Abstract
A protective or deleterious role of CD8+T cells in human cutaneous leishmaniasis (CL) has been debated. The present report explores the participation of CD8+T cells in disease pathogenesis as well as in parasite killing. CD8+T cells accumulated in CL lesions as suggested by a higher frequency of CD8+CD45RO+T cells and CD8+CLA+T cells compared with peripheral blood mononuclear cells. Upon Leishmania braziliensis restimulation, most of the CD8+T cells from the lesion expressed cytolytic markers, CD107a and granzyme B. Granzyme B expression in CL lesions positively correlated with lesion size and percentage of TUNEL-positive cells. We also observed a significantly higher percentage of TUNEL-positive cells and granzyme B expression in the biopsies of patients showing a more intense necrotic process. Furthermore, coculture of infected macrophages and CD8+T lymphocytes resulted in the release of granzyme B, and the use of granzyme B inhibitor, as well as z-VAD, Fas:Fc, or anti-IFN-γ, had no effect upon parasite killing. However, coculture of infected macrophages with CD4+T cells strongly increased parasite killing, which was completely reversed by anti-IFN-γ. Our results reveal a dichotomy in human CL: CD8+ granzyme B+T cells mediate tissue injury, whereas CD4+IFN-γ+T cells mediate parasite killing.
Introduction
Leishmaniasis is caused by protozoan parasites of the genus Leishmania transmitted by sand fly vectors. Sand flies inject the parasite along with their saliva, favoring the establishment of Leishmania infection (Kaye and Scott, 2011). In host cells, parasites transform into amastigotes that live and multiply within parasitophorous vacuoles, mainly in macrophages (McConville and Naderer, 2011). Recent reports have shown that neutrophils are able to uptake Leishmania parasites early after infection (Kaye and Scott, 2011; Ribeiro-Gomes and Sacks, 2012). Cytokines and chemokines secreted by these cells modulate the development of anti-Leishmania immune response (Ribeiro-Gomes and Sacks, 2012). Leishmania infection in resistant strains of mice induces a T-cell helper type 1 (Th1) response, characterized by IFN-γ production that activates macrophages (Mougneau et al., 2011) and nitric oxide and reactive oxygen species production, which are the main mediators responsible for the killing of parasites (McConville and Naderer, 2011). In susceptible mice, Leishmania infection elicits predominantly a T-cell helper type 2 response that allows parasite proliferation (Kaye and Scott, 2011).
Human cutaneous leishmaniasis (CL) caused by L. braziliensis is characterized by strong cellular responses and scarce numbers of parasites in the lesions (Carvalho et al., 2012). The presence of activating cytokines, such as IFN-γ and tumor necrosis factor-α, is decisive for the control of parasite dissemination, but an exaggerated Th1 response has been associated with the severe inflammation observed in CL lesions (Bacellar et al., 2002; Faria et al., 2005; Carvalho et al., 2007).
The role of CD8+T cells in human CL is not fully elucidated. Upon Leishmania infection in an in vitro priming system of human peripheral blood mononuclear cells (PBMC), CD8+T cells are stimulated earlier compared with CD4+T cells and produce large amounts of IFN-γ, possibly driving the differentiation of Th1 lymphocytes (Pompeu et al., 2001). An enrichment of Leishmania-reactive CD8+T cells in older lesions suggests that they also have an important role in the healing process (Da-Cruz et al., 2005). Our group also demonstrated that CD8+T cells are implicated in the pathogenesis of mucocutaneous leishmaniasis (Barral-Netto et al., 1995; Brodskyn et al., 1997). These data suggest that CD8+T cells participate in the healing process as well as in the development of active disease.
Herein, we show that the majority of CD8+T cells in the lesions display cytotoxic characteristics and the frequency of granzyme B+ cells correlates positively with lesion size. In addition, in the biopsies showing a more intense process of necrosis, a higher percentage of TUNEL-positive cells and Granzyme B+ cells were observed. Moreover, IFN-γ produced by CD4+T cells is essential for parasite killing, but its expression was not correlated with tissue injury.
Together, our data reveal a dichotomy in CL: CD8+Granzyme B+T cells mediate tissue injury, whereas CD4+IFN-γ+T cells mediate parasite killing.
Results
Recruitment of CD8+T cells to lesions of CL patients
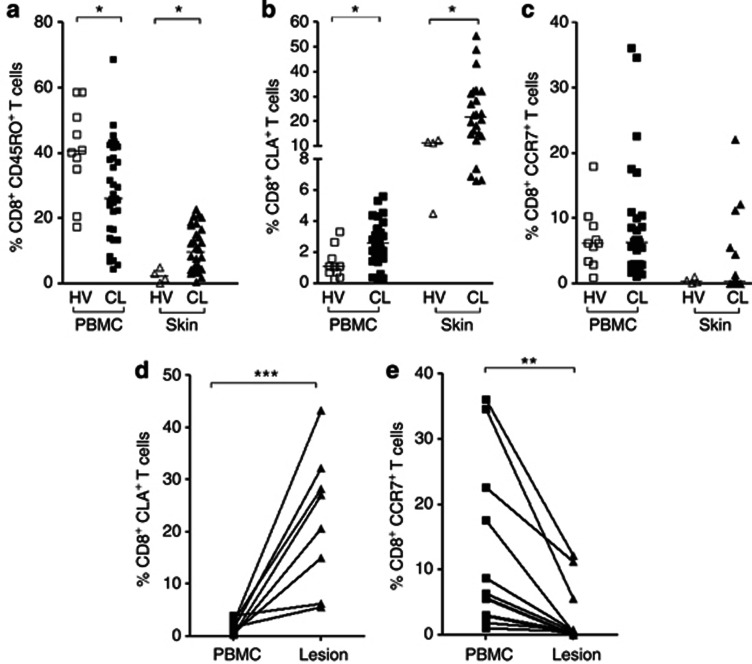
CL patients showed a significantly lower percentage of CD8+CD45RO+T cells in PBMC (28.29%±14.73%) compared with healthy individuals (40.67%±13.97% Figure 1a-left). However, there was a significantly higher percentage of CD8+CD45RO+T cells at the lesion site (skin; 10.50%±6.73%) compared with that of normal skin tissue (2.41%±2.03; Figure 1a-right), suggesting a specific recruitment of CD8+T cells to the site of infection. By using the same markers, we also showed recruitment of CD4+T cells to the site of infection (Supplementary Figure S1a online).
Figure 1.
Recruitment and expression of homing markers in CD8+ T cells from cutaneous leishmaniasis (CL) lesions. (a) Ex vivo analysis of CD8+CD45RO+ T cells, (b) CD8+CLA+ T cells, and (c) CD8+CCR7+ T cells in peripheral blood mononuclear cells (PBMC) from healthy volunteers (HV) (n=10, open squares), from CL patients (n=35, closed squares), and in skin biopsies from healthy controls (n=4, open triangles) and lesions (n=30, closed triangles) from CL patients. (d, e) Ex vivo analysis of homing markers in PBMC (closed squares) and lesion cells (closed triangles) from CL patients (n=9). Each symbol represents one individual and the lines represent results obtained in the same patient. *P<0.05. Statistical comparisons were done using the Mann–Whitney U-test. **P<0.01, ***P<0.001.
The expression of homing molecules was also analyzed ex vivo. There was an increase in the frequency of CD8+T cells expressing cutaneous leukocyte–associated antigen (CLA; skin homing) in both PBMC (2.58%±1.43, Figure 1b-left) and at the lesion site (skin; 23.31%±12.99, Figure 1b-right) from CL patients compared with healthy individuals (PBMC=1.28%±0.98; normal tissue=11.02%±3.73). However, there were no differences in chemokine (C-C motif) receptor 7 (CCR7) expression (lymph node homing), either in PBMC (8.71%±9.00) or at the lesion site (skin; 2.37%±5.17) from CL patients compared with healthy individuals (PBMC=6.89%±4.77; normal tissue=0.43%±0.40; Figure 1c). The difference in CLA expression between the healthy individuals and the CL patients was not observed in the CD4+T-cell population (data not shown). Comparing CLA and CCR7 expression in cells from the same patient, we observed a significantly increased expression of CLA (23.38%±12.65; Figure 1d) and a decreased expression of CCR7 (2.50%±4.33; Figure 1e) in CD8+T cells obtained from the lesion site compared with PBMC (CLA=1.70%±1.24; CCR7=11.44%±12.32). The expression of these markers on CD4+T cells followed the same behavior observed on CD8+T cells (Supplementary Figure S1b and c online). These results suggest a specific homing of effector T cells to the lesion site in CL.
Cells from CL lesions display a high frequency of CD8+T cells expressing cytolytic markers
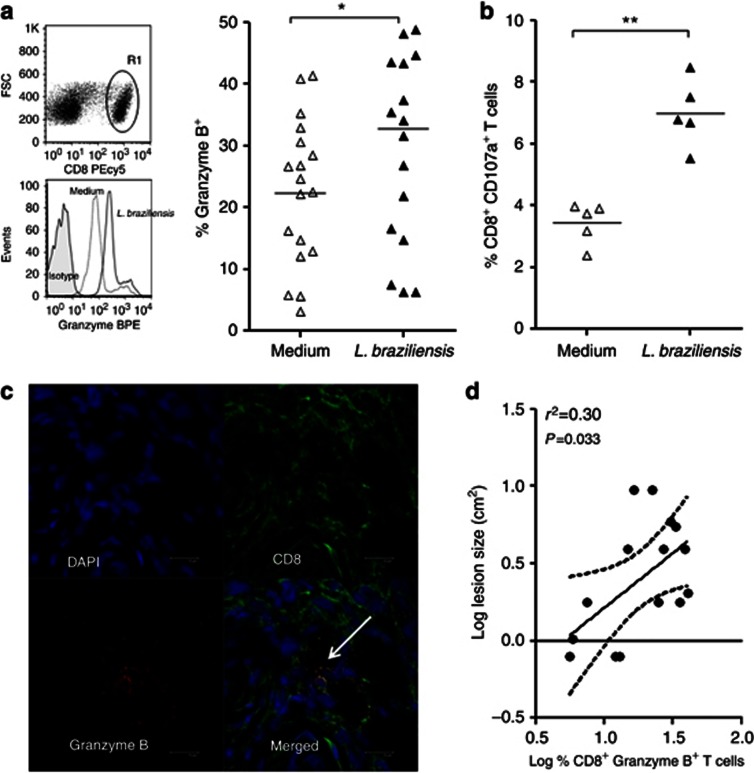
In order to investigate whether in situ CD8+T cells exhibit cytotoxic activity, cutaneous lesion cells were stimulated for 24 hours with L. braziliensis promastigotes and CD8+T cells were stained for granzyme B. Although the frequency of CD8+T cells expressing granzyme B was high in unstimulated cultures, there was an upregulation of this cytotoxic enzyme after restimulation with L. braziliensis (Figure 2a).
Figure 2.
Expression of cytolytic markers by lesion-derived CD8+ T cells from cutaneous leishmaniasis (CL) patients after stimulation. (a) R1 was analyzed from the CD8 versus forward scatter (FSC) dot plot (top). Histograms are representative of 18 CL patients and the graph displays the frequency of CD8+ T cells expressing granzyme B. (b) Expression of CD107a in CD8 T cells. (c) Representative images from confocal microscopy. 4',6-Diamidino-2-phenylindole (DAPI) counterstained (top left panel); CD8 staining (top right panel); granzyme B staining (bottom left panel); overlay showing expression of granzyme B in CD8+ T cells (white arrow; bottom right panel), bar=10 μm. (d) Correlation analysis between the frequency of CD8+Granzyme B+ T cells and lesion size. *P<0.05, **P<0.01. Statistical comparisons were done using the Mann–Whitney U-test and the Spearman's (r2) rank test. PE, phycoerythrin.
Another important marker of cytotoxic activity is CD107a, a lysosomal protein that is transiently exposed at the cell surface upon degranulation (Betts et al., 2003). As shown in Figure 2b, stimulation with L. braziliensis also leads to an increase of CD107a expression on CD8+T cells compared with unstimulated cells. Taken together, these data point at the role of CD8+T cells as effector cytotoxic cells in the lesion.
To further explore the presence of CD8+Granzyme B+T cells at the lesion site, confocal microscopy was performed. In Figure 2c, we observed the presence of CD8+ T cells (right-top panel) and granzyme B (left-bottom panel). There was a colocalization between CD8+T cells and granzyme B (merged image showed in right-bottom panel), confirming that these cells are present in the lesions. A significant positive correlation was observed between the frequency of intralesional CD8+Granzyme B+T cells and lesion size (Figure 2d). We also evaluated the frequency of CD4+T cells expressing IFN-γ. Although these cells are more abundant in the lesion compared with CD8+T cells, there was no correlation between the frequencies of CD4+IFN-γ+T cells and lesion size (Supplementary Figure S2a online), suggesting that CD8+T cells are key factors in tissue destruction.
In situ quantification of tissue injury in lesions of CL patients
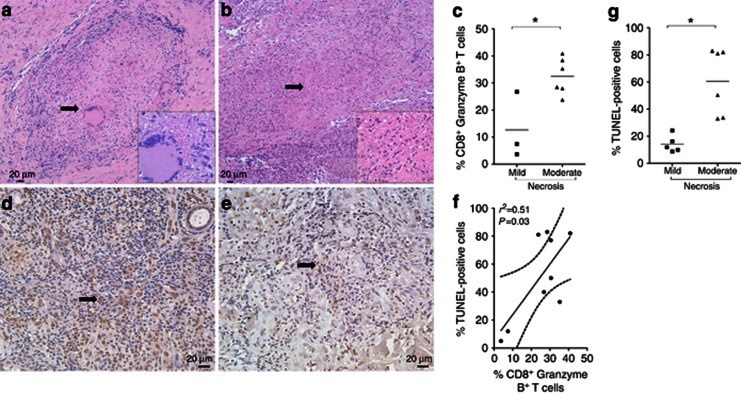
Analysis of tissue sections showed the inflammatory infiltrate did not vary in composition among the biopsies, showing the presence of lymphocytes, macrophages, and plasma cells. Focal necrosis predominated among the biopsies, although a diffuse distribution of necrosis was occasionally noted. Figure 3a is an example of mild necrosis (score 1, arrowhead and inset in Figure 3a) where fibrous connective tissue was characterized by the presence of inflammatory mononuclear cells and a well-defined granuloma with multinucleated giant cells. In Figure 3b, we observed a moderate intensity of necrosis (score 2, arrowhead and inset in Figure 3b) characterized by a dermoepidermal junction mononuclear band with an inflammatory infiltration, composed by lymphocytes and a focal moderate necrosis area with apoptotic neutrophils and eosinophils. Therefore, our next question was to investigate the relation between the necrosis intensity and the CD8+T-cell function, measured by the expression of granzyme B. In Figure 3c, it was shown that biopsies presented a higher intensity of necrosis (score 2) and also displayed a significantly higher percentage of CD8+Granzyme B+T cells.
Figure 3.
Histopathology and TUNEL-positive cells in cutaneous leishmaniasis (CL) lesions. (a, b) Necrosis scores 1 and 2 showed by arrowhead and inset. (c) Analysis between intensity of necrosis and percentage of CD8+Granzyme B+ T cells. DNA damage in biopsies with (d) higher and (e) lower percentages of TUNEL-positive cells, which are indicated by black arrows. (f) Correlation analysis between the percentage of CD8+Granzyme B+ T cells in the tissue and the percentage of TUNEL+ cells. (g) Analysis between the intensity of necrosis and the number of TUNEL+ cells. Bar=20 μm. *P<0.05. Statistical comparisons were done using the Mann–Whitney U-test and the Spearman's (r2) rank test. Pictures represented a magnification of × 200, insets × 1,000, and TUNEL+ cells × 400.
Upon encounter with the target cell, granzyme B is released from cytotoxic cells, leading to DNA fragmentation (Lieberman, 2010). Thereafter, we quantified DNA damage in CL lesions, using a TUNEL assay. The stain revealed a different percentage of TUNEL-positive cells in CL biopsies, as shown in Figure 3d (high percentage) and Figure 3e (low percentage). Moreover, positive correlation was detected between the frequency of CD8+Granzyme B+T cells in the tissue and the percentage of TUNEL-positive cells (Figure 3f). We also observed that biopsies displaying moderate intensity of necrosis showed a higher percentage of TUNEL-positive cells (Figure 3g), which reflects a positive correlation between the intensity of necrosis and the percentage of TUNEL-positive cells. However, no correlation was observed between the percentage of TUNEL-positive cells and the frequency of CD4+IFN-γ+T cells in the tissue (Supplementary Figure S2b online). Together, these results suggest that cytotoxic T cells in lesions from CL patients could mediate lysis of target cells, through the release of granzyme B, leading to necrosis and tissue injury.
CD8+ T cell–mediated cytotoxicity does not contribute to parasite killing in CL patients
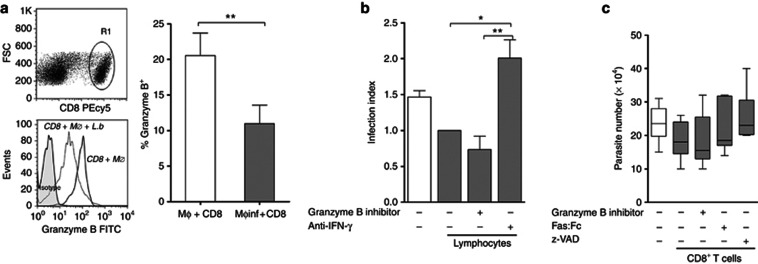
As CD8+T cells are present at the lesion site of CL patients and exert cytotoxic activity, causing cell death, an important question is whether CD8+T cells could also be able to kill the parasites through destruction of infected cells. Therefore, we analyzed the relationship between the cytotoxicity exerted by CD8+T cells and Leishmania killing. Macrophages from PBMC of CL patients were infected with L. braziliensis promastigotes and cocultivated with peripheral autologous effector lymphocytes for 48 hours. In fact, a significant decrease in the expression of granzyme B was observed in CD8+T cells upon coculture with infected macrophages (Figure 4a) compared with controls (uninfected macrophages), suggesting release of granules in the presence of target cells. Furthermore, we investigated whether the presence of granzyme B contributed to parasite killing. Peripheral blood lymphocytes were cocultivated with autologous infected macrophages, in the presence or absence of granzyme B inhibitor or anti-IFN-γ. The percentage of infected macrophages and the number of amastigotes were determined, and the infection index was calculated. Cocultures of infected macrophages and lymphocytes in the presence of granzyme B inhibitor did not alter the infection index (Figure 4b). Cocultures in the presence of anti-IFN-γ significantly increased the infection index compared with controls (cocultures of macrophages and lymphocytes without addition of inhibitors; Figure 4b).
Figure 4.
Cytotoxic CD8+ T cell does not contribute to parasite killing in cutaneous leishmaniasis (CL) patients. Macrophages from peripheral blood mononuclear cells (PBMC) of CL patients were infected or not infected with parasites and cocultured with autologous lymphocytes or peripheral CD8+ T cells for 48 hours. (a) R1 was analyzed from the CD8 versus forward scatter (FSC) dot plot (top). Histograms are representative of six CL patients. The graph shows the expression of granzyme B in CD8+ T cells. (b, c) Infected macrophages cocultured (closed bars) or not (open bar) with peripheral lymphocytes (b) or with purified CD8+ T cells (closed bars) (c) in the absence or presence of inhibitors. *P<0.05; **P<0.01. Statistical comparisons were done using the Mann–Whitney U-test, the Kruskal–Wallis test, or the Friedman test, followed by the Dunn's multiple comparison test.
Furthermore, to confirm this data, we analyzed the number of viable parasites in the system. We cocultivated infected macrophages with peripheral autologous blood CD8+ T cells, in the presence or absence of granzyme B inhibitor for 48 hours. Moreover, to evaluate possible molecular mechanisms of CD8+-triggered apoptosis, we added z-VAD, a pan-caspase inhibitor or Fas:Fc, to inhibit the interaction between Fas and Fas-L in the culture. After this period, the medium was replaced by Schneider's medium and the number of viable parasites was evaluated. There were no differences in the number of viable parasites in the cocultures of infected macrophages with CD8+T cells in the presence or absence of any inhibitors tested when compared with the control, i.e., infected macrophages (Figure 4c). As a negative control for zVAD, we used its diluent DMSO, which had no significant effect upon CD8+-dependent parasite killing (data not shown).
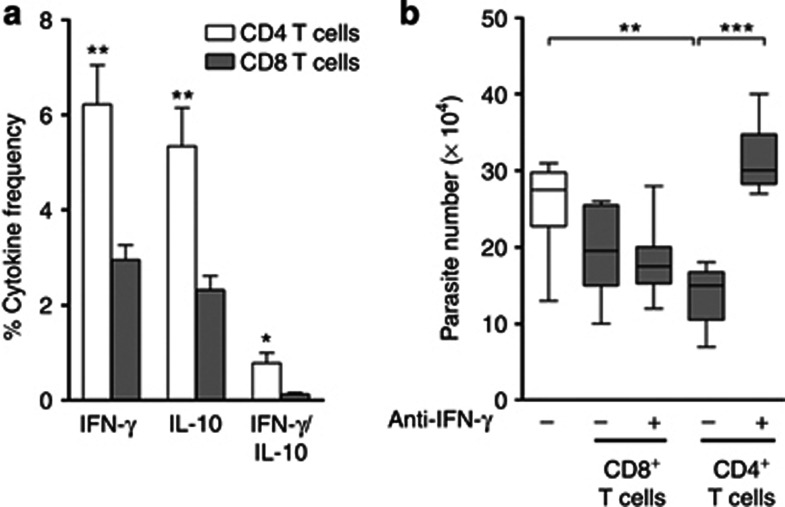
Besides exerting cytotoxicity, CD8+T cells also exert immunomodulatory effects through cytokine production. Therefore, we analyzed cytokine expression by both CD8+ and CD4+T cells obtained from the lesion site after restimulation with L. braziliensis promastigotes. As shown in Figure 5a, the frequency of CD4+T cells producing IFN-γ and/or IL-10 is significantly higher compared with CD8+T cells, and we also observed a lower frequency of CD4+ and CD8+ T cells expressing both cytokines. These results indicate CD4+T cells as the main source of cytokines in CL in situ. It has been known that IFN-γ production is necessary to activate macrophages and kill intracellular parasites (Mougneau et al., 2011). Therefore, we quantified parasite killing in cocultures of infected macrophages with purified peripheral CD8+ or CD4+T cells, in the presence or absence of anti-IFN-γ. We did not observe differences in the number of viable parasites in the cocultures of CD8+T cells and infected macrophages in the presence or absence of anti-IFN-γ compared with the control, that is, infected macrophages alone (Figure 5b). There was a significant decrease in parasite viability in cocultures of CD4+T cells and infected macrophages, compared with the control (Figure 5b). In addition, the presence of anti-IFN-γ in these cocultures led to a significant increase in viable parasite numbers (Figure 5b), pointing out the decisive role of CD4+IFN-γ+T cells in parasite killing.
Figure 5.
CD4+, but not CD8+, T cells are the principal source of cytokines in cutaneous leishmaniasis (CL) lesions and are responsible for the killing of parasites. (a) The frequency of lesional CD4+ T cells (open bars) and CD8+ T cells (closed bars) expressing IFN-γ and IL-10 or coexpressing these cytokines, after restimulation in vitro with L. braziliensis. (b) Parasite load in infected macrophages (open bar) and in coculture of infected macrophages with peripheral CD8+ or CD4+ T cells (closed bars) in the absence or presence of anti-IFN-γ. *P<0.05; **P<0.01; ***P<0.001. Statistical comparisons were done using the Mann–Whitney U-test or the Friedman test, followed by the Dunn's multiple comparison test.
Discussion
Our results showed a recruitment of CD8+T cells to the infection site, as shown by the higher percentage of CD8+CD45RO+T cells in the lesions compared with healthy skin. Most possibly, extravasation and retention of these cells in the lesion are mediated by CLA as shown by other reports in the literature (Costa et al., 2003; Mendes-Aguiar Cde et al., 2009; Lieberman, 2010).This preferential migration of CD8+CLA+T cells to the lesion and their permanence at this site was confirmed by the decrease in the percentage of CD8+CCR7+T cells in the tissue. CCR7 expression drives lymphocyte migration to the lymph nodes and downregulation of this receptor occurs mainly in effector cells (Costa et al., 2003; Sallusto and Mackay, 2004). Similarly, cutaneous CD8+CLA+T cells in atopic dermatitis lesions have been shown to display an effector memory phenotype (Akdis et al., 1999). In biopsies from CL patients infected by L. braziliensis, these effector cells are predominantly cytotoxic, and our correlations between cytotoxic markers, intensity of necrosis, and lesion size point out the harmful role played by these cells, contributing to the tissue injury.
Although many reports in the literature point toward cytotoxicity as an immune mechanism favoring a protective response, the presence of cytotoxic CD8+T cells has also been associated with tissue damage in mucocutaneous leishmaniasis (Brodskyn et al., 1997) as well as lesion progression in CL patients (Faria et al., 2009). Moreover, data on the development of experimental cerebral malaria suggest that cytotoxic CD8+T cells may contribute to immunopathology (Haque et al., 2011). The cytolytic function of CD8+T cells could induce rapid DNA fragmentation, a hallmark feature of cell death, which is an important biological function of granzyme B (Lord et al., 2003). Recently, Hernandez-Ruiz et al. (2010) observed a large number of apoptotic macrophages in CL lesions from patients infected with L. mexicana, possibly induced by CD8+T cells. In fact, in our study, positive correlations between the lesional CD8+Granzyme B+T cells and the lesion size and between the percentage of cells expressing granzyme B and the TUNEL-positive cells reveal the participation of these cells in tissue damage in CL caused by L. braziliensis. These data are also reinforced by the association between intensity of necrosis and the percentage of CD8+Granzyme B+T cells observed in these tissues. Recently, Sharron et al. (2012) using a mouse sepsis model, found that platelet granzyme B–mediated cell death occurs in spleen and lung, whereas the absence of granzyme B slows sepsis progression, clearly demonstrating the harmful role played by granzyme B.
In human CL caused by L. major and L. mexicana, an increase in granzyme B activity was associated with a good prognosis (Bousoffara et al., 2004; Hernandez-Ruiz et al., 2010). In these studies, cytotoxicity observed in the coculture of infected macrophages with peripheral blood lymphocytes was mediated by granzyme B. However, the effect of this cytotoxicity on parasite killing was not explored. In L. braziliensis-infected macrophages, we also observed a release of granzyme B, but the inhibition of this serine protease or even apoptosis inhibitors did not lead to changes in the number of viable parasites, suggesting that these cells do not participate in the control of parasite growth.
Besides exerting cytotoxicity, CD8+T cells are important source of cytokines (Bourreau et al., 2007; Jordan and Hunter, 2010; Nateghi Rostami et al., 2010). In human CL caused by L. major, CD8+T and Th1 cells are important for the resolution of the infection, mainly producing IFN-γ (Da-Cruz et al., 2005; Nateghi Rostami et al., 2010). Our results in cells from biopsies of CL patients caused by L. braziliensis demonstrated that CD4+T cells are the main source of IFN-γ, and this outcome was related to parasite killing rather than lesion development. Although CD8+T cells also produce IFN-γ, they have as principal function the cytotoxicity, contributing to the lesion injury. However, some reports in the literature have shown that IFN-γ could be responsible for the intense inflammatory response observed in CL patient lesions (Ribeiro-de-Jesus et al., 1998; Antonelli et al., 2005; Faria et al., 2009), and no correlation was observed between CD4+IFN-γ+T cells and lesion size, suggesting CD8+T cells are really the key cells involved in tissue destruction. These results are important for the understanding of immunopathogenesis of CL and could contribute to the delineation of different therapeutic treatments. However, the activation of CD8+T cells in the early events of infection seems to be important to induce a protective response (Pompeu et al., 2001) and even in the efficacy of different schedules of immunization (Jayakumar et al., 2011). Therefore, the fine regulation of CD8+T cells in CL is a key factor to induce protection or lesion injury.
In summary, we show that CD8+T cells in CL patients have a peculiar profile of effector cells migrating to the lesion, exerting cytotoxicity, and, therefore, contributing to tissue destruction rather than parasite control, which is exerted mainly by CD4+T cells. These results reveal a dichotomy in human CL: CD8+Granzyme B+ T cells mediate tissue injury, whereas CD4+IFN-γ+ T cells mediate parasite killing.
Materials and Methods
Ethics
This study is a part of a project approved by Centro de Pesquisas Gonçalo Moniz (CPqGM/FIOCRUZ-Bahia)-Institutional Review Board number 124/2007. Informed written consent was obtained from all participants or legal guardians before entering the study. Clinical investigations were conducted in accordance with principles expressed in the Declaration of Helsinki Principles.
Subjects
Sixty CL patients (47 male patients and 13 female patients; 31±17-year old) were recruited at our field clinic in the municipality of Jiquiriça (State of Bahia, Brazil). All patients presented active lesion (lesion size, 4.14±4.08 cm2) with less than 60 days of infection (a mean of 39 days of disease duration). The control group consisted of eight healthy individuals (five male individuals and three female individuals, 27±3-year old) from a nonendemic area for L. braziliensis infection. Diagnostic criteria for CL included clinical and histological characteristics of skin lesion and a positive result in anti-Leishmania delayed-type hypersensitivity or serology. None of the individuals had reported prior infection with Leishmania. Blood and tissue specimens were obtained before patients received treatment with antimoniate-N-methyl-glucamine (15 mg Sb−1 d−1 for 20 days). Normal skin tissue samples were obtained after plastic surgery. Tissue samples were incubated in RPMI-1640 medium for cell culture.
Isolation of PBMC and leukocytes from skin lesions
Following the Ficoll–Hypaque gradient centrifugation, PBMC were resuspended in RPMI-1640 supplemented with 2 mℳℒ-glutamine, penicillin (100 U ml−1), streptomycin (100 μg ml−1; Invitrogen, Carlsbad, CA), and heat-inactivated human serum AB Rh+ (Sigma Chemical, St Louis, MO; complete medium). Tissue samples were incubated with 50 μg ml−1 of liberase (Roche Diagnostics, GmbH, Mannheim, Germany) for 1 hour at 37 °C. Cell suspensions were obtained after mechanical disruption (Medimachine, BD Biosciences, San Jose, CA), according to the manufacturer's instructions. Tissue homogenates were filtered using a 30-μm cell strainer. Leukocyte viability was evaluated by trypan blue exclusion.
Lymphocytes culture and flow cytometry
PBMC and lesion cells were stained directly for ex vivo detection of CLA-FITC (HECA-452), CCR7-FITC (3D12), CD45RO-PE (UCHL1), CD4-FITC (RPA-T4), CD4-Pecy7 (SK3), and CD8-PeCy5 (RPA-T8). Alternatively, the cells were plated in 48-well plates (Corning Incorporated Life Sciences, Lowell, MA) and incubated at 37 °C, 5% CO2, and in the presence of L. braziliensis (MHOM/BR/00/BA788; 1 cell:1 parasite) for 24 hours. During the last 4 hours of culture, brefeldin A (BD Biosciences) was added to the cultures. The cells were fixed and permeabilized using cytofix/cytoperm solution (BD Biosciences) and stained for 30 minutes at 4 °C using monoclonal antibodies directly conjugated to fluorochromes, against granzyme B (FITC or phycoerythrin (PE), both GB11), IFN-γ (Alexa 488-B27, PE-4S.B3), or IL-10 (PE-JE53-19F1 or APC-JE53-9D7). In some experiments, fluorochrome conjugated to anti-LAMP (CD107a-FITC-eBioH4A3) antibody (eBioscience, San Diego, CA) was added before the stimulation. FITC-, PE-, APC, and Alexa 488-labeled immunoglobulin control antibodies were included in all experiments. In all cases, 10,000 events/samples for cutaneous lesion cells and 100,000 events/samples for PBMC were acquired in a FACSort_flow cytometer (Franklin Lakes, NJ) (Becton Dickinson, Immunocytometry) and a FASCsAria cell sorter (BD Biosciences). All data were analyzed using FlowJo software (Ashland, OR).
Confocal microscopy
Confocal analysis was used to identify CD8 lymphocytes expressing granzyme B in cutaneous lesions. Rabbit anti-CD8 (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-granzyme B were used as primary antibody. Secondary antibodies were biotin anti-mouse IgG (Vector Laboratories, Burlingame, CA) or anti-rabbit Alexa 488 (Molecular Probes, Eugene, OR). Streptavidin Cy3 (Sigma, Buchs, Switzerland) was used after biotin antibody and the sections were counterstained with 4',6-diamidino-2-phenylindole. Multiple images representing positive staining and negative controls were acquired using a confocal microscopy (Leica TCS SP2 SE and SP5 AOB5). The images were acquired using the software Image-Pro Plus (Media Cybernetics, Silver Spring, MD).
Histopathology
Cutaneous punch biopsy samples (4 mm) were obtained from the border of ulcer and immediately fixed in 10% formalin-buffered solution. Three tissue sections of 3-μm to 5-μm thick were stained with hematoxylin and eosin. The necrosis process was quantified according to its extension on histological sections and attributed the following scores: 0 (zero), if the extension did not exceed 25% of the section; 1 (mild necrosis), if the extension of necrosis was between 25 and 50% of section; 2 (moderate necrosis), if the necrosis involved more than 50% of histological section; and 3 (severe necrosis), if the entire section contains necrosis. To perform this analysis, we selected one of the three sections, analyzing the quality of staining as well as the absence of artifacts.
TUNEL assay
A TUNEL assay was performed using the In Situ Cell Death Detection Kit (Boehringer Mannheim, Mannheim, Germany), according to the manufacturer's instructions. The number of TUNEL-positive cells was counted as 1,000 cells distributed in five different microscopic fields with a magnification power of × 400. Digital images of tissue sections were captured using a Q-Color 1 Olympus digital camera (Olympus, Melville, NY). Measurement of stained areas was performed using Image Pro-Plus software (Media Cybernetics).
Functional assays
PBMC (1 × 106 cells per ml) from CL patients were plated onto glass coverslips placed within the wells of a 24-well plate at 1 ml per well and incubated for 40 minutes, at 37 °C, and with 5% CO2. Nonadherent cells were collected and adherent cells (1 × 105 cells per ml) were cultured at 37 °C with 5% CO2 for additional 7 days. Afterwards, L. braziliensis promastigotes were added or not added in the cultures at a parasite/cell ratio of 5:1 for 4 hours. Infected macrophages were cocultivated for 48 hours with effector lymphocytes (1 × 106 cells per ml, ratio 10:1) or with peripheral CD8 or CD4 T cells (1 × 106 cells per ml, ratio 10:1) obtained using magnetic cell sorting (Mini Macs, Miltenyi Biotec, Auburn, CA) at 37 °C with 5% CO2. Nonadherent cells were then collected and stained, as previously described, with anti-CD8 and anti-granzyme B. For inhibitory assay, cultures were performed in the presence of 3,4-dichloroisocoumarin (100 μmol l−1; Sigma-Aldrich, St Louis, MO), a reactive serine esterase inhibitor, anti-human IFN-γ (5 μg ml−1; eBioscience), z-VAD-FMK (Sigma; 100 μℳ) to block caspase activation, or Fas:Fc chimeric fusion protein (1 μg ml−1; BD Pharmingen). DMSO (vehicle) 0.4% was used as control.
Parasite load
Glass coverslips containing infected macrophages cocultured for 48 hours with effector lymphocytes were washed, stained with hematoxylin and eosin, and analyzed using light microscopy. Results were shown as the infection index, which was calculated dividing the number of amastigotes counted in 100 macrophages obtained in different experimental approaches by the number obtained in the control group that corresponded to the coculture of infected macrophages and lymphocytes without inhibitors. Alternatively, after 48 hours, infected macrophage cocultured with peripheral CD8 or CD4 T cells were washed and medium was replaced by 0.5 ml of the Schneider medium (Sigma-Aldrich) supplemented with 10% fetal calf serum, to quantify the number of viable parasites. The plates were cultured at 26 °C for an additional 5 days. Viable number of L. braziliensis was estimated by proliferation of extracellular motile promastigotes in the Schneider's medium.
Statistical analysis
Comparisons between the two unpaired groups were performed by the Mann–Whitney U-test and among multiple groups by the Kruskal–Wallis test or the Friedman test followed by Dunn's multiple comparison tests. Spearmann correlation analysis tests were also applied. Analyses were conducted using Graph Prism 5 software (GraphPad Software, San Diego, CA) and a P<0.05 was considered significant.
Acknowledgments
The authors are grateful to all patients and control subjects who participated in this study. This study was supported by grants from Fundação de Amparo a Pesquisa do Estado da Bahia (FAPESB) e/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant number PPSUS # SUS0025/2009) and from Pronex (FAPESB/CNPq grant number 738712006). CdaSS has a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil; NT and MJL receive fellowships from CNPq Brazil and CIdO, VMB, JvW, AB, MB-N, and CIB are senior investigators of CNPq.
Glossary
- CCR7
chemokine (C-C motif) receptor 7
- CD107a
lysosomal-associated membrane protein 1
- CL
cutaneous leishmaniasis
- CLA
cutaneous leukocyte–associated antigen
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- Th1
T-cell helper type 1
- z-VAD-FMK
N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Akdis M, Simon HU, Weigl L, et al. Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol. 1999;163:466–475. [PubMed] [Google Scholar]
- Antonelli LR, Dutra WO, Almeida RP, et al. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M, Barral A, Brodskyn C, et al. Cytotoxicity in human mucosal and cutaneous leishmaniasis. Parasite Immunol. 1995;17:21–28. doi: 10.1111/j.1365-3024.1995.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Bourreau E, Ronet C, Couppie P, et al. IL-10 producing CD8+ T cells in human infection with Leishmania guyanensis. Microbes Infect. 2007;9:1034–1041. doi: 10.1016/j.micinf.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bousoffara T, Louzir H, Ben Salah A, et al. Analysis of granzyme B activity as a surrogate marker of Leishmania-specific cell-mediated cytotoxicity in zoonotic cutaneous leishmaniasis. J Infect Dis. 2004;189:1265–1273. doi: 10.1086/382031. [DOI] [PubMed] [Google Scholar]
- Brodskyn CI, Barral A, Boaventura V, et al. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J Immunol. 1997;159:4467–4473. [PubMed] [Google Scholar]
- Carvalho LP, Passos S, Bacellar O, et al. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LP, Passos S, Schriefer A, et al. Protective and pathologic immune responses in human tegumentary leishmaniasis. Front Immunol. 2012;3:301. doi: 10.3389/fimmu.2012.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RP, Gollob KJ, Machado PR, et al. Adhesion molecule expression patterns indicate activation and recruitment of CD4+ T cells from the lymph node to the peripheral blood of early cutaneous leishmaniasis patients. Immunol Lett. 2003;90:155–159. doi: 10.1016/j.imlet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Da-Cruz AM, Bertho AL, Oliveira-Neto MP, et al. Flow cytometric analysis of cellular infiltrate from American tegumentary leishmaniasis lesions. Br J Dermatol. 2005;153:537–543. doi: 10.1111/j.1365-2133.2005.06647.x. [DOI] [PubMed] [Google Scholar]
- Faria DR, Gollob KJ, Barbosa J, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria DR, Souza PE, Duraes FV, et al. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009;31:432–439. doi: 10.1111/j.1365-3024.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Best SE, Unosson K, et al. Granzyme B expression by CD8+ T cells is required for the development of experimental cerebral malaria. J Immunol. 2011;186:6148–6156. doi: 10.4049/jimmunol.1003955. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ruiz J, Salaiza-Suazo N, Carrada G, et al. CD8 cells of patients with diffuse cutaneous leishmaniasis display functional exhaustion: the latter is reversed, in vitro, by TLR2 agonists. PLoS Negl Trop Dis. 2010;4:e871. doi: 10.1371/journal.pntd.0000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar A, Castilho TM, Park E, et al. TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia) PLoS Negl Trop Dis. 2011;5:e1204. doi: 10.1371/journal.pntd.0001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KA, Hunter CA. Regulation of CD8+ T cell responses to infection with parasitic protozoa. Exp Parasitol. 2010;126:318–325. doi: 10.1016/j.exppara.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Anatomy of a murder: how cytotoxic T cells and NK cells are activated, develop, and eliminate their targets. Immunol Rev. 2010;235:5–9. doi: 10.1111/j.0105-2896.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Lord SJ, Rajotte RV, Korbutt GS, et al. Granzyme B: a natural born killer. Immunol Rev. 2003;193:31–38. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Naderer T. Metabolic pathways required for the intracellular survival of Leishmania. Annu Rev Microbiol. 2011;65:543–561. doi: 10.1146/annurev-micro-090110-102913. [DOI] [PubMed] [Google Scholar]
- Mendes-Aguiar Cde O, Gomes-Silva A, Nunes E, et al. The skin homing receptor cutaneous leucocyte-associated antigen (CLA) is up-regulated by Leishmania antigens in T lymphocytes during active cutaneous leishmaniasis. Clin Exp Immunol. 2009;157:377–384. doi: 10.1111/j.1365-2249.2009.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougneau E, Bihl F, Glaichenhaus N. Cell biology and immunology of Leishmania. Immunol Rev. 2011;240:286–296. doi: 10.1111/j.1600-065X.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- Nateghi Rostami M, Keshavarz H, Edalat R, et al. CD8+ T cells as a source of IFN-gamma production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010;4:e845. doi: 10.1371/journal.pntd.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeu MM, Brodskyn C, Teixeira MJ, et al. Differences in gamma interferon production in vitro predict the pace of the in vivo response to Leishmania amazonensis in healthy volunteers. Infect Immun. 2001;69:7453–7460. doi: 10.1128/IAI.69.12.7453-7460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-de-Jesus A, Almeida RP, Lessa H, et al. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–148. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Sacks D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front Cell Infect Microbiol. 2012;2:59. doi: 10.3389/fcimb.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr Opin Immunol. 2004;16:724–731. doi: 10.1016/j.coi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Sharron M, Hoptay CE, Wiles AA, et al. Platelets induce apoptosis during sepsis in a contact-dependent manner that is inhibited by GPIIb/IIIa blockade. PLoS One. 2012;7:e41549. doi: 10.1371/journal.pone.0041549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.