuously monitor the delivered CO2, O2, and isoflurane concentrations. We treated the cells with 2% isoflurane or 4.1% sevoflurane for 6 h in serum free media,33,34 which has been shown to increase cytosolic calcium,17 caspase-3 activation,31 Aβ accumulation,31 and mitochondrial dysfunction.7 In some studies, the cells were treated with 10 μM PDTC35 (Sigma, St. Louis, MO, USA) or 10 mM 2-DG18 (Sigma) 1 h before treatment with 2% isoflurane. The control conditions for isoflurane and PDTC or 2-DG was 5% CO2 plus 21% O2 and saline.
Mouse primary neurones and microglia
The protocol was approved by the Massachusetts General Hospital Standing Committee on the Use of Animals in Research and Teaching. The harvest of neurones was performed as described.34 Microglia cells were harvested as described.9 After 7–10 days in culture, the cells were treated with 2% isoflurane for 6 h as described.34
Immunoblotting
Immunoblot analysis was performed as described.7 Briefly, cell pellets were detergent-extracted on ice using immunoprecipitation buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM Ethylenediaminetetraacetic acid (EDTA), 0.5% Nonidet P-40) plus protease inhibitors (1 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, and 1 µg ml−1 pepstatin A). The lysates were collected, centrifuged at 12 000×g for 10 min, and proteins determined with a bicinchoninic acid protein assay kit (Pierce, Iselin, NJ, USA). Antibodies to NF-κB (1:1000; sc-109 Santa Cruz, CA, USA), IL-6 (1:1000; ab6672, Abcam, Cambridge, MA, USA), or β-actin (1:5000, Sigma) were used to detect NF-κB, IL-6 and β-actin, respectively.
Real-time polymerase chain reaction
The effects of isoflurane on IL-6 mRNA were determined by real-time polymerase chain reaction (RT-PCR) in H4 cells as described.32 RNA was isolated using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA), with concentration determined using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Primers of human IL-6 (ID: QT00083720) and human glyceraldhyde 3-phosphate dehydrogenase (GAPDH) (ID: QT01192646) were purchased from Qiagen. RT-PCR was carried out using the QuantiTect SYBR Green RT-PCR Kit (Qiagen). IL-6 mRNA levels were determined and standardized with GAPDH as internal controls.
Nuclear extraction
A nuclear extraction kit (SK-0001, Signosis, Inc., Sunnyvale, CA, USA) was used for the preparation of nuclear extracts from H4 cells. Cells were washed three times with phosphate buffered saline. Then, buffer I working reagent was added into the cells. The culture dish was put into an icebox and rocked at 200 rpm for 10 min on a shaking platform. Cells were released from the dish using a sterile scraper and transferred to a 1.5 ml microcentrifuge tube to centrifuge at 12 000×g for 5 min at 4°C. The supernatant was discarded completely and buffer II working reagent was added to the pellets and mixed gently. The tube was put into an icebox and shaken at 200 rpm on a platform for 2 h. The sample was centrifuged at 12 000×g for 5 min at 4°C, and the nuclear extract was obtained in the supernatant.
Electrophoretic-mobility shift assay
An electrophoretic-mobility shift assay (EMSA) kit (GS-0030, Signosis) was used to assess the transcription binding activity of NF-κB. Nuclear extract (5 µg) was incubated with 1 µl poly d(I-C), 2.0 μl 5X Binding Buffer and 1.0 μl of transcription factor (TF) probe in a 0.5 ml microcentrifuge tube (PCR tube) at 20–23°C for 30 min in a PCR machine. For the cold probe control, 1.0 μl of cold TF probe was added into this reaction. Samples were loaded onto a 6.5% non-denaturing polyacrylamide gel, which was run at 100 V and transferred at 60 V for 1 h at 4°C. The membrane was imaged using a chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA).
Statistics
Data are expressed as mean (sd). Normality test showed that the data were normally disturbed (data not shown). Student's t-test, and one-way and two-way analysis of variance (anova) were used to compare differences from the control group, followed by the Bonferroni post hoc test where appropriate. P<0.05 (* or #) and P<0.01 (** or ##) were considered statistically significant. The significance testing was two-tailed, and the Prism 6 software (La Jolla, CA, USA) was used to analyse the data.
Results
Isoflurane increases levels of IL-6 in the H4 cells
H4 cells were treated with 2% isoflurane for 6 h, harvested, and subjected to immunoblot analysis. IL-6 immunoblotting revealed that isoflurane (lanes 4–6 in Fig. 1) increased IL-6 compared with the control group (lanes 1–3 in Fig. 1). There was no significant difference in β-actin between isoflurane-treated and control cells. These results suggest that isoflurane is able to increase the levels of IL-6 in H4 cells.
Fig 1.
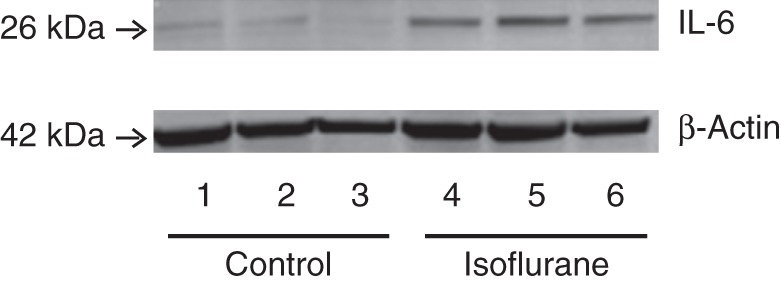
Isoflurane increases IL-6 in H4 cells. (a) Isoflurane (lanes 4–6) increases IL-6 levels compared with control (lanes 1–3) normalized to β-actin. IL, interleukin.
Isoflurane increases nuclear levels of NF-κB and its transcription binding activity in H4 cells and mouse microglia
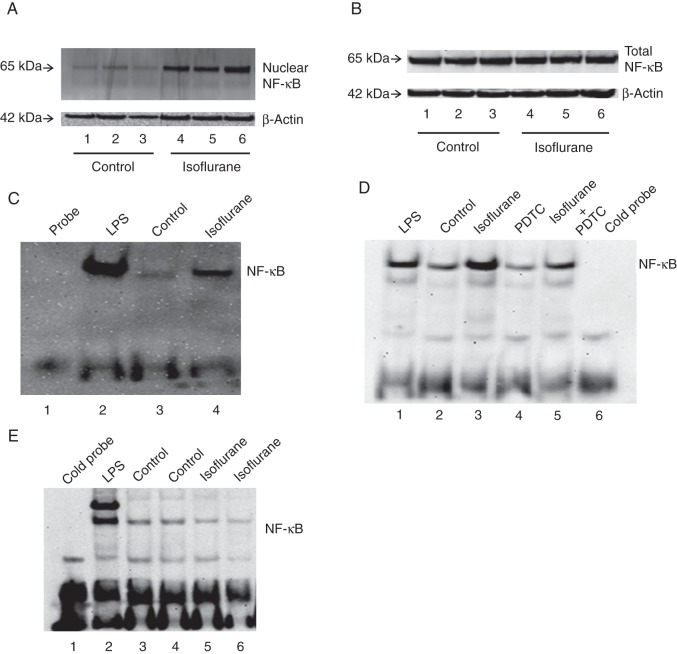
Activated NF-κB translocates to the nucleus where it binds to the promoter region of multiple genes, including cytokine genes,21–26,36 leading to expression of cytokine mRNA. Thus, we assessed the effects of isoflurane on the nuclear levels of NF-κB in H4 cells. H4 cells were harvested at the end of isoflurane treatment, or nuclear extract prepared, and subjected to immunoblotting. Immunoblotting of NF-κB showed that isoflurane (lanes 4–6, Fig. 2a) increased nuclear levels of NF-κB compared with the control group (lanes 1–3, Fig. 2a). There was no significant difference in β-actin between isoflurane-treated and control H4 cells. Next, we assessed the effects of the isoflurane treatment on total NF-κB levels in H4 cells. Isoflurane did not significantly alter total NF-κB levels (Fig. 2b). These findings suggest that isoflurane facilitates nuclear translocation of NF-κB.
Fig 2.
Isoflurane increases nuclear NF-κB levels and the transcription binding activity of NF-κB. (a) Isoflurane (lanes 4–6) increases NF-κB levels in nuclear extracts from H4 cells relative to control (lanes 1–3). (b) Isoflurane does not change total NF-κB levels in H4 cells. (c) Isoflurane enhances the transcription binding activity of NF-κB in H4 cells. (d) Isoflurane enhances the transcription binding activity of NF-κB in microglia from mice. PDTC attenuates the isoflurane-induced enhancement of the transcription binding activity of NF-κB in mouse microglia. (e) Isoflurane does not enhance the transcription binding activity of NF-κB in primary neurones from mice. NF-κB, nuclear factor-kappa B; LPS, lipopolysaccharide; PDTC, pyrrolidine dithiocarbamate.
Finally, we determined the effect of isoflurane on transcription binding activity of NF-κB in nuclear extracts prepared from H4 cells using EMSA.21 In Figure 2c, lane 1 is the probe, lane 2 is treatment with lipopolysaccharide, a positive control that enhances transcription binding activity of NF-κB,37 lane 3 is control, and lane 4 is isoflurane treatment. Isoflurane enhanced the transcription binding activity of NF-κB compared with the control group (Fig. 2c). We then assessed whether isoflurane could enhance the transcription binding activity of NF-κB in mouse primary neurones and microglia. Treatment with 2% isoflurane enhanced the transcription binding activity of NF-κB in microglia (Fig. 2d), but not primary neurones (Fig. 2e). The NF-κB inhibitor PDTC attenuated isoflurane-induced enhancement of the transcription binding activity of NF-κB in microglia (Fig. 2d).
PDTC attenuates isoflurane-induced increase in IL-6 levels in H4 cells
We assessed whether the NF-κB inhibitor PDTC might attenuate the isoflurane-induced increase in IL-6 in H4 cells. Two-way anova demonstrated that there was a significant interaction of group (control and isoflurane) and treatment (saline and PDTC) (F=24.78, P=0.0001). RT-PCR showed that isoflurane increased mRNA levels of IL-6 in H4 cells (Fig. 3a): 4.8 (1.8)-fold P<0.01 (one-way anova with Bonferroni correction). PDTC alone did not significantly alter IL-6 mRNA levels, but PDTC attenuated the isoflurane-induced increase in IL-6 mRNA levels in H4 cells: 1.1 (0.7) vs 4.8 (1.8) (Fig. 3a). PDTC also attenuated the isoflurane-induced increase in protein levels of IL-6 (F=17, P=0.0045, two-way anova): 410% (54) vs 76% (44) (Fig. 3b and c). These data suggest that isoflurane increases the mRNA and protein levels of IL-6 via NF-κB signalling.
Fig 3.
PDTC attenuates isoflurane-induced increases in the mRNA and protein levels of IL-6 in H4 cells. (a) Isoflurane increases IL-6 mRNA level in the H4 cells, and PDTC attenuates this increase. (b) Isoflurane increases protein levels of IL-6, and PDTC attenuates this increase. (c) Quantification of the immunoblots shows that PDTC attenuates the isoflurane-induced increase in the protein levels of IL-6. IL, interleukin; PDTC, pyrrolidine dithiocarbamate (n=9).
PDTC attenuates isoflurane-induced activation of NF-κB signalling
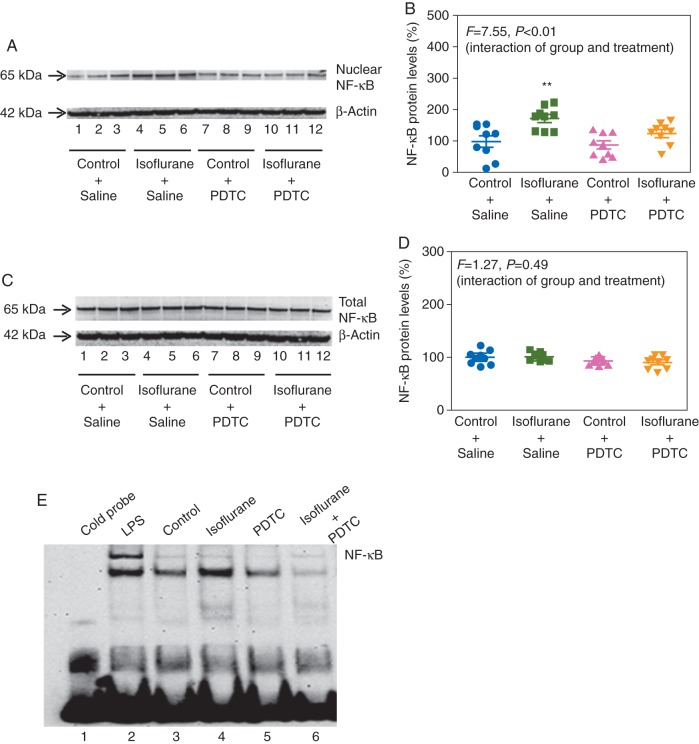
Immunoblotting of NF-κB showed that isoflurane increased nuclear levels of NF-κB in H4 cells (Fig. 4a). Two-way anova showed a significant interaction between the group (control and isoflurane) and treatment (saline and PDTC): F=7.55, P=0.0055 (Fig. 4b). PDTC attenuated the isoflurane-induced increase in nuclear NF-κB levels: 130% (34) vs 170% (36) (Fig. 4b). PDTC did not significantly alter total NF-κB levels (Fig. 4c and d, F=1.265, P=0.49, two-way anova) (Fig. 4c and d). Finally, PDTC attenuated the isoflurane-induced enhancement of transcription binding activity of NF-κB (Fig. 4e).
Fig 4.
PDTC attenuates the isoflurane-induced changes in NF-κB in H4 cells. (a) Isoflurane increases nuclear levels of NF-κB, and PDTC attenuates the increase. (b) Quantification of the immunoblots shows that PDTC attenuates the isoflurane-induced increase in nuclear NF-κB levels. (c) PDTC does not alter total NF-κB levels. (d) Quantification of the immunoblots shows that PDTC does not alter total NF-κB levels. (e) PDTC inhibits the isoflurane-induced activation of the transcription binding activity of NF-κB. PTDC, pyrrolidine dithiocarbamate; NF-κB, nuclear factor-kappa B (n=9); LPS, lipopolysaccharide.
Sevoflurane increases levels of IL-6, nuclear levels of NF-κB and the transcription binding activity of NF-κB in H4 cells
Given the findings that isoflurane might increase mRNA and protein levels of IL-6 through NF-κB signalling pathway, we determined whether sevoflurane has similar effects as sevoflurane induces caspase-3 activation and Aβ accumulation in H4 cells.38 IL-6 immunoblotting revealed that 4.1% sevoflurane for 6 h (lanes 4–6 in Fig. 5a) increased IL-6 compared with the control group (lanes 1–3 in Fig. 5a). There was no significant difference in the levels of β-actin between sevoflurane-treated and control cells. Quantification of the immunoblots showed that sevoflurane increased protein levels of IL-6 compared with control; 320% (30), P=0.0001 (Fig. 5b).
Fig 5.
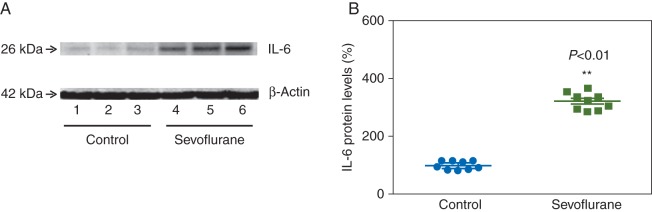
Sevoflurane increases IL-6 levels in H4 cells. (a) Sevoflurane treatment (lanes 4–6) increases IL-6 levels. (b) Quantification of the immunoblots shows that sevoflurane increases IL-6 levels. IL, interleukin (n=9).
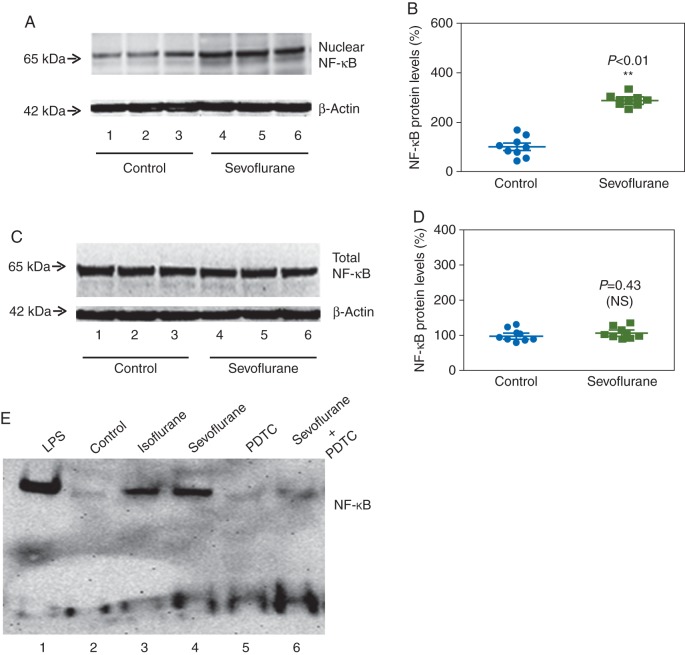
Immunoblotting of NF-κB showed that sevoflurane (lanes 4–6, Fig. 6a) increased nuclear levels of NF-κB in H4 cells compared with the control group (lanes 1–3, Fig. 6a). There was no significant difference in levels of β-actin. Quantification of the immunoblots showed that sevoflurane increased nuclear levels of NF-κB in H4 cells: 290% (23), P=0.0001 (Fig. 6b). Sevoflurane did not significantly alter total NF-κB levels (Fig. 6c and d). These findings suggest that sevoflurane facilitates NF-κB translocation to the nucleus, rather than affecting total levels. Sevoflurane enhanced transcription binding activity of NF-κB compared with the control group (Fig. 6e). These data suggest that sevoflurane, like isoflurane, increases IL-6 levels via NF-κB activation of IL-6 gene, which was attenuated by PDTC.
Fig 6.
Sevoflurane increases nuclear NF-κB levels and the transcription binding activity of NF-κB in H4 cells. (a) Sevoflurane treatment (lanes 4–6) increases NF-κB levels in nuclear extracts. (b) Quantification of the immunoblots shows that sevoflurane increases NF-κB levels in nuclear extracts. (c) Sevoflurane does not change total NF-κB levels. (d) Quantification of the immunoblots shows that sevoflurane does not change total NF-κB levels. (e) Sevoflurane enhances the transcription binding activity of NF-κB, and PDTC attenuates the sevoflurane-induced enhancement of the transcription binding activity of NF-κB. NF-κB, nuclear factor-kappa B; LPS, lipopolysaccharide; PDTC, pyrrolidine dithiocarbamate (n=9).
2-DG reduces isoflurane-induced increases in IL-6 and nuclear NF-κB in H4 cells
2-DG attenuates isoflurane-induced elevation of cytosolic calcium,18 thus, we assessed whether 2-DG could attenuate the isoflurane-induced increases in IL-6 and nuclear NF-κB in H4 cells. Quantitative immunoblotting showed that 2-DG reduced the isoflurane-induced increase in IL-6 (Fig. 7a). Two-way anova demonstrated a significant interaction of group (control and isoflurane) and treatment (saline and 2-DG): F=4.382, P=0.044 (Fig. 7b). Specifically, 2-DG attenuated the isoflurane-induced increase in IL-6 levels: 180% (23) vs 120% (34).
Fig 7.
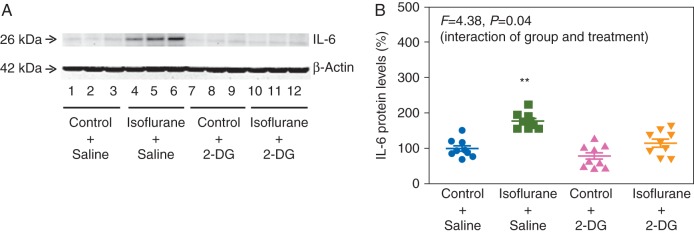
2-DG reduces the isoflurane-induced increase of IL-6 in H4 cells. (a) 2-DG attenuates the isoflurane-induced increase in IL-6 levels. (b) Quantification of the immunoblots shows that 2-DG attenuates the isoflurane-induced increase in IL-6 levels. 2-DG, 2-deoxy-d-glucose; IL, interleukin (n=9).
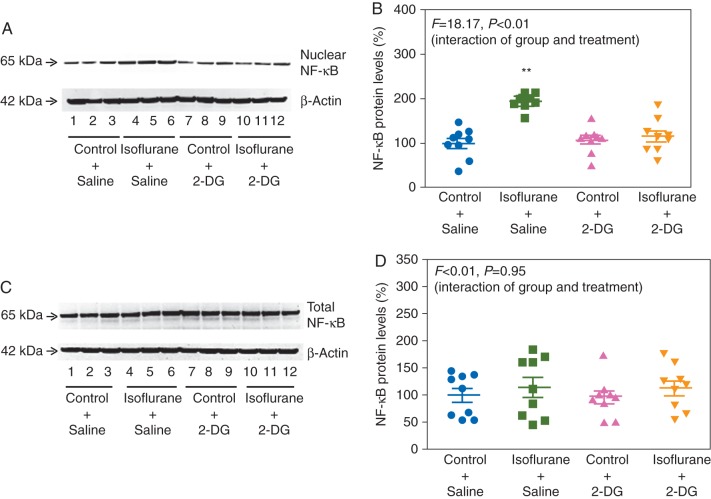
2-DG also attenuated the isoflurane-induced increase in nuclear NF-κB levels (F=18.17, P=0.0002, two-way anova): 200% (18) vs 120% (38) (Fig. 8a and b). Finally, there was no significant interaction between group (control and isoflurane) and treatment (saline and 2-DG) on total NF-κB (F=0.0048, P=0.95) (Fig. 8c and d). Taken together, these findings suggest that 2-DG reduces the isoflurane-induced increase in IL-6 by decreasing nuclear NF-κB levels.
Fig 8.
2-DG reduces the isoflurane-induced increases in nuclear levels of NF-κB in H4 cells. (a) 2-DG inhibits the isoflurane-induced increase in nuclear NF-κB. (b) Quantification of the immunoblots shows that 2-DG inhibits the isoflurane-induced increase in nuclear levels of NF-κB. (c) 2-DG does not alter total NF-κB levels. (d) Quantification of the immunoblots shows that 2-DG treatment does not alter total NF-κB levels. NF-κB, nuclear factor-kappa B; 2-DG, 2-deoxy-d-glucose (n=9).
Discussion
The commonly used inhalation anaesthetic isoflurane can induce neuroinflammation (e.g. increasing IL-6 levels in the brain tissues of mice),9 which could contribute to the isoflurane-associated decline in cognitive function in rodents5–7 and possibly humans.8 However, it is largely unknown how isoflurane increases IL-6 levels. We, therefore, established a cellular system to determine the mechanism by which isoflurane increases IL-6 using H4 cells. Treatment with isoflurane increased IL-6 in H4 cells. These data, together with our previous findings that isoflurane increases cytosolic calcium,17 mitochondrial dysfunction,7 caspase-3 activation,31 and Aβ accumulation31 in H4 cells, suggested that we could use these cells to perform mechanistic studies. We found that isoflurane increased NF-κB in nucleus, but not in whole cells. NF-κB belongs to a family of inducible dimeric transcription factors that recognizes a consensus DNA sequence and regulates many target genes, especially genes involved in inflammation, injury, and stress.39 NF-κB is usually present in the cytoplasm as a p65 and p50 heterodimer. Activated NF-κB enters the nucleus and promotes transcription of its target genes, including the pro-inflammatory cytokine IL-6.40 The finding that isoflurane increased nuclear levels of NF-κB but not total NF-κB levels suggests that isoflurane might facilitate translocation of NF-κB to the nucleus. Moreover, isoflurane enhances the transcription activity of NF-κB. These findings suggest that isoflurane induces activation of NF-κB signalling by facilitating translocation of NF-κB into the nucleus and transcription.
Interestingly, the isoflurane-induced increase in transcription activity of NF-κB was cell-type dependent, occurring in mouse microglia, but not neurones. These findings suggest that isoflurane specifically targets microglia to induce neuroinflammation. The ‘neuroinflammation AD hypothesis’ suggests that microglia-associated neuroinflammation impairs axonal transport by inducing mitochondrial dysfunction,41 and isoflurane can induce mitochondrial dysfunction.7 It would be interesting to know whether isoflurane can induce microglia-dependent neuroinflammation, which then leads to impairment of axonal transport through mitochondrial dysfunction. Moreover, it is important to systematically compare the effects of different anaesthetics (e.g. isoflurane, sevoflurane, desflurane, propofol, and ketamine) on neuroinflammation, its up-stream mechanisms such as NF-κB signalling, and down-stream consequences such as impairment of axonal transport in future studies.
These findings cannot specifically determine the cause–effect relationships between isoflurane-induced increases in IL-6 and isoflurane-induced activation of NF-κB signalling. The findings that the NF-κB pathway inhibitor PDTC inhibited both isoflurane-induced increase in IL-6 and activation of NF-κB signalling further suggest that isoflurane increases IL-6 levels via activation of NF-κB signalling. Finally, we found that 2-DG attenuated the isoflurane-induced increase in IL-6 levels by inhibiting activation of NF-κB signalling.
Sevoflurane, another commonly used inhalation anaesthetic, also increased the levels of IL-6 and nuclear NF-κB, but not total NF-κB. These findings suggest that sevoflurane, like isoflurane, increases IL-6 protein levels via NF-κB-mediated activation of IL-6 expression. Future research should include in vivo relevance experiments, including studies to determine whether PDTC and 2-DG can also attenuate isoflurane or sevoflurane-induced cognitive impairment in vivo.
These studies have several limitations. First, we did not determine isoflurane effects on the levels of other pro-inflammatory cytokines (e.g. tumour necrosis factor-α), which itself can activate NF-κB signalling. We, therefore, chose IL-6 to establish a cellular system to investigate anaesthetic effects on pro-inflammatory cytokines. Secondly, we did not study the effects of isoflurane on NF-κB signalling in brain tissue in vivo. It is technically difficult to determine the effects of isoflurane on the transcription binding activity of NF-κB using EMSA in brain tissue. Nevertheless, our findings illustrate an up-stream mechanism by which isoflurane might increase levels of the pro-inflammatory cytokine IL-6. Future studies are required to determine the in vivo relevance of these in vitro findings. Thirdly, cytokines are normally generated and released from glial cells. We did not assess the effects of isoflurane on IL-6 levels and the nuclear level of NF-κB in microglia. However, our studies illustrate that isoflurane enhanced transcription activity of NF-κB in cultured mouse microglia, but not neurones. Finally, our studies did not systematically assess the time- and dose-dependent effects of isoflurane and sevoflurane on NF-κB signalling. The main goal of the current studies was to establish a cellular model to determine up-stream mechanisms by which anaesthetics increase pro-inflammatory cytokines. These findings should facilitate future studies of the potential effects of anaesthetics on neuroinflammation and the underlying mechanisms.
In conclusion, we have established a cellular system to study the mechanisms by which anaesthetics affect the pro-inflammatory cytokine IL-6. Either isoflurane or sevoflurane can increase IL-6 levels via activation of NF-κB signalling, which can be attenuated by inhibition of the NF-κB signalling pathway or glycolysis. This suggests potential therapeutic approaches to prevent or treat anaesthesia neurotoxicity.
Authors' contributions
Conceived and designed the experiments: L.Z.; J.Z., Y.Z., and Z.X. Performed the experiments: L.Z., J.Z., L.Y., and Y.D. Analysed the data: J.Z., Y.D. Wrote the paper: Z.X. and Y.Z.
Declaration of interest
None declared.
Funding
This research was supported by R21AG038994, R01 GM088801 and R01 AG041274 from National Institutes of Health, Bethesda, MD, USA; investigator-initiated research grant from Alzheimer's Association, Chicago, IL, USA; and Cure Alzheimer's Fund, Wellesley, MA, USA to Z.X. Isoflurane and sevoflurane were generously provided by the Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. These studies are attributed to the Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School.
References
- 1.Liu LL, Leung JM. Predicting adverse postoperative outcomes in patients aged 80 years or older. J Am Geriatr Soc. 2000;48:405–12. doi: 10.1111/j.1532-5415.2000.tb04698.x. [DOI] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. doi:10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl. 1):i41–6. doi: 10.1093/bja/aep291. doi:10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. doi:10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 5.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–7. doi: 10.1213/01.ANE.0000135408.14319.CC. table of contents doi:10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi SL, Tran T, Liu C, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–10. doi: 10.1016/j.neurobiolaging.2007.02.009. doi:10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. doi:10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Tian M, Zhen Y, et al. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–5. doi: 10.1213/ANE.0b013e31823b2602. doi:10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33:1364–78. doi: 10.1016/j.neurobiolaging.2010.11.002. doi:10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braida D, Sacerdote P, Panerai AE, et al. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153:423–9. doi: 10.1016/j.bbr.2003.12.018. doi:10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Cao XZ, Ma H, Wang JK, et al. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1426–32. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–53. doi: 10.1016/j.neurobiolaging.2007.04.012. doi:10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patanella AK, Zinno M, Quaranta D, et al. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. 2010;88:1106–12. doi: 10.1002/jnr.22276. [DOI] [PubMed] [Google Scholar]
- 14.Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25:1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- 15.Schuitemaker A, Dik MG, Veerhuis R, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–9. doi: 10.1016/j.neurobiolaging.2008.01.014. doi:10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Katsumata Y, Harigai M, Kawaguchi Y, et al. Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheumatol. 2007;34:2010–7. [PubMed] [Google Scholar]
- 17.Zhang G, Dong Y, Zhang B, et al. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–60. doi: 10.1523/JNEUROSCI.5694-07.2008. doi:10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Dong Y, Xu Z, et al. 2-Deoxy-d-glucose attenuates isoflurane-induced cytotoxicity in an in vitro cell culture model of H4 human neuroglioma cells. Anesth Analg. 2011;113:1468–75. doi: 10.1213/ANE.0b013e31822e913c. doi:10.1213/ANE.0b013e31822e913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei H, Liang G, Yang H, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. doi:10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. doi:10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–8. doi: 10.1038/nn1110. doi:10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 22.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31:378–93. doi: 10.1159/000232556. doi:10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. doi:10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–92. doi: 10.1179/016164104X2357. doi:10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 25.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–9. doi: 10.1038/8432. doi:10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. doi:10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Cho SH, Kim JS, et al. Human astrocytic bradykinin B(2) receptor modulates zymosan-induced cytokine expression in 1321N1 cells. Peptides. 2010;31:101–7. doi: 10.1016/j.peptides.2009.10.011. doi:10.1016/j.peptides.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Kim KS, Oh da H, Choi HM, et al. Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes. Eur J Pharmacol. 2009;613:167–75. doi: 10.1016/j.ejphar.2009.04.026. doi:10.1016/j.ejphar.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Snyder JG, Prewitt R, Campsen J, Britt LD. PDTC and Mg132, inhibitors of NF-kappaB, block endotoxin induced vasodilation of isolated rat skeletal muscle arterioles. Shock. 2002;17:304–7. doi: 10.1097/00024382-200204000-00011. doi:10.1097/00024382-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 30.He HJ, Zhu TN, Xie Y, et al. Pyrrolidine dithiocarbamate inhibits interleukin-6 signaling through impaired STAT3 activation and association with transcriptional coactivators in hepatocytes. J Biol Chem. 2006;281:31369–79. doi: 10.1074/jbc.M603762200. doi:10.1074/jbc.M603762200. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Dong Y, Maeda U, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. doi:10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. doi:10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–6. doi: 10.1093/gerona/61.12.1300. doi:10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 34.Zhen Y, Dong Y, Wu X, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111:741–52. doi: 10.1097/ALN.0b013e3181b27fd4. doi:10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CF, Liu SH, Lin-Shiau SY. Pyrrolidine dithiocarbamate augments Hg(2+)-mediated induction of macrophage cell death via oxidative stress-induced apoptosis and necrosis signaling pathways. Toxicol Lett. 2012;214:33–45. doi: 10.1016/j.toxlet.2012.08.006. doi:10.1016/j.toxlet.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J. 2007;21:2642–54. doi: 10.1096/fj.06-7615rev. doi:10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 37.Mazumdar T, Gorgun FM, Sha Y, et al. Regulation of NF-kappaB activity and inducible nitric oxide synthase by regulatory particle non-ATPase subunit 13 (Rpn13) Proc Natl Acad Sci USA. 2010;107:13854–9. doi: 10.1073/pnas.0913495107. doi:10.1073/pnas.0913495107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–31. doi: 10.1001/archneurol.2009.48. doi:10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilmore TD, Wolenski FS. NF-kappaB: where did it come from and why? Immunol Rev. 2012;246:14–35. doi: 10.1111/j.1600-065X.2012.01096.x. doi:10.1111/j.1600-065X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z, Xiao SB, Xu P, et al. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-kappaB. J Biol Chem. 2011;286:21401–12. doi: 10.1074/jbc.M110.198630. doi:10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]